Energy from sugar-sweetened beverages (SSB) has increased in the USA during the last three decades(Reference Nielsen and Popkin1). Paralleling this trend has been the well-known increased prevalence of obesity(Reference Wang and Beydoun2). In particular, beverages sweetened with high-fructose corn syrup (HFCS) have been implicated in relation to the development of obesity(Reference Forshee, Storey and Allison3), and longitudinal data support the notion that SSB consumption is associated with increased BMI(Reference Bleich, Wang and Wang4). Emerging data have also linked SSB intake to central obesity(Reference Dhingra, Sullivan and Jacques5), high blood pressure(Reference Dhingra, Sullivan and Jacques5–Reference Jalal, Smits and Johnson7), dyslipidaemia(Reference Dhingra, Sullivan and Jacques5) and a constellation of these metabolic risk factors, i.e. the metabolic syndrome(Reference Dhingra, Sullivan and Jacques5, Reference Malik, Popkin and Bray8). In addition, SSB intake has been associated with the incidence of type 2 diabetes(Reference Malik, Popkin and Bray8–Reference Odegaard, Koh and Arakawa10). Recently, the metabolic syndrome has been linked with cognitive impairment cross-sectionally(Reference Dik, Jonker and Comijs11) and prospectively(Reference Yaffe, Kanaya and Lindquist12–Reference Ho, Niti and Yap14). Type 2 diabetes is also considered to be a risk factor for impaired cognitive function(Reference van den Berg, Kloppenborg and Kessels15). However, it remains unclear whether habitual consumption of SSB is associated with cognitive dysfunction.
HFCS has gained much attention, because food industry sweeteners have shifted from sucrose to HFCS(Reference Jones16). Limited data from animal studies suggest that HFCS may have a role in cognitive impairment. Stranahan et al. (Reference Stranahan, Norman and Lee17) found that rats fed a high-fat, high-glucose diet supplemented with HFCS showed impaired hippocampal synaptic and cognitive function, possibly via development of insulin resistance(Reference Stranahan, Norman and Lee17). Hamsters fed a high-fructose diet have been shown to exhibit insulin resistance in the hippocampus, but cognitive function in these animals has not been examined(Reference Mielke, Taghibiglou and Liu18). Nevertheless, due to the similar composition of widely used HFCS to sucrose(Reference White19), it remains unclear whether cognitive function is associated with HFCS, with sucrose, or with both.
Although studies have been conducted to investigate immediate or short-term effects of pure glucose and other types of carbohydrates on cognitive performance(Reference Gilsenan, de Bruin and Dye20), to our knowledge no epidemiological studies have examined associations of habitual consumption of fructose and other sugars with cognitive function among middle-aged and older adults(Reference Stephan, Wells and Brayne21). In addition, it remains unclear whether SSB differ in their associations with cognitive function, relative to sugar-sweetened solid foods or 100 % fruit juice. In the present study we, therefore, examined habitual intakes of total sugars, added sugars, SSB, 100 % fruit juice and sugar-sweetened solid foods, as well as sucrose and added fructose, and their associations with cognitive function among a subsample of Puerto Rican adults without diabetes, living in the greater Boston area.
Materials and methods
Participants
The Boston Puerto Rican Health Study is an ongoing study funded by the National Institutes of Health as one of their Centers for Population Health and Health Disparities(Reference Tucker, Mattei and Noel22). Detailed information regarding the design of the study has been described previously(Reference Tucker, Mattei and Noel22). Briefly, data collection began in 2004 and was completed in 2009. Census tracts containing at least twenty-five Puerto Rican adults, aged 45–75 years, in the year 2000 census were identified in the greater Boston area. All blocks with ten or more Hispanic adults of the same age range were enumerated. One Puerto Rican adult, aged 45–75 years, per household was randomly recruited from qualified households in selected blocks. Most (77·4 %) participants were recruited this way, with an additional 9·8 % identified through partnerships with community organisations and random approach at major community events, 7·2 % from referrals from community members and 5·6 % from calls responding to posted flyers. A total of 2170 individuals were identified. Of these, seventy-seven participants were excluded because they were unable to answer questions due to a serious health condition or advanced dementia, they planned to move from the area within 2 years, or they did not have a permanent address. All others (n 2093) were invited to participate and 1811 (86·5 %) agreed to be interviewed. After excluding nine participants with low Mini-Mental State Examination (MMSE) score ( ≤ 10), 1802 participants were eligible to continue the survey. A total of 1500 (83·2 %) individuals finally completed the baseline interview whereas others (n 302) did not complete the interview due to difficulty in scheduling, or unsuccessful follow-up. Cleaned data for cognitive tests, dietary intake and plasma biomarkers were available for 1300 participants at the time of analysis. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board at Tufts Medical Center. Written informed consent was obtained from all participants. In the present study, we excluded participants (n 520) who took medications for diabetes or those who had fasting glucose ≥ 7·0 mmol/l, because diabetes has been linked to cognitive impairment(Reference van den Berg, Kloppenborg and Kessels15), and because those with diabetes may change their dietary pattern, especially intake of added sugars. We also excluded participants reporting implausible energy consumption ( < 2512 kJ (600 kcal) per d and >20 093 kJ (4800 kcal) per d) (n 43), resulting in 737 participants in the present analyses.
During the home interview, data about social-demographic status, lifestyle, dietary intake, cognitive function, self-reported diagnosed health conditions and medication use were collected by bilingual trained research assistants in Spanish or English depending on the primary language spoken by the participant at home.
Assessment of usual food and nutrient consumption
Usual food and nutrient intakes were estimated for the last 12 months from a semi-quantitative FFQ with 246 food items(Reference Noel, Newby and Ordovas23). This FFQ has been validated against plasma carotenoids(Reference Bermudez, Ribaya-Mercado and Talegawkar24) and vitamin B12(Reference Kwan, Bermudez and Tucker25) in Hispanics including Puerto Ricans aged 60 years and over. Nutrient intakes were calculated using the Nutrition Data System for Research software version 2007 developed from the Food and Nutrient Database 2007 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA).
Total sugars were defined as the sum of three free monosaccharides (glucose, fructose and galactose) and three free disaccharides (sucrose, lactose and maltose). Data for these mono- and disaccharides in the nutrient database include those from naturally occurring sources, such as fruit and vegetables, and from added sources, such as sucrose and HFCS. Added sugars included sugars and syrups added to foods during food preparation or commercial food processing(26), and did not include mono- and disaccharides occurring naturally in foods, such as lactose in milk or fructose in fruit. Ingredients designated as added sugars were white sugar (sucrose), brown sugar, powdered sugar, honey, molasses, pancake syrup, corn syrup, malt syrup, fructose, glucose (dextrose), galactose and lactose(26).
In the FFQ, nineteen items were designed to collect information about intakes of specific types of beverages, including 100 % fruit juice, fruit nectars, fruit drinks, soft drinks and teas. In the present study, SSB included regular fruit drinks with added sugars (fruit drinks, fruit juices and fruit nectars) and soft drinks (carbonated beverages with added sugars). Sugar-sweetened solid foods included sweets (candy, cookies, cakes, pies, doughnuts, sweet rolls, energy or granola bars) and dairy desserts (ice cream, sherbet, frozen yogurt and pudding). Included in 100 % fruit juice were fruit juices (orange, apple and other) with no added sugars. We defined naturally occurring fructose as fructose from fruit and fruit products without added sugars (such as 100 % fruit juice, fresh, frozen, canned and dried fruits) and vegetables and vegetable products without added sugars (for example, onions, peppers, tomatoes and canned plain tomato sauces). Fructose from other sources was defined as added fructose.
Assessment of cognitive function
As described previously, seven cognitive tests were administered to each participant in their home on the same day that dietary intake was assessed with the FFQ by a qualified research assistant(Reference Gao, Scott and Falcon27). Briefly, the MMSE was administered to assess general cognitive function(Reference Folstein, Folstein and McHugh28) (scores ranged from 12 to 30 in the present study). Also administered were: a sixteen-word list learning task to assess verbal memory, with subscores including learning and immediate recall (the sum of words recalled over five learning trials), recognition (the number of words discriminated correctly from a longer word list after a 25–35 min delay) and percentage retention (calculated by dividing the number of words recalled after the delay by the number of correct responses on the fifth learning trial)(Reference Artiola Fortuny, Hermosillo Romo and Heaton29); digit span forward and backward, to assess attention and working memory(Reference Artiola Fortuny, Hermosillo Romo and Heaton29); the Stroop test, to measure cognitive flexibility, response inhibition and processing speed(Reference Artiola Fortuny, Hermosillo Romo and Heaton29); verbal fluency, to assess the speed at which one can provide exemplars to a category (initial letter of a word)(Reference Artiola Fortuny, Hermosillo Romo and Heaton29); and clock drawing(Reference Wolf-Klein, Silverstone and Levy30) and figure copying(Reference Beery31), both to assess visual-spatial organisation. Scores for figure copying were weighted for the complexity of the figure copied; one point for easy figures and four points for the most difficult. Higher scores on each of these seven tests signify better cognition.
Factor analysis was used to conduct data reduction based on the scores of the seven cognitive tests, as described previously(Reference Gao, Scott and Falcon27). We identified three factors with Kaiser's criterion (Eigenvalue >1)(Reference Kaiser32). A varimax rotation was performed to improve the interpretation of these factors. These factors were then labelled as memory, executive function and attention, respectively (see Supplemental Table 1; available at http://www.journals.cambridge.org/bjn). In the present study, the MMSE scores were used as the primary outcome because the MMSE test reflects general cognitive function. We also examined whether intakes of total and added sugars were associated with the three derived cognitive function factors and with scores from individual tests.
Assessment of covariates
Educational attainment was categorised into five groups by their highest degree (below 5th grade, 5th–8th grade, 9th–12th grade, college, or graduate school). Smoking status was categorised as never smoking ( < 100 cigarettes in entire life), former smoking or current smoking. Alcohol use was classified as not current, current moderate ( ≤ one drink per d for women and ≤ two drinks per d for men) or current heavy (> one drink per d for women and > two drinks per d for men). Physical activity was estimated as a score, based on a modified Paffenbarger questionnaire of the Harvard Alumni Activity Survey(Reference Paffenbarger, Hyde and Wing33). The score was constructed by weighting time spent in various physical activities by factors that reflect O2 consumption of related physical activities. Poverty was defined as ‘yes’ if a participant's total annual house income was below the threshold released each year by the US Department of Health and Human Services. Acculturation score was calculated based on answers to seven questions regarding the extent of use of English and/or Spanish at work, in watching television, listening to the radio, reading newspapers/books, speaking with neighbours, talking to friends and talking to family members: a summed score ranged from 0 (fully unacculturated, only using Spanish) to 100 (fully acculturated, only using English)(Reference Falcon and Tucker34).
Anthropometric measures were taken in the home with standard methods(Reference Noel, Newby and Ordovas23). BMI was calculated as weight in kilograms divided by squared height in metres. Blood pressure was measured at three time points during the home interview, and the latter two measures were averaged. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or use of antihypertensive medications.
Statistical analyses
Intakes of total sugar, added sugar, specific sugars, types of beverages and food were adjusted for total energy intake using the regression residual method(Reference Willett, Howe and Kushi35). For example, adjusted total sugar intake was the sum of the regression residuals and a constant equal to the expected total sugar intake at the mean total energy intake. The same procedure was used to calculate adjusted values for other types of sugars, beverages and food before categorisation into quintiles for statistical analyses. General linear models were used to calculate adjusted means of cognitive scores by quintile of energy-adjusted sugar consumption, after controlling for age, sex, educational attainment (below 5th grade, 5th–8th grade, 9th–12th grade, college, or graduate school), poverty (yes or no), smoking (never, former, or current), alcohol use (not current, current moderate, current heavy), physical activity score, BMI (kg/m2; < 25, 25–29·9, or ≥ 30) and presence of hypertension. Acculturation score was also adjusted, because language use (Spanish v. English) might influence the results of cognitive tests(Reference Escobar, Burnam and Karno36) and also be related to dietary intake(Reference Ayala, Baquero and Klinger37). Means were compared with Tukey's adjustment for multiple comparisons. In secondary analyses, we further adjusted for intake of dietary fibre, total cholesterol, fat (saturated, monounsaturated, polyunsaturated and trans), vitamin supplement use (yes or no) and plasma concentrations of vitamin C, total carotenoids and total homocysteine. Because there are no well-established age- and education-based cut-off points for cognitive impairment specifically for this population, we used logistic regression models to estimate OR for cognitive impairment defined by the traditional cut-off point of 24 of the MMSE score (MMSE score < 24) in the upper four quintiles relative to the first quintile of total and added sugar intakes, respectively. Because a large proportion of individuals (45·3 %) had an education of less than 9 years, we repeated our analyses using a modified definition of cognitive impairment (MMSE score < 21)(Reference Robison, Gruman and Gaztambide38). Continuous values of sugar consumption were used for trend tests. All statistical analyses were conducted with SAS (version 9.1.3; SAS Institute Inc., Cary, NC, USA). A value of P < 0·05 (two-sided) was considered statistically significant.
Results
Among participants without diabetes (mean age 56·3 (sd 7·6) years), the averages of total and added sugar intakes were 115 (sd 62) and 64·0 (sd 45·4) g/d, respectively. Total sugars contributed to 21·4 (sd 8·0) % of energy intake, and added sugars contributed to 11·8 (sd 8·0) %. Sucrose was the most consumed sugar (46·7 (sd 27·9) g/d), followed by fructose (25·2 (sd 19·3) g/d) and glucose (25·0 (sd 17·9) g/d). Together, sucrose, glucose and fructose contributed 84·4 % of total sugar consumption. Fruit drinks, soft drinks, dairy desserts and sweets provided 22·1, 12·9, 11·3 and 10·3 % of added sugars, respectively. The mean MMSE score was 23·7 (sd 3·4). Approximately 47·0 % had a MMSE score < 2 4 and 16·7 % had a MMSE score < 21 in this population.
Participants in the highest quintile of energy-adjusted total sugar intake were more likely to be older, women, and to have lower prevalence of heavy drinkers, lower BMI, lower prevalence of hypertension, and higher plasma vitamin C concentration, relative to participants with the lowest energy-adjusted total sugar consumption (Table 1). As expected, participants in the highest quintile of energy-adjusted total sugar consumption had significantly lower intake of fat, but higher intakes of added sugars, sucrose, glucose, natural and added fructose, galactose, maltose, lactose, maltose, SSB, sugar-sweetened solid foods and 100 % fruit juice (all P < 0·05; Table 2) when compared with those in the lowest quintile of energy-adjusted total sugar consumption.
Table 1 Characteristics of participants without diabetes from the Boston Puerto Rican health study by quintile of energy-adjusted total sugar intake, 2004–9†
(Mean values with their standard errors or percentages)
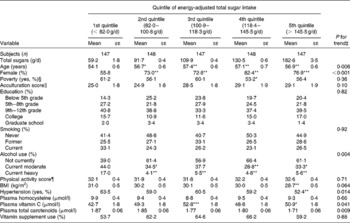
Value was significantly different from that of the first quintile: * P < 0·05, ** P < 0·01, *** P < 0·001.
† Values for continuous variables were adjusted for age (years) and sex. Means were compared using the general linear model procedure (PROC GLM) in SAS, with Tukey's adjustment for multiple comparisons. Percentages of categorical variables across quintiles of total sugar intake were compared using logistic regression (PROC LOGISTIC) in SAS after adjustment for age (years) and sex.
‡ Continuous value of total sugar intake was used for the trend test.
§ Poverty was defined as ‘yes’ if a participant's total annual house income was below the poverty threshold released each year by the US Department of Health and Human Services.
∥ Acculturation score was calculated based on answers of questions regarding the extent of use of English and/or Spanish at work and in ordinary life: a summed score ranged from 0 (fully unacculturated: only using Spanish) to 100 (fully acculturated: only using English).
¶ Physical activity score was constructed by weighting time spent in various physical activities by factors that reflect O2 consumptions of related physical activities.
Table 2 Intakes of selected nutrients, sugars and food by quintile of energy-adjusted total sugar intake in participants without diabetes from the Boston Puerto Rican health study, 2004–9†
(Mean values with their standard errors)
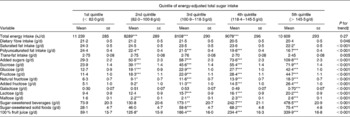
Mean value was significantly different from that of the first quintile: * P < 0·05, ** P < 0·01, *** P < 0·001.
† Values were adjusted for age (years) and sex. Means were compared using the general linear model procedure (PROC GLM) in SAS, with Tukey's adjustment for multiple comparisons.
‡ Continuous value of total sugar intake was used for the trend test.
Intake of total sugars was strongly associated with intakes of added sugars, sucrose, glucose, added fructose and SSB (r 0·63 to 0·83; all P < 0·001), moderately associated with natural fructose and 100 % fruit juice (r 0·43 and 0·46, respectively; P < 0·001) and weakly associated with sugar-sweetened solid foods, lactose, maltose and galactose (r 0·15 to 0·30; all P < 0·001) (see Supplemental Table 2; available at http://www.journals.cambridge.org/bjn).
Total and added sugar intakes were each inversely associated with MMSE score, after adjusting for several covariates (Table 3). The MMSE score was 0·4 points lower for each 60 g of total sugar intake (β − 0·41; se 0·15; P = 0·007), after adjusting for age, sex, educational attainment, poverty, acculturation score, smoking, alcohol use, physical activity score, BMI and the presence of hypertension. This difference in MMSE score was approximately comparable with an associated difference in MMSE score per 10-year increase in age (β − 0·39; se 0·17; P = 0·020) in the same model. The MMSE score was 0·4 points lower for each 50 g of added sugar intake (β − 0·43; se 0·16; P = 0·005), which also equalled the difference in MMSE score per 10-year increase in age (β − 0·43; se 0·17; P = 0·011). These associations were attenuated after further adjustment for intakes of dietary fibre and fat (saturated, polyunsaturated and trans), vitamin supplement use and serum concentrations of vitamin C, total carotenoids and total homocysteine, but still remained significant for total sugar (P for trend = 0·024) and marginally significant for added sugar (P for trend = 0·057).
Table 3 Mini-Mental State Examination scores by quintile of energy-adjusted sugar and food intake in participants without diabetes from the Boston Puerto Rican Health Study, 2004–9†
(Mean values with their standard errors)
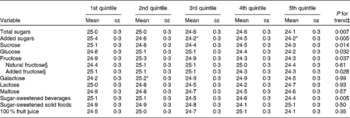
* Mean value was significantly different from that of the first quintile (P < 0·05).
† Values were adjusted for age (years), sex, educational attainment ( < 5th grade, 5th–8th grade, 9th–12th grade, college, or graduate school), poverty (yes or no), acculturation score, smoking (never, former, or current), alcohol use (not current, current moderate, current heavy), physical activity score, BMI (kg/m2; < 25, 25–29·9, or ≥ 30) and the presence of hypertension. Means were compared by using the general linear model procedure in SAS, with Tukey's adjustment for multiple comparisons.
‡ Continuous value of sugar consumption was used for the trend test.
§ Non-added fructose and added fructose were included in the model simultaneously.
Similar inverse trends were observed for sucrose, glucose and fructose, after adjusting for covariates (Table 3). Further analysis showed that added fructose, but not natural fructose, was significantly associated with lower MMSE score. The association between sucrose and MMSE score was attenuated after further adjustment for the sum of glucose, fructose, galactose, lactose and maltose (P for trend = 0·059). The associations between glucose (P for trend = 0·083), as well as fructose (P for trend = 0·093) and added fructose (P for trend = 0·088), and MMSE score were also attenuated after further adjustment for the sum of sucrose, galactose, lactose and maltose.
No significant associations between intakes of galactose, lactose or maltose and MMSE score were observed (Table 3). SSB, but not sugar-sweetened solid foods or 100 % fruit juice, were inversely associated with MMSE score. Higher intakes of total and added sugars were also associated with a higher likelihood of having cognitive impairment (MMSE score < 24) (Fig. 1); the multiple-adjusted OR for cognitive impairment were 2·23 (95 % CI 1·24, 3·99) and 2·28 (95 % CI 1·26, 4·14) for the highest v. lowest quintiles of total and added sugar consumption, respectively. A higher intake of fructose was also marginally associated with lower cognitive impairment (OR 1·57; 95 % CI 0·91, 2·73) when comparing extreme quintiles (P for trend = 0·072). The relative OR for cognitive impairment defined by a more conservative cut-off point (MMSE score < 21) were 1·71 (95 % CI 0·81, 3·61) for total sugars (P for trend = 0·029), 2·42 (95 % CI 1·08, 5·39) for added sugars (P for trend = 0·029), and 1·43 (95 % CI 0·70, 2·93) for fructose (P for trend = 0·078) when comparing extreme quintiles, respectively.
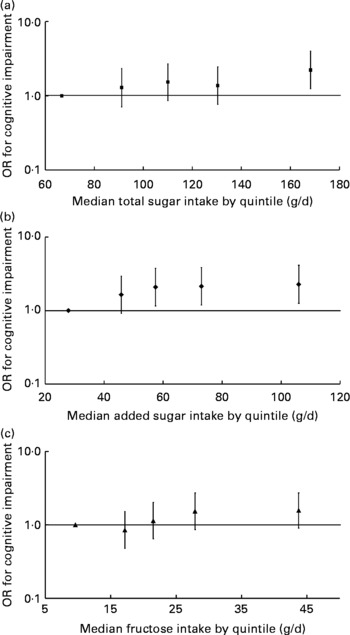
Fig. 1 Multivariate-adjusted OR and 95 % CI for cognitive impairment according to quintile of energy-adjusted total sugar (P for trend = 0·015) (a), added sugar intakes (P for trend = 0·040) (b) and fructose intake (natural plus added) (P for trend = 0·072) (c) among participants without diabetes from the Boston Puerto Rican Health Study, 2004–9. OR and 95 % CI were plotted against the median of sugar intake of each quintile. The median (range) of total sugar intake (g/d) across quintiles of total sugar intake were: 66·7 ( < 82·0), 91·2 (82·0–100·8), 110·0 (100·9–118·3), 130·4 (118·4–145·5) and 168·3 (>145·5), respectively. The median (range) of added sugar intake (g/d) across quintiles of added sugar intake were: 27·9 ( < 37·9), 45·8 (38·0–51·0), 57·4 (51·1–64·6), 73·0 (64·7–85·1) and 106·0 (>85·1), respectively. The median (range) of fructose intake (g/d) across quintiles of fructose intake were: 9·6 ( < 14·2), 17·2 (14·2–19·3), 21·5 (19·4–24·9), 27·9 (25·0–33·8) and 43·7 (>33·8), respectively. Cognitive impairment was defined as a Mini-Mental State Examination score < 24. OR was calculated with logistic regression, after adjustment for age (years), sex, educational attainment ( < 5th grade, 5th–8th grade, 9th–12th grade, college, or graduate school), poverty (yes or no), acculturation score, smoking (never, former, or current), alcohol use (never, former, or current), physical activity score, BMI (kg/m2; < 25, 25–29·9, or ≥ 30), and the presence of hypertension.
Total sugar intakes were inversely associated with memory function and a similar trend with marginal significance was observed for added sugar intakes (Table 4). Inverse associations were also observed for total sugar intake with letter fluency and word list percentage recognition, delayed recognition, short- and long-term recall scores; and for added sugar intake with letter fluency and word list long-term recall score (all P < 0·05) (data not shown).
Table 4 Major cognitive function factors* by quintile of intake of energy-adjusted total sugars and added sugars in participants without diabetes from the Boston Puerto Rican health study, 2004–9†
(Mean values with their standard errors)
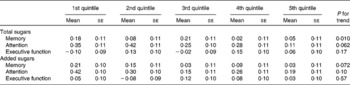
* Three major cognitive function factors were derived from seven cognitive tests by factor analysis (see Supplemental Table 1; available at http://www.journals.cambridge.org/bjn).
† Values were adjusted for age (years), sex, educational attainment ( < 5th grade, 5th–8th grade, 9th–12th grade, college, or graduate school), poverty (yes or no), acculturation score, smoking (never, former, or current), alcohol use (not current, current moderate, current heavy), physical activity score, BMI (kg/m2; < 25, 25–29·9, or ≥ 30) and the presence of hypertension. Means were compared by using the general linear model procedure in SAS, with Tukey's adjustment for multiple comparisons.
Discussion
In this sample of Puerto Rican adults without diabetes, aged 45–75 years, approximately 21 % of energy intake was obtained from total sugars and 12 % from added sugars. Total sugars, added sugars, sucrose, glucose and added fructose were each significantly inversely associated with cognitive function. SSB, but not sugar-sweetened solid foods, were inversely associated with cognitive function. Higher intakes of total sugars were also significantly associated with lower memory function, but not with measurable differences in executive function or attention.
The percentage contribution of added sugar to total energy intake in the present study was similar to that of the general US population with a similar age range, as shown in the 1994–6 Continuing Survey of Food Intakes by Individuals (CSFII)(Reference Guthrie and Morton39) and the National Health and Nutrition Examination Survey (NHANES 1999–2002)(Reference Cook and Friday40). However, in the 1994–6 CSFII, soft drinks provided 33 % of total added sugars, and fruit drinks provided 10 %(Reference Guthrie and Morton39); and in the NHANES 2001–2, soft drinks provided 37 % and fruit drinks 12 %(Reference Bachman, Reedy and Subar41). In contrast, the percentage contributions of added sugars from fruit drinks were higher than from soft drinks in the present study population.
The mean MMSE score (23·7) was generally low when compared with other studies. For example, in the Northern Manhattan Study of 3298 stroke-free subjects aged 40 years and older, the mean MMSE score for Hispanic subjects was 25·3, which was significantly lower than that for white subjects (27·7)(Reference Wright, Lee and Paik42). The low mean MMSE score and high prevalence of cognitive impairment (MMSE score < 24) in the present study may be due to differences in language use, low educational attainment(Reference Escobar, Burnam and Karno36) and high prevalence of risk factors and diseases that may affect cognitive function.
Previous studies have shown that high sugar consumption is a risk factor for obesity(Reference Forshee, Storey and Allison3, Reference Bleich, Wang and Wang4), the metabolic syndrome(Reference Dhingra, Sullivan and Jacques5, Reference Malik, Popkin and Bray8) and type 2 diabetes(Reference Bleich, Wang and Wang4, Reference Malik, Popkin and Bray8–Reference Odegaard, Koh and Arakawa10, Reference Laville and Nazare43, Reference Johnson, Segal and Sautin44). Obesity, the metabolic syndrome and type 2 diabetes are risk factors for cognitive impairment(Reference Yaffe, Kanaya and Lindquist12–Reference van den Berg, Kloppenborg and Kessels15, Reference Greenwood45). Cognitive impairment in type 2 diabetes has been more evident for verbal memory than for attention, concentration or executive function(Reference Greenwood45). We observed that higher sugar consumption was more significantly associated with lower memory function, than with other cognitive measures. Findings from animal studies have also suggested that diets with high sugar may reduce memory function(Reference Stranahan, Norman and Lee17, Reference Cao, Lu and Lewis46). For example, Cao et al. (Reference Cao, Lu and Lewis46) found that long-term consumption of sucrose-sweetened water induced insulin resistance and exacerbated memory deficits in a transgenic mouse model of Alzheimer's disease. Stranahan et al. (Reference Stranahan, Norman and Lee17) showed that rats fed high-fructose diets developed insulin resistance, resulting in impaired hippocampal synaptic plasticity and hippocampus-dependent memory. More recently, Rasgon et al. (Reference Rasgon, Kenna and Wroolie47) reported that insulin resistance was inversely associated with right and total hippocampal volume among non-diabetic, cognitively intact middle-aged women at risk for Alzheimer's disease(Reference Rasgon, Kenna and Wroolie47).
Nevertheless, an inverse association between BMI and total sugar intake observed in the present study deserves attention, and could be explained by several possible mechanisms. In a 10-week intervention in overweight subjects, Raben et al. (Reference Raben, Vasilaras and Moller48) showed that those in the sucrose-supplemented group had an increased fat mass of 1·3 kg (total weight increased by 1·6 kg), compared with those receiving artificial sweetener, where fat mass decreased by 0·3 kg (total weight decreased by 1·0 kg)(Reference Raben, Vasilaras and Moller48). These results suggest that sucrose may contribute more to change in body fat than in total weight. Because BMI has its own limitation in assessing fatness among elder individuals(Reference Kyle, Genton and Hans49), further studies are needed to verify whether sugar intake is more correlated to body fat than to BMI among older individuals. We also cannot exclude the possibility that those with higher BMI may under-report sugar intake and sweetened food and beverages than their counterparts(Reference Mendez, Wynter and Wilks50). Those with the highest BMI were younger in the present study, and it is possible that they may have been more concerned about their body weight than their older counterparts, and may have tried to reduce their sugar intake. Prospective studies are needed to verify the role of sugar intake in the development of obesity in this population.
Importantly, however, the observed associations between sugar intake and MMSE were independent of age and BMI. Sugar intake and cognitive function may also be connected through other mechanisms(Reference Stephan, Wells and Brayne21). Sucrose and HFCS have been shown to increase the production of uric acid in human subjects(Reference Akhavan and Anderson51). An association between elevated uric acid and cognitive decline was recently reported in older individuals(Reference Schretlen, Inscore and Jinnah52, Reference Ruggiero, Cherubini and Lauretani53). In addition, a dietary pattern with high SSB was related to elevated inflammatory status(Reference Schulze, Hoffmann and Manson54). A 10-week intervention study demonstrated that sugar-sweetened drinks and food tend to increase inflammatory activity(Reference Sorensen, Raben and Stender55). Inflammation, a major risk factor for diabetes and CVD, is also correlated with cognitive decline(Reference Weaver, Huang and Albert56, Reference Yaffe, Lindquist and Penninx57) and dementia(Reference Engelhart, Geerlings and Meijer58) even after adjustment for cardiovascular metabolic disorders. Fructose, one of the major ingredients of SSB, has been linked to increased de novo lipogenesis, glycogenesis, oxidative stress and uric acid production, and to reduced NO production(Reference Stephan, Wells and Brayne21). As reviewed by Stephan et al. (Reference Stephan, Wells and Brayne21), these metabolic changes have been associated with the metabolic syndrome or its components which, in turn, may lead to cognitive decline and Alzheimer's disease(Reference Dik, Jonker and Comijs11–Reference Ho, Niti and Yap14, Reference Vanhanen, Koivisto and Moilanen59, Reference Razay, Vreugdenhil and Wilcock60).
Interestingly, added fructose, but not natural fructose, was related to low MMSE scores. Natural fructose is from fruit and vegetables, which also contribute protective nutrients for cognitive function, including B vitamins and antioxidants(Reference Tucker, Qiao and Scott61, Reference Akbaraly, Faure and Gourlet62), whereas added fructose is mainly added to SSB. Importantly, 100 % fruit juice, unlike SSB, was not associated with lower MMSE score or memory function. Other nutrients in 100 % fruit juice have been positively linked with cognitive function(Reference Tucker, Qiao and Scott61, Reference Akbaraly, Faure and Gourlet62) and, therefore, may counteract the potential effects of naturally occurring sugars in 100 % fruit juice.
It is important to note that SSB, but not sugar-sweetened solid foods, were associated with lower cognitive function. One potential interpretation could be the differential contributions of sucrose, glucose and fructose in beverages v. solid foods. SSB were strongly associated with intakes of glucose and added fructose, whereas sugar-sweetened solid foods were only associated with the total intake of sucrose.
Intake of total sugars was independently associated with lower cognitive function, after adjusting for supplemental use of vitamins, dietary fibre and fat, and plasma concentrations of several nutrients as was the association of intake of added sugars with cognitive function, although marginally significant (P for trend = 0·065). These findings suggest that the inverse associations between sugar consumption and cognitive function were not fully explained by other aspects of a poorer dietary pattern that may be associated with higher sugar consumption.
Alternatively, our findings could also be explained by potentially altered dietary selections because of chemosensory changes with ageing and cognitive decline(Reference Murphy63). Older participants have been reported to have a preference for higher concentrations of sugar relative to their younger counterparts(Reference Murphy63). A high proportion of participants had evidence of cognitive impairment (MMSE score < 24) in the present study. Some of these low scores are probably related to low education levels(Reference Crum, Anthony and Bassett64). Although we excluded those with MMSE score ≤ 10, it is possible that some with scores between 10 and 24 could, if clinically diagnosed, be found to have mild cognitive impairment or early-stage Alzheimer's disease. A few studies have suggested that patients with Alzheimer's disease may have a greater preference for sweet foods than non-impaired controls(Reference Mungas, Cooper and Weiler65). Due to the cross-sectional design of available studies, prospective studies are needed to clarify causal associations between sweet foods and cognitive decline.
The present study has several strengths. We conducted analyses among individuals without diabetes, which reduces the potential confounding effects of diabetes and its complications, and other important confounding factors were controlled. We used a series of cognitive function tests that allowed the assessment of different domains of cognitive function. Limitations include the cross-sectional design, which precludes conclusions regarding the direction of causality. We were also not able to separate the specific associations of fructose with cognitive function from glucose because they were highly correlated with each other. Further, although we controlled for several possible confounders in our analyses, we cannot exclude the possibility of residual confounding.
To our knowledge, this is the first population-based study to simultaneously investigate the associations between habitual intakes of sugars, SSB and sugar-sweetened solid foods with cognitive function among middle-aged and older adults. Total sugars, added sugars, sucrose and added fructose were each inversely associated with cognitive performance. Consistently, a higher intake of SSB was significantly associated with lower cognitive performance. Prospective studies are needed to confirm whether long-term consumption of SSB and/or sugar-sweetened foods is a risk factor for cognitive impairment.
Acknowledgements
The present study was supported by the National Institute on Aging of the National Institutes of Health (NIH) (no. P01AG023394 and R01AG02708), the National Heart Lung and Blood Institute of NIH (no. P50HL105185) and the US Department of Agriculture, Agricultural Research Service contract (no. 58–1950–7–707).
X. Y. contributed to data analysis, interpretation of data and writing the manuscript. X. G., T. S. and K. L. T contributed to the interpretation of data and critical revision of the manuscript. K. L. T obtained funding, conceived of the study and supervised the data collection. All authors read and approved the final manuscript.
None of the authors had any conflicts of interest.







