High-protein diets are popular because they help to control body fat( Reference Moran, Noakes and Clifton 1 , Reference Noakes, Keogh and Foster 2 ). This is related to the fact that high protein intake together with low carbohydrate intake control appetite and increase the need for irreversible N disposal, thermogenesis and gluconeogenesis, processes that are highly energy consuming( Reference Halton and Hu 3 , Reference Kuhla, Kucia and Görs 4 ). However, studies in pregnant women showed that high protein together with low carbohydrate intake can result in fetal growth retardation( Reference Campbell-Brown, Johnston and Kerr Grieve 5 – Reference Rush 7 ), with a greater risk of the offspring developing adult-onset diseases (Barker hypothesis)( Reference Barker 8 , Reference Singhal, Kennedy and Lanigan 9 ). It has been confirmed by pig and rodent studies that intra-uterine growth restriction can be a result of excess protein intake of the pregnant dam( Reference Daenzer, Ortmann and Klaus 10 , Reference Rehfeldt, Lang and Görs 11 ) which also occurs as a consequence of low dietary protein intake( Reference Davis, Fiorotto and Burrin 12 – Reference Fernandez-Twinn, Ekizoglou and Wayman 14 ).
In the case of very-low-protein diets, intra-uterine growth restriction is probably the result of fetal undersupply with indispensable amino acids (AA)( Reference Wu, Pond and Ott 13 ), as has also been reported for natural low-birth-weight fetuses( Reference Finch, Yang and Nwagwu 15 ). It has been shown that a low-protein–high-carbohydrate (LP-HC) intake in pregnant rats activates the mammalian AA response pathway in the placenta, indicating the involvement of the placenta in the response to an imbalanced diet during pregnancy( Reference Strakovsky, Zhou and Pan 16 ). Less is known about how exposure to maternal excess protein–low carbohydrate intake might cause intra-uterine growth restriction. Generally, consumption of high-protein–low-carbohydrate (HP-LC) diets results in an induction of protein catabolism and causes a characteristic plasma AA profile with increased concentrations of branched-chain AA and reduced levels of Thr, Glu, Ala, Ser and Gln( Reference Moundras, Rémésy and Demigné 17 , Reference Noguchi, Zhang and Sugimoto 18 ). This may be seen as a reflection of the organism's effort to maintain N homeostasis while providing precursors for gluconeogenesis. Whether this translates to AA concentration profiles suboptimal for normal fetal growth is not clear. Therefore, we hypothesised that a maternal HP-LC diet may specifically modify maternal, umbilical and fetal AA profiles during pregnancy.
The objective of the present study was to investigate whether a HP-LC diet as compared with diets with a LP-HC or a standard (ST) protein and carbohydrate composition during pregnancy changes AA composition in maternal, umbilical and fetal plasma as well as placental and fetal extraction of AA in mid and late pregnancy of nulliparous sows. Changes in maternal plasma AA concentrations were monitored from early pregnancy on. Moreover, fetal mass was determined in mid and late pregnancy to estimate the effect of the imbalanced maternal diets on fetal growth.
Experimental methods
The research protocol was in accordance with the guidelines issued by the German animal protection law and approved by the relevant authorities (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei, Mecklenburg-Vorpommern, Germany; LVL M-V/TSD/7221.3-1.1-006/04; LALLF M-V/TSD/7221.3-1.2-05/06; LALLF M-V/TSD/7221.3-1.2-013/06).
Animals and diets
In total, fifty-six German Landrace nulliparous sows bred at the pig facility of the Leibniz Institute for Farm Animal Biology (FBN) were used in five replicates with six sows each per replicate (two sows per diet per replicate). Housing and breeding management were as recently described in detail( Reference Rehfeldt, Lang and Görs 11 ). To prevent effects on maternal, umbilical and fetal AA concentrations by the daily energy intake among feeding groups, sows were fed isoenergetic maize–barley–soyabean meal-based diets (13·7 MJ metabolisable energy/kg) with LP-HC (6·5 % crude protein), ST (12·1 % crude protein) or HP-LC (30·0 % crude protein) levels (Table 1). Diets were fed throughout pregnancy beginning 1 d before insemination( Reference Rehfeldt, Lang and Görs 11 ). Crystalline l-AA were added to the LP-HC and HP-LC diets to achieve AA proportions similar to the ST diet so that only the total amount of AA differed. Sows were restrictively fed to achieve a mean energy intake of 34 MJ metabolisable energy/d during pregnancy according to recommendations( 19 ). They were fed twice daily at 07.00 and 15.00 hours and had free access to water.
Table 1 Ingredients and composition of isoenergetic diets with different protein:carbohydrate ratios fed to sows throughout pregnancy (as-fed basis, unless otherwise indicated)( Reference Rehfeldt, Lang and Görs 11 )

LP-HC, low-protein–high-carbohydrate; ST, standard; HP-LC, high-protein–low-carbohydrate.
* Soyabean meal, 50 % crude protein.
† Mineral and vitamin mixture (ADB15 Prämix; Deutsche Vilomix Tierernährung GmbH) supplied the following amounts of vitamins and minerals (per kg complete diet): 3.75 mg vitamin A, 31.25 μg vitamin D3, 37·5 mg vitamin E, 1·875 mg vitamin K3, 2·5 mg vitamin B1, 6·25 mg vitamin B2, 3·75 mg vitamin B6, 25 μg vitamin B12, 31·25 mg niacin, 75 μg biotin, 0·312 mg folic acid, 15·625 mg pantothenic acid, 125 mg choline, 5869 mg Ca, 1625 mg P, 1250 mg Na, 250 mg Mg, 125 mg Fe, 125 mg Cu, 178·75 mg Zn, 75 mg Mn, 0·5 mg Co, 0·75 mg I and 0·25 mg Se.
‡ Protein:carbohydrate ratio based on N-free extracts and crude protein.
§ In complete air-dried diet.
Collection and processing of maternal, fetal umbilical and fetal endogenous blood
At 5 d before ( − 5), and 24, 59 and 80 d after insemination (days of pregnancy; dp), blood samples were withdrawn by venepuncture of the jugular vein after overnight feed withdrawal from nine LP-HC, nine ST and ten HP-LC sows, respectively, that underwent Caesarean section at 94 dp, to analyse post-absorptive plasma AA concentrations during the progression of pregnancy. Additionally, twenty-eight sows each underwent Caesarean section in mid (64 dp) and late pregnancy (94 dp). A blood sample was taken via an indwelling jugular vein catheter that was installed 9 d before Caesarean section( Reference Rodriguez and Kunavongkrit 20 ). Thereafter anaesthesia was induced with azaperon (Stresnil®, Janssen-Cilag; 0·05 ml/kg body weight (BW)) and ketamine (0·15 ml/kg BW; Serumwerk Bernburg AG). Afterwards, fetuses were withdrawn and fetal BW and length (crown to rump) were recorded. Because the anaesthetic and the section can influence blood circulation in the sow, four fetuses per sow balanced for sex were randomly selected for blood sampling among the first eight fetuses taken. Umbilical venous and arterial blood samples and simultaneously blood samples from the maternal vena jugularis were collected in heparinised tubes. Additionally, endogenous fetal blood was collected from the vena cava cranialis. Blood was centrifuged at 1580 g , 4°C, for 20 min to obtain plasma. Plasma AA concentrations were determined by HPLC( Reference Krömer, Fritz and Heinzle 21 ). Urea was analysed by HPLC with refractory index detection on a 300 × 7·8 mm Rezex RCM-Monosaccharide Ca column (Phenomenex) at 75°C and 0·5 ml/min flow rate with water as the eluent after protein precipitation with acetonitrile. All fetuses per sow were used to determine litter size, total litter weight, average fetal weight, percentage of males, fetal length, fetal mass:length ratio, BMI and ponderal index.
Calculations and statistics
Placental and fetal AA extraction was estimated according to equations 1 and 2, respectively:
 $$Placental\,AA\,extraction\,(\%) = \,((sow\,venous\,AA - umbilical\,venous\,AA)/\quad sow\,venous\,AA)\times 100; $$
$$Placental\,AA\,extraction\,(\%) = \,((sow\,venous\,AA - umbilical\,venous\,AA)/\quad sow\,venous\,AA)\times 100; $$
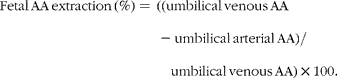 $$Fetal\,AA\,extraction\,(\%) = \,((umbilical\,venous\,AA - umbilical\,arterial\,AA)/\quad umbilical\,venous\,AA)\times 100. $$
$$Fetal\,AA\,extraction\,(\%) = \,((umbilical\,venous\,AA - umbilical\,arterial\,AA)/\quad umbilical\,venous\,AA)\times 100. $$
A positive fractional extraction indicates an uptake of AA, whereas a negative fractional extraction suggests a release of AA.
A total of 350 and 322 fetuses of twenty-eight sows each at 64 and 94 dp, respectively, were collected. Among these a subset of four fetuses per each sow balanced for sex was randomly selected at 64 dp (ten, nine and nine sows) and 94 dp (nine, ten and nine sows) with the LP-HC, ST and HP-LC diets, respectively. Hence, at 64 dp plasma AA concentrations of thirty-six, thirty-six and thirty-six fetuses, and at 94 dp, of thirty-four, forty and thirty-six fetuses derived from LP-HC, ST and HP-LC groups were analysed, respectively. Data were analysed by the MIXED procedure of SAS (version 9.2; SAS Institute Inc.). To determine any influential observation on the model, the Cook's distance (Cook's D) test was used. Any observation having a Cook's D greater than 0·1 was considered as influential and hence deleted from further analysis. Post hoc comparisons were performed using the Tukey–Kramer test. Significance was considered at P ≤ 0·05 and trends were discussed at 0·05 < P < 0·15. Results were reported as least square means with their standard errors of the mean.
The statistical model to analyse litter data included the fixed effects of diet and replicate and the interaction diet × replicate. AA concentrations in sow venous, umbilical venous, umbilical arterial and fetal venous plasma were analysed using the fixed factors of diet, blood vessel (sow venous, umbilical arterial, umbilical venous, and fetal venous plasma), replicate, fetal sex, fetal mass group calculated from the quartiles of frequency distribution (at 64 dp: 25 % light = 1–145·5 g, 50 % medium ≥ 145·6–185·3 g, 25 % heavy ≥ 185·4 g; at 94 dp: 25 % light = 230–572 g, 50 % medium ≥ 573–733 g; 25 % heavy ≥ 734 g), the collection group rank of the fetuses according to the order in which they were collected during Caesarean section (1 = fetuses no. 1–3; 2 = fetuses no. 4–6; 3 = fetuses no. 7–8), the interactions of diet × blood vessel, diet × replicate, diet × sex, diet × fetal mass group, and diet × fetus collection group rank, and the random factor sow nested in diet. The fractional extraction of plasma AA by the placenta and fetus was evaluated using the fixed factors of diet, replicate, fetal sex, fetal mass group, fetus collection group rank, the interactions of diet × replicate, diet × sex, diet × fetal mass group, and diet × fetus collection group rank, and the random factor sow nested in diet. In a separate model post-absorptive AA concentrations of sow venous plasma taken at − 5, 24, 59 and 80 dp were analysed considering the fixed factors of diet, replicate, day of pregnancy as a repeated measure, and the corresponding interactions. During the development of the statistical model, litter size was originally included as a covariate. Because its effect was not significant, it was not considered in the final models.
Results
Sows' food intake and litter data
Food intake of sows was increased between early to late pregnancy from 2·3 to 2·9 kg/d. Maternal diets differed in their protein:carbohydrate ratio as follows: 1:10·4, 1:5 and 1:1·3 for the LP-HC, ST and HP-LC diets, respectively (Table 1). Additionally, crude fat content was 28 % lower for the LP-HC diet compared with the ST and HP-LC diets. Fetal mass and fetal mass:length ratio were affected by maternal diet at 94 dp (Table 2). Fetal mass was highest in sows fed the ST diet and lowest for those fed the HP-LC diet resulting in a lower fetal mass:length ratio with the HP-LC diet (P ≤ 0·05). There were no diet-dependent differences in these traits in fetuses at 64 dp.
Table 2 Litter data of fetuses of sows fed experimental diets differing in protein:carbohydrate ratio throughout gestation (Least square means with their pooled standard errors)

LP-HC, low-protein–high-carbohydrate; ST, standard; HP-LC, high-protein–low-carbohydrate; dp, days of pregnancy.
a,b Within a row, least square means with unlike superscript letters were significantly different (P ≤ 0·05; Tukey post hoc test).
* Litter data for 64 dp have already been presented in Rehfeldt et al. ( Reference Rehfeldt, Lang and Görs 11 ).
† Replicate influenced (P < 0·01) fetal mass, fetal length, fetal mass:length ratio, BMI and ponderal index. Diet × replicate interaction was not significant.
Amino acid concentration in sow venous plasma with progressing pregnancy
Except for Leu, Arg, Asn, Pro, Ser, Cit and Orn, maternal diet affected (P ≤ 0·05) AA concentrations in venous plasma of post-absorptive sows with progressing pregnancy (Table 3). Likewise, progressing time during pregnancy changed (P ≤ 0·05) venous plasma AA concentrations except for His, Leu, Phe, Thr, Trp, Asn and Gly. For instance, venous plasma concentrations of Ile and Tau decreased from 5 d before insemination to 59 dp in sows fed the ST diet. A significant diet × time interaction (P ≤ 0·05) indicated that diet affected sow venous plasma concentrations of His, Ile, Lys, Met, Thr, Val, Ala, Gln, Glu, Gly, Cit and Tau differently at different days of pregnancy. Some glucogenic AA such as Ala, Glu and Gly were markedly lower at all time points with the HP-LC compared with the ST and LP-HC diets.
Table 3 Concentrations (μmol/l) of venous plasma amino acids at −5, 24, 59 and 80 days of pregnancy (dp) in sows fed isoenergetic diets with different protein:carbohydrate ratios throughout gestation* (Least square means with their pooled standard errors)

LP-HC, low-protein–high-carbohydrate; ST, standard; HP-LC, high-protein–low-carbohydrate; D, diet; T, blood sampling time.
a,b,c Within a diet, least square means with unlike lower-case superscript letters were significantly different among time points (P ≤ 0·05; Tukey post hoc test).
d,e Within a diet, least square means with unlike lower-case superscript letters tended to be different among time points (P < 0·15; Tukey post hoc test).
A,B,C Within a time point, least square with unlike capital superscript letters were significantly different among diets (P ≤ 0·05; Tukey post hoc test).
D,E Within a time point, least square means with unlike capital superscript letters tended to be different among diets (P < 0·15; Tukey post hoc test).
* Replicate effect: His, Lys, Met, Ala, Arg, Asn, Asp, Cys, Glu, Pro, Ser, Orn, Tau (P ≤ 0·05).
Amino acid concentration in sow venous, umbilical venous, umbilical arterial and fetal venous blood at day 64 of pregnancy
At 64 dp, diet affected (P ≤ 0·05) the plasma concentrations of indispensable His, Met and Val (Table 4) and of dispensable Ala, Gly and Tyr (Supplemental Table 1, available online at http://www.journals.cambridge.org/bjn). Furthermore, plasma concentrations of all AA varied greatly (P < 0·001) among the blood vessels investigated. For some AA, variations in plasma concentrations among blood vessels were substantial. For instance, Gln concentration was about twice as high in umbilical venous than in sow venous plasma (P ≤ 0·05). Additionally, concentrations of Asp and Glu were eleven and eight to eighteen times higher in fetal venous compared with sow venous plasma with the ST and HP-LC diets (P ≤ 0·05), respectively. The diet × blood vessel interaction for indispensable AA (i.e. His, Ile, Leu, Met, Phe, Thr, Trp and Val) and some dispensable AA (Ala, Gln, Gly and Tyr) indicated that the effect of diet depended on the respective blood vessel. Specifically, mainly sow venous plasma AA concentrations were changed but not those in umbilical venous, umbilical arterial and fetal venous plasma.
Table 4 Concentration (μmol/l) of indispensable amino acids of sow venous, umbilical venous and arterial and fetal venous plasma at 64 days of pregnancy (dp) after feeding experimental diets with different protein:carbohydrate ratios from the beginning of gestation* (Least square means with their pooled standard errors)

LP-HC, low-protein–high-carbohydrate; ST, standard; HP-LC, high-protein–low-carbohydrate; D, diet; BlV, blood vessel; D × BlV; SV, sow venous plasma; UV, umbilical venous plasma; UA, umbilical arterial plasma; FV, fetal venous plasma.
a,b Mean values within a row with unlike lower-case superscript letters were significantly different (P ≤ 0·05; Tukey post hoc test).
d,e Mean values within a row with unlike lower-case superscript letters tended to be different (P < 0·15; Tukey post hoc test).
A,B Mean values within a column with unlike capital superscript letters were significantly different (P < 0·05; Tukey post hoc test).
D,E Mean values within a column with unlike capital superscript letters tended to be different (P < 0·15; Tukey post hoc test).
* Effect of fetal mass group: His (P ≤ 0·05); effect of collection group rank during Caesarean section: Ile, Leu, Phe (P ≤ 0·05); diet × sex interaction: Lys, Met (P ≤ 0·05). Least square means are shown in Supplemental Tables 3 and 4 (available online at http://www.journals.cambridge.org/bjn).
Amino acid concentration in sow venous, umbilical venous, umbilical arterial and fetal venous blood at day 94 of pregnancy
At 94 dp, diet affected (P ≤ 0·05) plasma concentrations of indispensable Ile, Leu, Phe, Trp and Val (Table 5) and dispensable Ala, Arg, Asp, Glu, Gly and Tyr (Supplemental Table 2, available online at http://www.journals.cambridge.org/bjn). Additionally, plasma concentrations of indispensable and dispensable AA varied greatly (P < 0·001) among the various blood vessels. For instance, the concentration of Glu in sow venous plasma was only about half of that in umbilical arterial and one-third to one fifth (HP-LC) of that in fetal venous plasma (P < 0·001). Also, the concentration of Gln was about twice as high in umbilical venous plasma and about 1·5 times as high in umbilical arterial and fetal venous plasma as in sow venous plasma (P ≤ 0·05). Diet × blood vessel interactions (P ≤ 0·05) for all indispensable AA, except Phe, and dispensable Ala, Cys, Gln, Glu, Gly, Ser, Tyr, Cit, Orn and Tau suggested that diet affected plasma AA concentrations differently in the various blood vessels. As for 64 dp, for most AA only sow venous plasma AA concentrations were different among the diets. Interestingly, the sow venous plasma concentration of Val was highest with the HP-LC, intermediate with the ST and lowest with the LP-HC diet (P ≤ 0·05), whereas the opposite was observed for sow venous concentrations of Ala and Gly (P ≤ 0·05) (Table 5). Only at 94 dp, an effect on fetal venous plasma concentration was detected, as fetal venous Glu was less (P ≤ 0·05) with the LP-HC compared with the ST diet (Supplemental Table 2, available online at http://www.journals.cambridge.org/bjn).
Table 5 Concentrations (μmol/l) of indispensable amino acids of sow venous, umbilical venous and arterial and fetal venous plasma at 94 d of pregnancy after feeding experimental diets with different protein:carbohydrate ratios from the beginning of gestation* (Least square means with their pooled standard errors)

LP-HC, low-protein–high-carbohydrate; ST, standard; HP-LC, high-protein–low-carbohydrate; D, diet; BlV, blood vessel; SV, sow venous plasma; UV, umbilical venous plasma; UA, umbilical arterial plasma; FV, fetal venous plasma.
a,b,c Mean values within a row with unlike lower-case superscript letters were significantly different (P ≤ 0·05; Tukey post hoc test).
d,e Mean values within a row with unlike lower-case superscript letters tended to be different (P < 0·15; Tukey post hoc test).
A,B,C Mean values within a column with unlike capital superscript letters were significantly different (P < 0·05; Tukey post hoc test).
D,E Mean values within a column with unlike capital superscript letters tended to be different (P < 0·15; Tukey post hoc test).
* Effect of replicate: His, Lys (P ≤ 0·05); effect of sex: Lys (P ≤ 0·05); effect of fetal mass group: Lys (P ≤ 0·05); effect of collection group rank during Caesarean section: His, Leu, Lys, Phe (P ≤ 0·05); diet × replicate interaction: His (P ≤ 0·05); diet × sex interaction: His, Ile, Leu, Met (P ≤ 0·05); diet × fetal mass group interaction: His (P ≤ 0·05); diet × collection group rank during Caesarean section interaction: Ile, Leu, Met, Phe (P ≤ 0·05). Least square means are shown in Supplemental Tables 5 and 6 (available online at http://www.journals.cambridge.org/bjn).
Fractional extraction of amino acids by the placenta at day 64 of pregnancy
Diet affected (P ≤ 0·05) placental uptake of His, Ile, Trp, Val and Gly as well as placental release of Thr, Ala, Gln, Glu and Tau (Fig. 1(A) and (B)). When least square means were compared among diets, a greater placental release was observed with the glucogenic AA Ala, Asp, Gln and Glu with the HP-LC diet than with the ST diet (P ≤ 0·05). Moreover, the HP-LC diet reduced (P ≤ 0·05) placental uptake of His and Gly, whereas it increased (P ≤ 0·05) that of Val compared with the ST diet. Interestingly, Thr was taken up by the placenta in sows fed the HP-LC diet, but was secreted in sows fed the ST and LP-HC diets (P ≤ 0·05). The HP-LC diet decreased (P ≤ 0·05) placental secretion of Tau compared with the LP-HC diet. In addition, the LP-HC diet reduced (P ≤ 0·05) placental uptake of Trp and Val compared with the ST diet.

Fig. 1 Fractional placental amino acid extraction (%, uptake or release) at 64 days of pregnancy of sows fed low-protein–high-carbohydrate (
![]() ), standard (
), standard (
![]() ) and high-protein–low-carbohydrate (
) and high-protein–low-carbohydrate (
![]() ) diets throughout pregnancy. (A) Indispensable amino acids; (B) dispensable amino acids. Values are least square means, with standard errors represented by vertical bars. Negative values suggest a placental release of amino acids towards the fetus. a,b,c Mean values with unlike letters were significantly different (P ≤ 0·05; Tukey post hoc test). c,d Mean values with unlike letters tended to be different (P < 0·15; Tukey post hoc test). Sex effect: Ala (P ≤ 0·05); fetal mass group effect: His, Cit (P ≤ 0·05); effect of collection group rank during Caesarean section: Ile, Leu, Phe, Pro, Tyr, Cit (P ≤ 0·05); diet × sex interaction: Met, Ala, Gly (P ≤ 0·05); diet × fetal mass group interaction: Cit (P ≤ 0·05); diet × collection group rank during Caesarean section interaction: Ile (P ≤ 0·05).
) diets throughout pregnancy. (A) Indispensable amino acids; (B) dispensable amino acids. Values are least square means, with standard errors represented by vertical bars. Negative values suggest a placental release of amino acids towards the fetus. a,b,c Mean values with unlike letters were significantly different (P ≤ 0·05; Tukey post hoc test). c,d Mean values with unlike letters tended to be different (P < 0·15; Tukey post hoc test). Sex effect: Ala (P ≤ 0·05); fetal mass group effect: His, Cit (P ≤ 0·05); effect of collection group rank during Caesarean section: Ile, Leu, Phe, Pro, Tyr, Cit (P ≤ 0·05); diet × sex interaction: Met, Ala, Gly (P ≤ 0·05); diet × fetal mass group interaction: Cit (P ≤ 0·05); diet × collection group rank during Caesarean section interaction: Ile (P ≤ 0·05).
Fractional extraction of amino acids by the placenta at day 94 of pregnancy
Diet affected (P ≤ 0·05) placental uptake of Trp, Val, Ala, Cys, Glu, Pro and Orn and placental release of Lys, Thr, Gln and Tau (Fig. 2(A) and (B)). Comparison of least square means showed that placental Lys release was substantially lower (P ≤ 0·05) with the HP-LC and LP-LC diets compared with the ST diet. The HP-LC diet further increased (P ≤ 0·05) placental release of the glucogenic Gln compared with the ST and LP-HC diets. Placental uptake was higher for Val and lower for Pro in sows fed the HP-LC diet compared with those fed the ST diet (P ≤ 0·05). Ala and Glu were taken up by the placenta of sows fed the LP-HC and ST diets, whereas the placenta of sows fed the HP-LC diet released the glucogenic AA Ala and Glu (P ≤ 0·05). The LP-HC diet increased (P ≤ 0·05) placental release of Thr and Tau compared with the ST diet. Furthermore, the LP-HC diet lowered placental uptake of Trp and increased that of Ala and Orn (P ≤ 0·05).

Fig. 2 Fractional placental amino acid extraction (uptake or release) at 94 d of pregnancy of sows fed low-protein–high-carbohydrate (
![]() ), standard (
), standard (
![]() ) and high-protein–low-carbohydrate (
) and high-protein–low-carbohydrate (
![]() ) diets throughout pregnancy. (A) Indispensable amino acids; (B) dispensable amino acids. Values are least square means, with standard errors represented by vertical bars. Negative values suggest a placental release of amino acid towards the fetus. a,b,c Mean values with unlike letters were significantly different (P ≤ 0·05; Tukey post hoc test). d,e Mean values with unlike letters tended to be different (P < 0·15; Tukey post hoc test). Replicate effect: His, Lys, Ala, Arg, Gly, Orn, Tau (P ≤ 0·05); fetal mass group effect: Trp, Tyr, Cit (P ≤ 0·05); effect of collection group rank during Caesarean section: all amino acids except Thr, Ala, Cys, Gln, Glu, Pro, Cit (P ≤ 0·05); diet × replicate interaction: His, Ala, Tau (P ≤ 0·05); diet × sex interaction: Gly (P ≤ 0·05); diet × fetal mass group interaction: His, Ser, Orn (P ≤ 0·05); diet × collection group rank during Caesarean section interaction: Lys, Asp (P ≤ 0·05).
) diets throughout pregnancy. (A) Indispensable amino acids; (B) dispensable amino acids. Values are least square means, with standard errors represented by vertical bars. Negative values suggest a placental release of amino acid towards the fetus. a,b,c Mean values with unlike letters were significantly different (P ≤ 0·05; Tukey post hoc test). d,e Mean values with unlike letters tended to be different (P < 0·15; Tukey post hoc test). Replicate effect: His, Lys, Ala, Arg, Gly, Orn, Tau (P ≤ 0·05); fetal mass group effect: Trp, Tyr, Cit (P ≤ 0·05); effect of collection group rank during Caesarean section: all amino acids except Thr, Ala, Cys, Gln, Glu, Pro, Cit (P ≤ 0·05); diet × replicate interaction: His, Ala, Tau (P ≤ 0·05); diet × sex interaction: Gly (P ≤ 0·05); diet × fetal mass group interaction: His, Ser, Orn (P ≤ 0·05); diet × collection group rank during Caesarean section interaction: Lys, Asp (P ≤ 0·05).
Fractional extraction of amino acids by fetuses
At 64 dp fractional extraction of AA by the fetuses was not influenced by maternal diet (Table 6). In general, indispensable AA were taken up by the fetus. With respect to dispensable AA, Ala, Arg, Asn, Cys, Gln, Pro and Tyr were taken up and Asp, Glu, Ser, Cit, Orn, and Tau were released by the fetus at 64 dp. At 94 dp, the fetus took up all indispensable AA and dispensable Ala, Arg, Asn, Cys, Gln, Ser and Thr, whereas it released Asp, Glu and Tau for all three diets (Table 7). Diet only affected (P ≤ 0·05) fetal extraction of Pro at 94 dp. Comparison of least square means demonstrated that the HP-LC and LP-HC diets caused a fetal uptake of Pro, whereas there was a fetal release of Pro with the ST diet. Fetal Pro uptake with the LP-HC diet differed (P ≤ 0·05) from the Pro release with the ST diet. Finally, fetal Lys uptake was lower (P ≤ 0·05) with the HP-LC compared with the ST diet.
Table 6 Fractional extraction of amino acids by the fetus (%) at 64 days of pregnancy when sows were fed experimental diets with different protein:carbohydrate ratios throughout gestation* (Least square means with their pooled standard errors)

LP-HC, low-protein–high-carbohydrate; ST, standard; HP-LC, high-protein–low-carbohydrate.
* Fetal mass group influenced fetal Glu extraction (P = 0·041); collection group rank during Caesarean section influenced fetal extraction of Ile, Leu, Phe and Asp (P < 0·05); diet × collection group rank during Caesarean section influenced Ile, Leu, Ser and Tyr (P < 0·05).
Table 7 Fractional extraction of amino acids by the fetus at 94 d of pregnancy when sows were fed experimental diets with different protein:carbohydrate ratios throughout gestation* (Least square means with their pooled standard errors)

LP-HC, low-protein–high-carbohydrate; ST, standard; HP-LC, high-protein–low-carbohydrate.
a,b Mean values within a row with unlike lower-case superscript letters were significantly different (P ≤ 0·05; Tukey post hoc test).
d,e Mean values within a row with unlike lower-case superscript letters tended to be different (P < 0·15; Tukey post hoc test).
* Replicate influenced fetal extraction of His, Gln and Glu (P < 0·05); fetal mass group influenced fetal Trp extraction (P = 0·03); collection group rank during Caesarean section influenced fetal Phe extraction (P = 0·030); diet × sex interaction influenced fetal Lys extraction (P < 0·002); diet × collection group rank during Caesarean section interaction influenced fetal extraction of Lys and Pro (P < 0·01).
Urea concentration in sow and fetal venous plasma
In accordance with the results for fetal AA concentrations and fetal fractional AA extraction we assumed that effects on protein metabolism may be more pronounced in the last trimester. Therefore, we only determined urea concentrations in sow and fetal venous plasma at 94 dp. Diet highly affected (P < 0·001) urea concentrations in sow (1·3, 2·7 and 6·6 mmol/l with the LP-HC, ST and HP-LC diets, respectively) and fetal (3·1, 4·7 and 8·8 with the LP-HC, ST and HP-LC diets, respectively) venous plasma. Urea concentration was greatest in sow and fetal venous plasma with the HP-LC diet and lowest with the LP-HC diet (P ≤ 0·05) compared with the ST diet. Urea concentration in fetal venous plasma was always higher (P < 0·001) than in sow venous plasma.
Discussion
Besides glucose, fatty acids and lactate, AA are critical for embryonic and fetal development( Reference Gardner, Pool and Lane 22 , Reference Angiolini, Fowden and Coan 23 ). By far, most studies have focused on effects of maternal protein restriction and its impact on fetal AA concentrations( Reference Wu, Pond and Ott 13 , Reference Strakovsky, Zhou and Pan 16 ). However, there is emerging evidence that excessive protein intake accompanied by a reduced carbohydrate intake during pregnancy may be as disadvantageous for fetal development as low protein intake( Reference Campbell-Brown, Johnston and Kerr Grieve 5 – Reference Rush 7 ). With isoenergetic diets an increase or reduction in daily protein intake is always associated with a change of intake of other nutrients, for example, carbohydrates and fat, as was the case in the present study. In this context, we compared the effects of maternal HP-LC with ST and LP-HC intake on maternal, umbilical and fetal plasma AA profiles in Pregnant sows. Novel findings of the present study imply that the maternal HP-LC diet mainly affected maternal plasma AA profiles with progressing pregnancy and placental extraction of many AA but did not influence umbilical and fetal plasma AA concentrations in mid and late pregnancy. Nevertheless, the HP-LC diet reduced fetal mass at 94 dp, thereby supporting previous observations in human subjects that maternal high protein intake accompanied by a reduced carbohydrate intake during pregnancy can restrict fetal growth( Reference Campbell-Brown, Johnston and Kerr Grieve 5 – Reference Rush 7 ). Similarly, the maternal LP-HC diet mainly affected maternal plasma AA concentrations and placental AA extraction but had, in contrast to previous studies( Reference Wu, Pond and Ott 13 ), only small effects on umbilical and fetal plasma AA profiles, which may be due to the less severe level of maternal protein restriction.
Effect of maternal diet on maternal plasma amino acids and urea
Effects of inadequate dietary protein and carbohydrate intake on maternal venous plasma AA profiles occurred from early pregnancy on, probably with impacts on placental growth and function, and eventually on fetal development( Reference Strakovsky, Zhou and Pan 16 , Reference Gardner, Pool and Lane 22 ). More AA were affected with progressing pregnancy, indicating that sows apparently compensated less well for the imbalanced protein and carbohydrate intake in late pregnancy consistent with a greater AA demand. Specifically, dispensable AA, such as Ala, Gln, Glu, Gly, Tau and Orn, are present in the female reproductive tract throughout pregnancy at relatively high concentrations( Reference Wu, Pond and Ott 13 , Reference Gardner, Pool and Lane 22 , Reference Wu, Bazer and Tuo 24 , Reference Kwon, Wu and Bazer 25 ). As a consequence, these AA may be affected first by aberrance in maternal dietary protein and carbohydrate intake. In the present study, the HP-LC diet reduced maternal plasma concentrations of Ala, Gly (24, 59, 64, 80, 94 dp), and Glu (24, 59, 80, 94 dp) and increased those of Ile (59, 64, 80, 94 dp), Val (24, 59, 64, 80, 94 dp), Thr (59, 64, 80 dp) and Lys (only 94 dp) compared with the ST diet. These findings are in line with earlier reports for non-reproducing rat models fed HP-LC diets( Reference Moundras, Rémésy and Demigné 17 , Reference Noguchi, Zhang and Sugimoto 18 , Reference Rémésy and Demigné 26 ) showing lower plasma concentrations of Ala, Glu, Gln, Gly, Ser and Thr due to greater utilisation of glucogenic AA as glucose precursors to maintain plasma glucose levels. However, in contrast to the previous reports, pregnant sows in the present study had a higher plasma Thr level, which might be related to differences in dietary protein source in the various studies( Reference Kuhla, Kucia and Görs 4 , Reference Moundras, Rémésy and Demigné 17 , Reference Rémésy and Demigné 26 ). The increased plasma urea concentration at 94 dp in HP-LC sows compared with the other two dietary groups indicates that considerably more AA were oxidised in sows fed the HP-LC diet in late pregnancy. Therefore, imbalances in the plasma AA profile of sows fed the HP-LC diet compared with those fed the ST diet are probably associated with N disposal and gluconeogenesis to compensate for the lower carbohydrate intake of these sows. The opposite effects of the HP-LC and LP-HC diets on maternal plasma concentrations of Ala, Val and Gly at 94 dp compared with the ST diet may have reflected the altered maternal availability of nutrients, i.e. protein, carbohydrates and fat, in late pregnancy. Except for increased plasma concentrations of Ala and Gly (24, 59, 64, 80, 94 dp), plasma concentrations of many AA (for example, Cys, Leu, Phe, Trp, Tyr and Val at 94 dp) were reduced in sows fed the LP-HC diet compared with the ST diet as a consequence of the lower dietary AA intake as previously reported( Reference Wu, Pond and Ott 13 ). Accordingly, maternal protein accretion and BW gain were reduced throughout pregnancy in nulliparous pregnant sows( Reference Rehfeldt, Lang and Görs 11 ), and, in order to spare AA, oxidation of AA was probably reduced in sows fed the LP-HC diet at 94 dp compared with the other two maternal diets as reflected by a lower plasma urea concentration. In another subset of pregnant nulliparous sows fed the three diets, post-absorptive plasma urea concentrations were found to be decreased in LP-HC sows compared with the other two groups while serum protein levels in LP-HC sows were only lower in late pregnancy( Reference Metges, Lang and Hennig 27 ). Carcass data supported observations made for maternal plasma AA concentrations, as carcass protein content was lower and carcass fat content was higher in sows fed the LP-HC diet compared with those fed the ST and HP-LC diets( Reference Rehfeldt, Lang and Görs 11 ). Overall, AA concentrations in sow venous plasma were in the range reported previously for reproducing and non-reproducing sows( Reference Wu, Pond and Ott 13 , Reference Hagemeister, Scholz-Ahrens and Schulte-Coerhe 28 , Reference Trottier and Easter 29 ).
Effect of maternal diet on umbilical and fetal plasma amino acids and urea
To our knowledge, this is the first report on data of umbilical venous and arterial and fetal venous plasma AA concentrations of sows fed an HP-LC diet. However, umbilical and fetal plasma AA concentrations were obviously not affected by this diet at 64 and 94 dp. Likewise, umbilical and fetal plasma AA concentrations were not as severely affected by the maternal LP-HC diet as previously reported with a virtually protein-free diet at days 40 and 60 of pregnancy in pigs( Reference Wu, Pond and Ott 13 ). For instance, using a diet with 0·5 % protein content, Wu et al. ( Reference Wu, Pond and Ott 13 ) observed decreased umbilical venous plasma concentrations of Ala, Arg, branched-chain AA, Gln, Gly, Lys, Orn, Pro, Tau, Thr and urea at day 60 of pregnancy. In contrast, the LP-HC diet used in the present study only tended to decrease umbilical venous and arterial plasma Phe concentrations, as well as reduce fetal venous plasma Glu concentration and, as a trend, those of Asp and Lys compared with the ST diet at 94 dp but not at 64 dp. The present findings may be related to the fact that the protein intake with the LP-HC diet was half of that recommended; thus, nulliparous pregnant sows could probably partly compensate the low dietary protein intake at the expense of BW gain( Reference Rehfeldt, Lang and Görs 11 ), presumably in favour of fetal AA provision. Yet, limited dietary protein intake comparable with that with the present LP-HC diet is potentially more widespread worldwide, i.e. in developing countries( Reference Père 30 ). Because Lys cannot be synthesised by mammals, and rate of Lys accretion is progressively increasing in porcine fetal tissue in the second half of pregnancy( Reference Wu, Ott and Knabe 31 ), a reduced concentration of this indispensable AA in the plasma of fetuses of sows at 94 dp fed the LP-HC diet may indicate limited fetal tissue protein synthesis( Reference Wu, Pond and Ott 13 ). Likewise, reduced fetal plasma Glu and Asp concentrations with the same diet may indicate changes in fetal endogenous C and N metabolism( Reference Wu 32 ) related to gluconeogenesis to maintain fetal plasma glucose. Moreover, reduced fetal extraction of Lys and Pro at 94 dp suggests impaired fetal growth as indicated by the observed lower fetal mass at 94 dp in the HP-LC group and lower birth weight of piglets of sows fed the HP-LC and LP-HC diets( Reference Rehfeldt, Lang and Görs 11 ).
Due to technical reasons, utero-umbilical blood flow was not measured in the present study; hence, it cannot be excluded that umbilical blood flow rate might have affected fetal availability of more AA with progressing pregnancy without obvious changes in AA concentrations in umbilical and fetal plasma. Transplacental nutrient exchange relies on uterine and umbilical blood flow which, in turn, depends on the vascularity of the placenta( Reference Itoh, Brawley and Wheeler 33 , Reference Redmer, Aitken and Milne 34 ). For instance, pregnant rats fed a low-protein diet had an attenuated uterine artery vasodilator response to vascular endothelial growth factor in uterine arteries( Reference Itoh, Brawley and Wheeler 33 ). Therefore, any impairment of the utero-umbilical blood flow caused by the HP-LC and LP-HC diets may have altered the total AA amount delivered to the fetus, thereby impairing fetal growth in late pregnancy. In any case, the direction of fractional extraction (uptake or release) of individual AA would be independent of the actual blood flow rate occurring with the different maternal diets. However, velocities of fetal mass accretion appeared to differ with the two imbalanced maternal diets in such a way that HP-LC fetuses seem to grow more slowly in the second half of pregnancy.
It is noteworthy that plasma concentrations distinctly differed among the various blood vessels (i.e. sow vein, umbilical vein, umbilical artery and fetal vein) for many AA, reflecting the AA demand for placental metabolism and synthesis of the various types of fetal body proteins with progressing pregnancy( Reference Trottier and Easter 29 ). For instance, the importance of Gln for the fetus in terms of cell proliferation and development( Reference Neu 35 ) was emphasised by the accumulation of Gln in umbilical venous plasma as compared with sow venous plasma. Therefore, changes in the differences in AA concentrations among sow, umbilical and fetal blood vessels with the HP-LC and LP-HC diets compared with the ST diet, as observed, for instance, for His, Met, Val, Cys and Tau at 94 dp, may be also an indicator for aberrant maternal nutrition.
Likewise, fetal plasma urea concentrations appeared to indicate changes in fetal AA availability, as fetal plasma urea concentration was increased with the HP-LC and reduced with the LP-HC compared with the ST diet, thereby reflecting the situation in their dams. Therefore, it seems possible that the higher maternal dietary protein but reduced dietary glucose availability with the HP-LC diet ( Reference Metges, Lang and Hennig 27 , Reference Metges, Lang and Hennig 36 ) may have programmed the fetus to catabolise more AA for energy supply. In contrast, fetuses of sows fed the LP-HC diet apparently spared AA from oxidation, thereby reducing fetal plasma urea concentration compared with the other two maternal diets. AA are generally used by the fetus not only for protein synthesis but also function as an important energy substrate( Reference Roos, Lagerlöf and Wennergren 37 ) that can contribute up to 30 % of the oxidative metabolism in the fetus( Reference Battaglia and Meschia 38 ). In this context, fetal plasma urea concentrations were above those of their dams regardless of the maternal diet. This may be related to the fact that fetal AA uptake exceeded the requirement for protein accretion relative to the available energy, resulting in a high rate of fetal AA oxidation which is indicated by an increased urea production( Reference Regnault, de Vrijer and Battaglia 39 ).
Placental amino acid extraction
Placental extraction of many AA (i.e. Ala, Asp, Cys, Glu, Gln, Gly, His, Ile, Leu, Lys, Orn, Phe, Pro, Ser, Tau, Thr, Trp, Tyr and Val) was altered with the HP-LC and LP-HC compared with the ST diet. This may suggest that placentas adjusted the AA uptake or release to compensate for the imbalanced maternal plasma AA concentrations. There is growing evidence from studies with sheep, mice and rats that the placenta can adapt its nutrient transfer capacity to meet the fetal nutrient demands for growth when the maternal diet is imbalanced( Reference Strakovsky, Zhou and Pan 16 , Reference Oumachigui 40 , Reference Constancia, Angiolini and Sandovici 41 ).
Maternal diet-related changes in placental extraction were especially evident for Ala at 94 dp; it was released by placentas of sows fed the HP-LC diet but taken up by placentas of sows fed the LP-HC diet. Although we did not measure the activity of the mammalian system A neutral AA transporter, which transports Ala, it has been reported that it is highly controlled by AA availability in placental and non-placental tissues and is up-regulated by AA restriction and imbalance( Reference Wu, Ott and Knabe 31 , Reference Gaccioli, Huang and Wang 42 ). Overall, greater placental extraction of most indispensable AA and Gly and greater placental release of Glu in mid compared with late pregnancy probably reflect specific fetal AA needs at different stages of pregnancy and might be interpreted to mean that fetal gluconeogenesis is supported by an increased maternal supply of glucogenic AA( Reference Wu, Pond and Ott 13 , Reference Wu, Ott and Knabe 31 , Reference Self, Spencer and Johnson 43 ).
Placental extraction of functional AA Cys, Gln, Pro, Trp and branched-chain AA was affected by the maternal diet. Functional AA play an important role in embryonic, placental and fetal development during pregnancy as they regulate key metabolic pathways( Reference Wu 32 ). Therefore, changes in placental extraction of functional AA may impair fetal nutrition and growth. For instance, Pro is a major AA for the placental synthesis of Arg which in turn is an important substance for the synthesis of polyamines and NO( Reference Wu, Bazer and Burghardt 44 ). Both polyamines and NO are crucial for angiogenesis, utero-placental blood flow, transfer of nutrients from the mother to the fetus, and fetal growth and development( Reference Wu, Bazer and Burghardt 44 ). Here, placental Pro uptake was lower with the HP-LC than with the ST diet and also fetal Pro extraction changed from a release in fetuses of sows fed the ST diet to an uptake with the HP-LC and LP-HC diets at 94 dp. Reduced placental extraction and availability of Pro and polyamines in the conceptus are assumed to contribute to reduced fetal growth during protein malnutrition of the pregnant dam( Reference Schoknecht, Newton and Weise 45 ) and in natural intra-uterine growth restriction( Reference Wu, Bazer and Wallace 46 ). The increased placental extraction of Val with the HP-LC compared with the ST and LP-HC diets may correspond to the higher release of Ala, Gln and Glu in mid and late pregnancy. In fact, branched-chain AA are catabolised by the porcine placenta in a concentration-dependent manner to form Glu, Gln and Ala( Reference Self, Spencer and Johnson 43 ), of which Gln, particularly, is important for fetal development( Reference Neu 35 ).
Generally, hormones such as insulin, cortisol, leptin and insulin-like growth factor (IGF)-1 link maternal nutrition to fetal growth by regulating placental nutrient transport and the placental endocrine response( Reference Jansson, Pettersson and Haafiz 47 , Reference Fowden, Ward and Wooding 48 ). Some AA, such as Arg, have been shown to enhance the secretion of insulin, growth hormone, IGF-1, prolactin, glucagon, progesterone and placental lactogen from their respective endocrine organs( Reference Wu 32 ). As well as AA, maternal glucose supply can influence placental AA transporter activity through changes in the endocrine response( Reference Jansson, Pettersson and Haafiz 47 ). Therefore, it may be reasonable to assume that, in addition to maternal protein intake, maternal dietary glucose availability, which was higher with the LP-HC diet and less with the HP-LC diet compared with the ST diet, may have resulted in an aberrant endocrine response, thereby signalling an imbalanced nutritional status to the placenta( Reference Rehfeldt, Lang and Görs 11 ). In a different subset of sows fed the three diets we found that in late pregnancy plasma glucose levels were lower in HP-LC compared with ST and LP-HC sows( Reference Metges, Lang and Hennig 27 , Reference Metges, Lang and Hennig 36 ).
In conclusion, maternal HP-LC as compared with isoenergetic LP-HC and ST diets during pregnancy altered the maternal plasma concentrations of many AA in mid and late pregnancy. In contrast to our hypotheses, as well as previous reports on protein-free diets during pregnancy, umbilical venous and fetal plasma AA profiles were only minimally affected by the maternal protein:carbohydrate ratio, indicating a compensatory role of the placenta. Placental AA extraction was adapted to the maternal plasma AA supply to provide a more balanced AA composition to the fetus in mid and late pregnancy. However, reduced fetal mass at 94 dp with the HP-LC diet and lower birth weight with the LP-HC and HP-LC diets( Reference Rehfeldt, Lang and Görs 11 ) indicated that probably additional factors, such as utero-umbilical blood flow and placental endocrine responses, are involved in signalling the maternal imbalanced nutritional status to the fetus.
Acknowledgements
The present study was supported by grants of the German Research Foundation (DFG) (PAK 24: ME 1420/8-1) and the German Federal Ministry of Research and Education (FUGATOplus, 0315132A), Germany, to C. C. M. The authors thank H. Sievert, Dr B. Stabenow, Dr O. Bellmann and colleagues (all Leibniz Institute for Farm Animal Biology) for animal feeding and care, and medical attention. S. Dwars, I. Brüning, K. Karpati, U. Lüdtke, A. Steinborn, M. Jugert-Lund, Dr. M. Hartung, M. Günther and R. Wal (all Leibniz Institute for Farm Animal Biology) are gratefully acknowledged for assistance with sampling and laboratory analysis. C. C. M., C. R. and W. O. designed the research, I. S. L., S. G., K.-P. B. and U. H. conducted the research, S. G. analysed the plasma AA concentrations, B. U. M.-Z., C. C. M. and G. N. performed statistical analysis, B. U. M.-Z. and C. C. M. wrote the paper, and C. C. M. had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors had any financial or personal conflicts of interest.











