α-Lactalbumin (α-LA) is a small, globular protein found in the whey fraction of milk in all mammals. It is composed of 123 amino acid residues with a resulting molecular weight of 14·2 kDa. It is a known component of lactose synthase in the mammary gland( Reference Brew, Vanaman and Hill 1 ). It is also known to be nutritionally significant in that it provides all the essential amino acids at their required concentrations( Reference Jensen 2 ). Recently, there have been reports that α-LA may protect somewhat against breast cancer, and thus it has become the focus of attention for the preparation of a potential vaccine against breast cancer( Reference Jaini, Kesaraju and Johnson 3 ). Once deemed to be a waste commodity or by-product of cheese production, the whey fraction of milk has received much interest from scientists and industry of late. Known for its traditional functions, including that of nutrition (whereby the amino acid sequence of the protein provides all the essential amino acids), it also possesses other functional properties. Whey has been used in gelation studies( Reference Kehoe, Morris and Brodkorb 4 ) as well as a carrier for the targeted delivery of probiotic bacteria to the intestines( Reference Doherty, Wang and Ross 5 ). Many other health-promoting benefits achieved through the consumption of whey proteins have been reported( Reference Chatterton, Smithers and Roupas 6 ), including the supplementation of α-LA in infant formulae to further ‘humanise’ infant formulae( Reference Rudloff and Lönnerdal 7 ) and the potential use of α-LA in the treatment of stress through the increase in serotonin concentrations after the consumption of tryptophan-rich α-LA( Reference Markus, Olivier and Panhuysen 8 ). More recently, whey proteins have been the focus of binding studies, whereby it has been shown that the binding of hydrophobic compounds to proteins can subsequently affect the bioactivity of the proteins, including the binding of sodium linoleate to β-lactoglobulin (β-LG)( Reference Le Maux, Giblin and Croguennec 9 ) and, more extensively, the binding of oleic acid (OA) to both α-LA( Reference Svensson, Hakansson and Mossberg 10 ) and β-LG( Reference Lišková, Auty and Chaurin 11 ) to form tumouricidal and antimicrobial( Reference Hakansson, Roche-Hakansson and Mossberg 12 ) complexes.
Extensive research into the tumouricidal complex between α-LA and the human milk fatty acid (OA), dubbed HAMLET (Human α-lactalbumin Made LEthal to Tumour cells), is ongoing( Reference Svensson, Hakansson and Mossberg 10 ). HAMLET is active against a range of over forty different cancer cell lines and exhibits certain selectivity and can differentiate between cancer cells and healthy cells( Reference Brinkmann, Heegaard and Petersen 13 ). It kills cancer cells through an apoptosis-like mechanism, activating caspases( Reference Duringer, Hamiche and Gustafsson 14 ), and induces microautophagy( Reference Aits, Gustafsson and Hallgren 15 ). HAMLET has been shown to be active in vivo in a range of clinical trials including bladder cancer( Reference Mossberg, Wullt and Gustafsson 16 , Reference Mossberg, Hou and Svensson 17 ), brain glioblastoma( Reference Fischer, Gustafsson and Mossberg 18 ) and colon cancer trials( Reference Puthia, Storm and Nadeem 19 ). It has previously been shown that a complex that is structurally and actively similar to BAMLET (Bovine α-lactalbumin Made LEthal to Tumour cells), the bovine analogue of HAMLET, is formed under simulated infant gastrointestinal conditions( Reference Sullivan, Mok and Brodkorb 20 ).
The initial concept of a HAMLET-like complex being formed in the gastrointestinal tract was described by Svensson et al. ( Reference Svensson, Fast and Mossberg 21 ). During the initial discovery of HAMLET, multimeric α-LA, which had a tumoricidal effect, dubbed MAL (multimeric α-lactalbumin), was found in the acidic fraction of precipitated human milk. This led to speculation that the conditions within the stomach of nursing infants may be favourable for the conversion of α-LA to its active form. First, high rates of gastric lipolysis occur in the gastric environment and thus free OA is present( Reference Roman, Carriere and Villeneuve 22 ). Second, upon changing of the pH to levels below the isoelectric point of the protein, it unfolds, potentially allowing the formation of a complex( Reference Kuwajima 23 ). Finally, low rates of proteolysis occur due to the pH gradient experienced in the stomach of infants( Reference Mitchell, McClure and Tubman 24 ). It has previously been shown that α-LA and fragments of α-LA produced through limited proteolysis of the protein can bind to the fatty acid and in turn exhibit cytotoxic activity( Reference Barbana, Pérez and Sánchez 25 ).
Given the previous in vitro work demonstrating the formation of a cytotoxic compound during simulated digestion, a subsequent in vivo study was undertaken. The aims of the present study were to determine whether the required unfolding of the protein occurs in vivo in the stomach of healthy adults and then to determine whether sufficient amounts of native protein remain during gastric digestion for the conversion of α-LA to its bioactive form and to test ex vivo the activity of gastric samples against cancer cells. To assess structural changes undergone by the protein in the stomach, it was necessary to use nasogastric tubes to allow sampling of the gastric contents. A novel approach was used to visualise changes in the protein drinks within the gastric environment during gastric transit. This allowed the assessment of gastric mixing.
Materials and methods
Materials
Commercially available BioPURE alpha-lactalbumin™ (95 % purity, 5 % β-LG) was purchased from Davisco Foods International Inc. Pharmaceutical grade OA was sourced from Sigma-Aldrich. Pharmaceutical grade ethanol was sourced from Ocon Chemicals. Bio-Rad molecular-weight markers for SDS–PAGE were purchased from Bio-Rad Laboratories. All other chemicals and reagents were purchased from Sigma-Aldrich. Nasogastric tubes (Ryles tubes with luer attachment, size 10) were sourced from Pennine Healthcare.
Methods
Subjects
A total of ten subjects (six females and four males) participated in the study (median age 32·5 (range 25–48) years). The subjects were recruited through an approved advertisement within the participating research facilities. All the recruited subjects were healthy with no pre-existing medical conditions that required daily medication. A pre-screening step was used to ensure that the subjects were healthy and exhibited no symptoms of prior gastrointestinal disease. Female subjects of child-bearing age were required to undergo a pregnancy test before participating in the study. All the subjects were informed of the protocol of the study orally and in writing. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Cork Research Ethics Committee (Cork University Hospital, Cork, Ireland). Written informed consent was obtained from all the subjects. The study was conducted under the medical supervision of the staff of the Clinical Trial Unit of the Alimentary Pharmabiotic Centre (UCC, Cork, Ireland). Medically qualified clinical research specialists from Mercy University Hospital (Cork, Ireland) inserted and removed the nasogastric tubes and the nasogastric pH probes and supervised the use of the capsule endoscope.
Gastric aspirate sampling
After an overnight fast, the subjects visited the Clinical Measurement Laboratory of Mercy University Hospital. A nasogastric tube was inserted into the stomach of the subjects. A 10 ml luer lock syringe was used to remove samples from the stomach. An aliquot of gastric juice was obtained and the pH was measured to confirm that the nasogastric tube was in the stomach. The void volume of the nasogastric tube was calculated to be 6·5 ml, and thus after each sample was collected through the nasogastric tube, 7 ml of air were injected through the tube to return the gastric content to the stomach. The subjects were then given 250 ml of a test drink to consume. The samples were collected every 3 min. Each sample measured 5 ml and the pH was recorded before aliquoting the samples into 1 ml vials. Each aliquot was immediately placed on ice after pH measurement and frozen within 2 h of sampling.
Test drinks
The subjects were given three test beverages that were prepared to a volume of 250 ml containing 50 g/l of sucrose and (1) 25 g/l of α-LA (only protein, pH = 6·7, drink 1), (2) 25 g/l of α-LA and 4 g/l of OA (protein and OA, pH = 6·5, drink 2), and (3) 4 g/l of OA (only OA, drink 3), herein referred to as α-LA, α-LA–OA and OA drinks, respectively. OA was dissolved in ethanol and mixed by agitation with the sucrose solution immediately before ingestion. The energy value of the three drinks was calculated using standard values per 1 g of protein (16·7 kJ), sugar (16·7 kJ), ethanol (22·3 kJ) and fat (37·6 kJ). The energy contents of drinks 1, 2 and 3 were 125·5, 192·5 and 150·6 kJ, respectively.
Fluorescence spectroscopy
Fluorescence spectroscopy measurements were taken using a Cary Eclipse Fluorescence spectrophotometer (Agilent Technologies) equipped with a multi-cell holder, a peltier unit and a temperature controller. Protein concentrations were determined using an extinction coefficient of 20·1 measured at 280 nm by absorbance. The protein content of the samples was diluted to 140 mg/l. The excitation wavelength was 280 nm and the emissions were recorded between 300 and 420 nm. Spectra were recorded at 25°C and at a scanning speed of 80 nm/min. The excitation and emission slits were set to 10 mm. The samples were measured in 1 × 1 cm quartz cuvettes. The spectrum of a blank (water) was subtracted from all the spectra before analysis.
Fourier transform infrared spectroscopy
Fourier transform IR spectroscopy measurements were taken using a Bruker Tensor 27 instrument equipped with a thermally controlled BioATR Cell™ II (Bruker Optik) that was designed for the analysis of protein in solution. The measurements were taken at 20°C, and an average of 180 scans at a resolution of 4 per cm were recorded. Protein samples were diluted to a concentration of 10 g/l and filtered through a 0·1 μm syringe filter. Data were processed by performing atmospheric compensation and then vector normalisation at 1600–1720 per cm for the amide I region and at 2800–2900 per cm for the C–H OA region. Spectrum-capturing software Opus (version 5.5; Bruker Optics) was used for data processing with vector normalisation after atmospheric compensation and offset correction.
HPLC
All HPLC analyses were carried out on the Waters 2695 Separations Module equipped with a dual-wavelength detector set at 214 and 280 nm. A sample volume of 20 μl of a 0·25 mg/ml protein solution was injected onto the column. Digestion progression was monitored through size-exclusion HPLC using a TSK G2000 column. The flow was isocratic and the solvent used was 30 % acetonitrile with 0·1 % (w/v) trifluoroacetic acid. The flow rate was 0·5 ml/min and the runtime was 60 min. Molecular-weight standards (molecular-weight range 67 000–262 Da) were subjected to the same run conditions to determine the elution time based on sample size.
Electrophoresis
SDS–PAGE was carried out as per the modified method of Laemmli( Reference Laemmli 26 ). The gels were prepared to a concentration of 15 % acrylamide. The samples were prepared to a concentration of 10 g/l and diluted 1:8 with a reducing SDS–PAGE buffer. The samples were heated at 95°C for 5 min and cooled at room temperature before loading onto the gels. Molecular-weight markers (molecular-weight range 250–10 kDa, catalogue no. 161-0373; Bio-Rad Laboratories) were used for electrophoresis.
Capsule endoscopy – Mirocam® technology
Capsule endoscopy was used for intragastric imaging of α-LA during digestion. A total of eight individual electrodes were attached to the torso of one subject. The camera wirelessly transmitted three images per second to the receptors/electrodes that were attached to the torso of the subject. The receptors were also connected to a workstation allowing real-time imaging of the gastric contents. The camera was equipped with a light-emitting diode, which transmitted light to allow correct imaging of the milk-like solution. A combination of the intragastric pH probe and the camera allowed real-time pH measurement and real-time imaging of the stomach, thus allowing the correlation of gastric pH with the gastric contents. The camera was swallowed simultaneously with the α-LA drink.
Intragastric pH monitoring
The intragastric pH levels were recorded using a nasogastric pH probe inserted through the nose into the stomach. The probe was connected to a recording unit that allowed real-time monitoring of the pH within the gastric environment.
Cell viability assay
Cytotoxicity measurements were taken using U937 cells – a suspension cell line extracted from a human diffuse histiocytic lymphoma. The cells were grown at a density of 1 × 105 in Roswell Park Memorial Institute (RPMI)-1640 supplemented with 10 % fetal bovine serum and incubated at 5 % CO2 at 37°C. For cytotoxicity assays, the cells were subcultured at a density of 2 × 105. For cytotoxicity analysis, the samples were dissolved in RPMI-1640 and sterile-filtered using a 0·1 μm syringe and diluted to a concentration of 352 μm. The samples were then diluted to the desired concentrations between 0 and 176 μm with RPMI-1640 to obtain a final volume of 50 μl. Then, cells at a density of 2 × 105 were added to the samples in the wells to obtain a final volume of 100 μl with the final concentration of fetal bovine serum being set at 5 %. The plates were incubated for 24 h, and 10 μl of alamarBlue reagent were added to each well and the plates were incubated for 4 h. The fluorescence intensities of the samples were measured at an excitation wavelength of 530 nm and emissions were recorded at 590 nm. For samples containing digestive enzymes, pepstatin, a potent pepsin inhibitor, was used to inhibit enzyme activity during bioactivity assay.
Pepsin activity assay
The in vivo activity of pepsin was measured using bovine Hb as a substrate. Briefly, bovine Hb was incubated at pH 2 at 37°C with the gastric aspirates. After 10 min, TCA was added to stop the reaction. The absorbance at 280 nm was measured, and the activity of pepsin was calculated as units per ml of gastric juice.
Statistical analysis
To compare the changes in λmax of the digests containing α-LA with those in λmax of digests containing α-LA and OA, the digests were grouped based on pH. Intervals of one pH unit were chosen when grouping the data. Student's t test was carried out using SigmaStat software to compare digests with and without OA at a given pH interval. All values given in this paper represent averages of triplicates and standard deviations.
Results
pH within the gastric environment
The pH of the gastric contents of the subjects after fasting was measured before the ingestion of each test drink. Typically, the pH of the stomach was acidic with average fasting pH levels of 2·31 ± 1·19, 2·22 ± 1·91 and 1·89 ± 1·80 before the ingestion of the α-LA, α-LA–OA and OA drinks, respectively. The activity of pepsin within the stomach was determined before the ingestion of the α-LA drink and it was found to be 39 (sd 12) units/ml of gastric juice (Table 1).
Table 1 Measured experimental values of the baseline pH and pepsin activity (n 10) (Mean values and standard deviations)
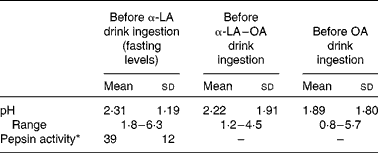
α-LA, α-lactalbumin; OA, oleic acid.
* Pepsin activity is defined as the units of pepsin per mg protein using bovine Hb as a substrate and expressed as units/ml of gastric juice.
The pH of the gastric aspirates was measured immediately after the ingestion of the test drinks and after their removal by suction through the previously inserted nasogastric tube (Fig. 1). The pH of the stomach increased due to the pH of the α-LA drink (pH 6·7) and the buffering capacity of the protein in the drink. A similar trend was observed upon ingestion of the α-LA–OA drink. No difference was observed between the pH curves of the α-LA and α-LA–OA drinks (Fig. 1). Before the ingestion of the OA drink, the resting pH of the stomach was 1·89 (sd 1·80) (range 0·8–5·7). After the ingestion of this drink, the pH of the stomach remained stable and no increase was observed (Fig. 1). This was probably due to the acidic nature of the OA and the absence of a buffer in the drink.
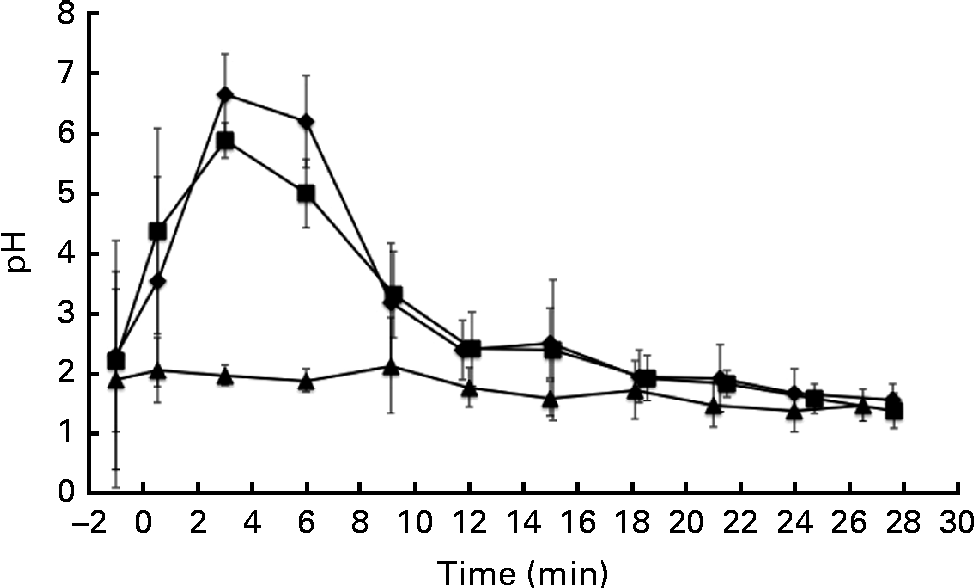
Fig. 1 Gastric pH levels in healthy human subjects (n 8) after the ingestion of 250 ml of test drinks: 50 g/l of sucrose in water with 25 g/l of α-lactalbumin (α-LA; ![]() ); 25 g/l of α-LA with oleic acid (α-LA–OA;
); 25 g/l of α-LA with oleic acid (α-LA–OA; ![]() ); OA alone (
); OA alone (![]() ); the energy values of the drinks were 126, 192 and 151 kJ (30, 46 and 36 kcal), respectively. The value at − 1 min is representative of the fasting pH before ingestion. Error bars are representative of the standard deviations of the pH measurement of each subject over a range of time points and are represented by vertical bars.
); the energy values of the drinks were 126, 192 and 151 kJ (30, 46 and 36 kcal), respectively. The value at − 1 min is representative of the fasting pH before ingestion. Error bars are representative of the standard deviations of the pH measurement of each subject over a range of time points and are represented by vertical bars.
For the purposes of the present study, gastric emptying was defined as the time when only air or gastric mucus was removed with a syringe via the nasogastric tube at the location accessible to the tube, with no sample remaining present at the insertion position of the nasogastric tube. Although it is a fair assumption that this is close to actual gastric emptying, it would need to be confirmed by other methods such as MRI scanning or scintigraphy. Gastric emptying was estimated to have occurred at 30 min for each of the three drinks. The energy values of the α-LA, α-LA–OA and OA drinks were 126, 192 and 151 kJ (30, 46 and 36 kcal), respectively. Gastric emptying rates are influenced by many factors, including the energy value of the drink. However, differences in the energy values of the drinks can be assumed to be negligible, as the gastric emptying times were not affected by the energy content of the drinks.
In addition, the pH of the gastric contents was also measured using a nasogastric pH probe in one subject who consumed two separate servings of the α-LA drink in a 2 h time period (Fig. 6(a)). The in vivo measurement of the pH of the stomach was effective and was comparable to that of the pH of the gastric aspirates discussed previously (Fig. 1). Small differences and several pH jumps were observed in the sample measured in vivo. Although measures were taken to ensure that the nasogastric tube remained in the same position (subject remained supine in a stationary position), movement of the stomach due to peristalsis could not be accounted for.
Structural changes within the protein
Using intrinsic fluorescence spectroscopy, it was possible to monitor protein unfolding as a function of the decrease in pH within the stomach. The isoelectric point of α-LA (approximately 4·8) is the point at which the protein partially unfolds, resulting in the alteration of the tertiary structure of the protein( Reference Fox and McSweeney 27 ). Upon protein unfolding, there is a change in the environment of the tryptophan residues, thus resulting in a change in the intrinsic fluorescence spectra of the protein, coincident with the alteration of the tertiary structure. Upon unfolding, there is a red shift in the wavelength at which the maximum intensity occurs and also an increase in the maximum intensity recorded (Fig. 2(a)). This confirms that the required structural change for the formation of a complex to occur happens during the in vivo gastric digestion of α-LA.
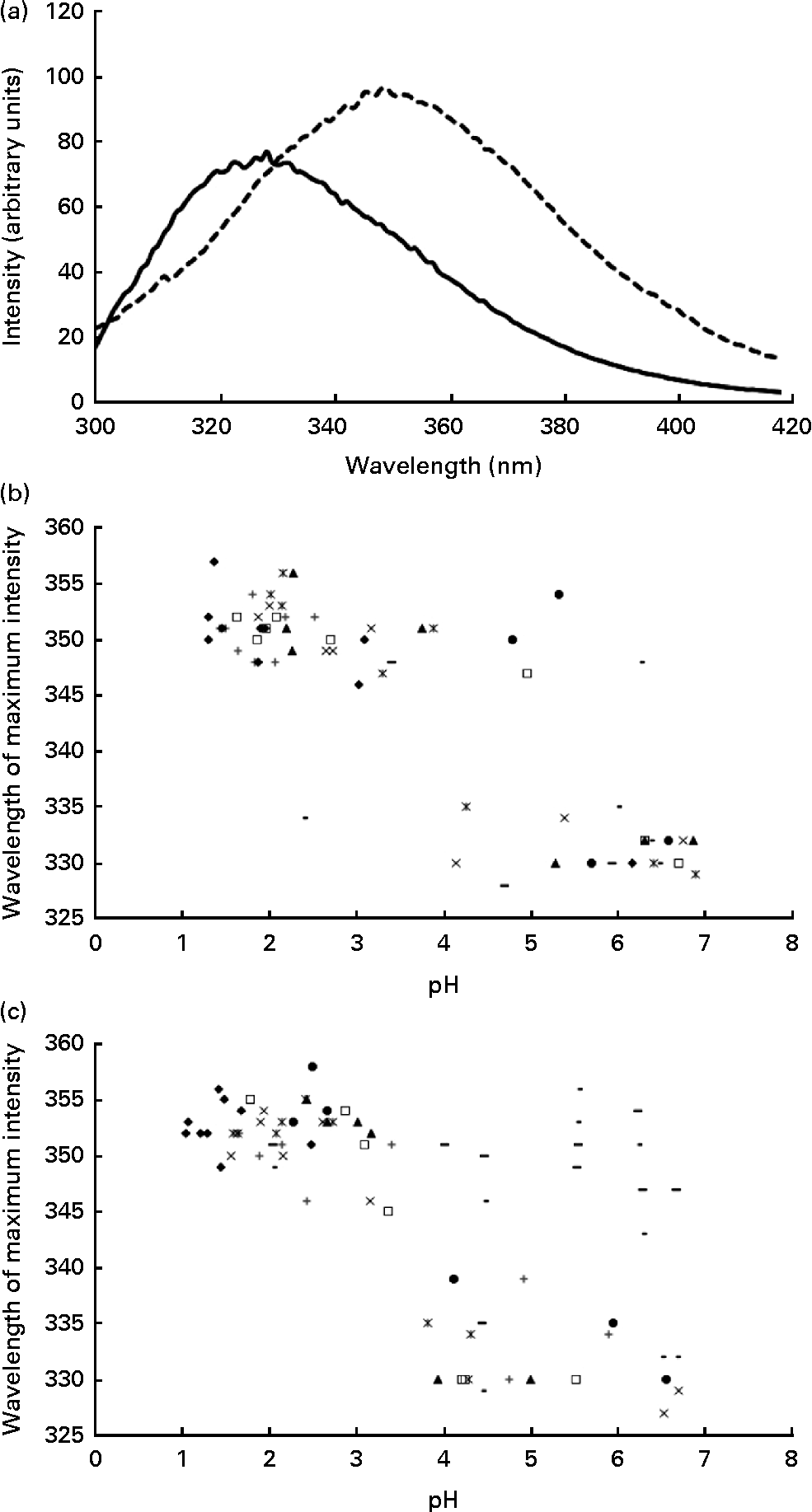
Fig. 2 (a) Typical intrinsic fluorescence spectra (71 mg/l of protein concentration, λex= 280 nm, measured at 25°C and at a scanning speed of 80 nm/min) of the α-lactalbumin (α-LA) drink before ingestion (![]() ) and the intragastric sample of the α-LA drink below its isoelectric point (
) and the intragastric sample of the α-LA drink below its isoelectric point (![]() ), at pH 3·9, with the shift in the wavelength at which the maximum intensity occurred being shown. All the spectra were measured in triplicate, and an average of the three measurements is shown. Plots of the wavelength (in nm) at which the maximum intensity was recorded as a function of pH (n 8) for (b) the α-LA drink and (c) the α-LA with oleic acid drink are shown. Different symbols are representative of different individuals.
), at pH 3·9, with the shift in the wavelength at which the maximum intensity occurred being shown. All the spectra were measured in triplicate, and an average of the three measurements is shown. Plots of the wavelength (in nm) at which the maximum intensity was recorded as a function of pH (n 8) for (b) the α-LA drink and (c) the α-LA with oleic acid drink are shown. Different symbols are representative of different individuals.
On plotting the wavelength at which the maximum fluorescence intensity was observed for the protein as a function of pH, two distinct regions with no statistically significant differences between the α-LA (Fig. 2(b)) or the α-LA–OA (Fig. 2(c)) drinks were observed. There are two distinct regions in the graph, folded and partially unfolded, as characterised by the clusters of points within the groups. Comparison of the plots confirmed that the structural change occurs irrespective of the presence of OA.
Fourier transform IR spectroscopy allows to evaluate the secondary structure of proteins through the measurement of vibrational changes within the amide I region of the spectra, 1600–1720 per cm, allowing differentiation between α-helices, β-sheets and random coil conformations. α-LA is mainly composed of α-helices, with only a small amount of β-sheets being present. Typically, there is a vibration at 1652 per cm corresponding to the α-helical structure. In the present study, this was observed in the original α-LA and α-LA–OA drinks before ingestion and in the samples above the isoelectric point of the protein. Molten globule-like structures have vibrational shifts at 1645 per cm, which is indicative of a random coil structure, which was observed in the samples below the isoelectric point, confirming that there was an alteration in the secondary structure of the protein along with changes in the tertiary structure (Fig. 3)( Reference Troullier, Reinstadler and Dupont 28 ).

Fig. 3 Typical Fourier transform IR spectra (measured at 25°C with an average of 180 scans with a resolution of 4 per cm) of the α-lactalbumin with oleic acid (α-LA–OA) drink before ingestion (![]() ) and the intragastric sample of the α-LA–OA drink below its isoelectric point (
) and the intragastric sample of the α-LA–OA drink below its isoelectric point (![]() ), at pH 3·9, after 8·5 min of gastric digestion. All the spectra were measured in triplicate, and an average of the three measurements is shown.
), at pH 3·9, after 8·5 min of gastric digestion. All the spectra were measured in triplicate, and an average of the three measurements is shown.
Polypeptide chain composition of α-lactalbumin during gastric transit
Molecular-weight calibration through size-exclusion HPLC allowed the identification and quantification of the α-LA peak at 23 min (Fig. 4(a)). Undigested monomers of α-LA were present in the stomach for each sample up to and during gastric emptying (Fig. 4(a)). However, the amounts of native protein remained low – approximately 5 % of the remaining protein content. The rate of proteolysis increased during gastric digestion, resulting in the production of a lower-molecular-weight substance. The pH rapidly decreased within 9 min of ingestion (Fig. 4(b)), reaching the optimum pH range for pepsin activity (pH 0·8–4)( Reference Minekus, Alminger and Alvito 29 ).

Fig. 4 (a) Typical size-exclusion HPLC chromatographs obtained for an α-lactalbumin drink as it was digested in the stomach of healthy adults (0–24 min) with the retention times of the molecular-weight standards. (b) Integrated peak area percentages as a function of digestion time showing peptides greater than 10 kDa (![]() ), 5–10 kDa (
), 5–10 kDa (![]() ), 1–5 kDa (
), 1–5 kDa (![]() ), 500 Da–1 kDa (
), 500 Da–1 kDa (![]() ) and less than 500 Da (
) and less than 500 Da (![]() ). Vertical error bars are representative of the standard deviations of HPLC measurements for each subject over a range of time points.
). Vertical error bars are representative of the standard deviations of HPLC measurements for each subject over a range of time points.
Similar results were obtained during SDS–PAGE (Fig. 5). Large peptides were easily visible in the initial gastric aspirate, with increasing amounts of peptides appearing as digestion progressed, coinciding with the decrease in the band intensity of α-LA. Residual β-LG remained intact even after 30 min of ingestion.
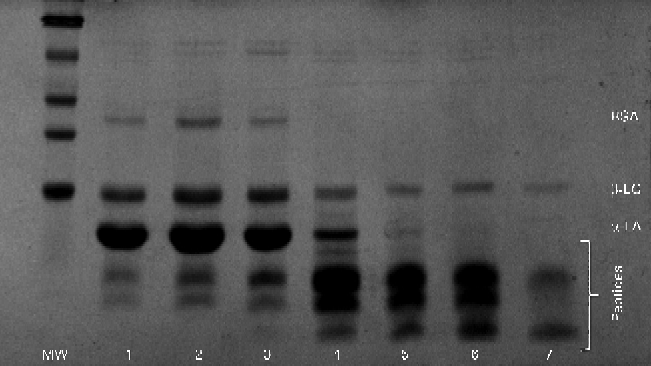
Fig. 5 Typical SDS–PAGE gel for the α-lactalbumin (α-LA) with oleic acid drink as digestion progressed. Lanes 1–7 (3, 6, 9, 12, 15, 18 and 21 min, respectively) showing bands of native α-LA, β-lactoglobulin (β-LG), bovine serum albumin (BSA) and peptides. MW, molecular weight markers.
In vivo imaging
In vivo gastric imaging using capsule endoscopy was performed in one subject. The resulting images are shown in Fig. 6. A pictorial overview of the entire gastric phase of the endoscopy recording is shown in Fig. 6(b). In Fig. 6(c), the nasogastric pH probe can be seen clearly within the stomach. The camera became lodged in the rugae of the stomach at the start of the fundus, allowing real-time imaging of the gastric contents before the camera entered the duodenum. The gastric pH decreased upon acid secretion within the stomach. Acid secretion could be visualised through the formation of white streaks within the gastric contents and also along the lining of the stomach wall (Fig. 6(d)). These white streaks were a result of the isoelectric precipitation of the protein, whereby apo-α-LA precipitates out of the solution forming a white mixture. Real-time measurement of the pH of the gastric contents was correlated with the visual appearance of the protein solution. The pyloric sphincter is the region where the highest shear within the stomach occurs and thus where the most mixing occurs. Based on the images (Fig. 6(e)) and videos, it was found that the shear within the stomach was not adequate for the formation of a homogeneous mixture similar to that formed before digestion. Gastric emptying time could also be determined with in vivo imaging for two consecutive protein drinks. Based on the images and videos, it was found that the drinks were emptied from the stomach after approximately 30 min of gastric digestion. Gastric emptying occurs by 30 min via the pyloric sphincter opening, allowing the gastric contents to enter the small intestine. This is achieved through the opening of the pyloric sphincter, allowing the entire contents of the pylorus to enter the small intestine, and does not occur in a stepwise manner. In Fig. 6(f), gastric emptying is shown.
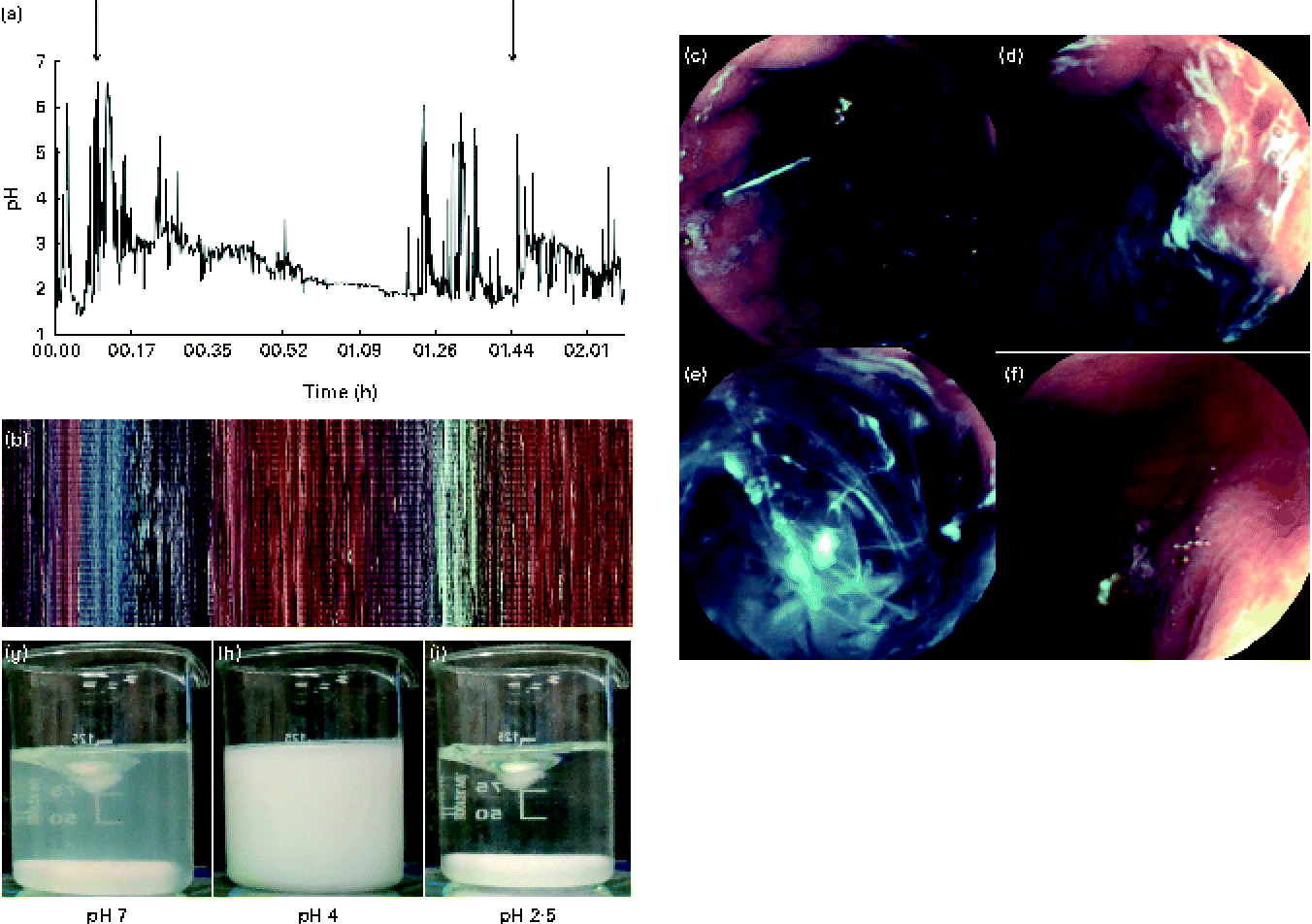
Fig. 6 (a) Intragastric pH as measured using a nasogastric pH probe (n 1), with the increase in the initial pH being shown upon the secretion of gastric acid with the two ingestion times highlighted. Capsule endoscopy images of 25 g/l of α-lactalbumin (α-LA) with 50 g/l of sucrose within the stomach. (b) Pixelated image of the entire video clip as a function of time. Selected images at different time points during gastrointestinal digestion: (c) before the ingestion of drink 1, visualisation of the pH probe from within the stomach; (d) 5 min after the ingestion of drink 1 (α-LA) at pH 5·2; (e) 8 min after the ingestion of the α-LA drink (pH 4·3); (f) after the occurrence of gastric emptying (camera located within the small intestine after passing the pyloric sphincter). For comparison, examples where homogeneous mixing occurred during equivalent in vitro digestion at (g) pH 7, (h) pH 4 and (i) pH 2·5 are shown. Images (c), (d), (e) and (f) are marked in (b) as the location/time at which the images were captured. Video files are available in the online supplementary material.
A real-time video was recorded, monitoring the fate of α-LA during gastric digestion. The subjects ingested two drinks at time 0 and 97 min. The camera passed the pyloric sphincter at approximately 150 min, where the typical yellow colouration of bile was observed. Slow but complete dissolution and digestion were observed within the jejunum. Recording was stopped after 200 min. Using the video function of the software MiroView, it was possible to observe peristalsis occurring within the stomach. Peristalsis occurred in the form of waves of stomach muscle contractions of the pyloric sphincter and was clearly visible when the stomach was empty around the pyloric sphincter.
Test beverages also underwent in vitro digestion and mixed at 150 rpm using a magnetic stirrer. It is generally recommended to mix samples sufficiently during digestion( Reference Dupont, Bordoni and Brodkorb 30 ). The turbidity of the samples changed as a function of pH. The initially clear samples became cloudy with the formation of a white precipitate, resulting in the formation of a homogeneous white solution, before the formation of a clear solution finally when the pH of the mixture reached 3·5 (Fig. 6(g)–(i)). This was in contrast to that observed in vivo where a homogeneous mixture was not obtained.
Selected video clips of the gastrointestinal digestion of α-LA are available in the online supplementary material.
Cytotoxicity measurements
The gastric aspirate samples were tested for their cytotoxic activity against human lymphoma U937 cells. From the in vitro digestion assay, it was found that protein folding and refolding occurred to allow the formation of a complex. Samples containing native, undigested protein that was deemed to have undergone unfolding by monitoring the intrinsic fluorescence spectra were pH-adjusted for cytotoxicity assays against the cancer cells and their activity was compared with that of BAMLET produced through chromatography( Reference Sullivan, Mok and Brodkorb 20 ). The effect of pepstatin, a potent pepsin inhibitor, on U937 cells was assessed and no detrimental effect was observed on the cells at the tested concentrations. BAMLET killed the cancer cells; however, the gastric aspirate samples did not affect the viability of the cells. Due to the overlapping of bands for sucrose and OA in their Fourier transform IR vibrations and the same chemical shift in NMR, ANS (8-anilino-1-naphthalenesulfonic acid) fluorescence analysis was used to estimate the presence of fatty acids. The data indicated that a fatty acid was present in the samples; however, it was not possible to determine whether the OA present in the sample was bound to the protein and was therefore cytotoxic.
To determine whether the sucrose present impedes the cytotoxicity of the gastric aspirates, a cell viability assay was performed against U2OS, a human osteosarcoma cell line that is sensitive to HAMLET and BAMLET( Reference Xie, Min and Harte 31 ), using BAMLET with and without added sucrose. No difference was observed in the activity of BAMLET after the addition of sucrose, indicating that the presence of sucrose in the samples did not affect the cytotoxicity of the samples and confirming that the complex was not formed under the in vivo conditions of the assay.
Discussion
There is no previous publication in the peer-reviewed literature that uses capsule endoscopy in conjunction with the consumption of food – typically, capsule endoscopies are used in the diagnosis of illnesses in the small intestine of people who present with abdominal pain or bleeding that cannot be diagnosed using either traditional endoscopy or colonoscopy techniques( Reference Kurien, Evans and Aziz 32 ). Capsule endoscopy has been found to allow a more detailed investigation of the gastric contents and provide important information on mixing levels, peristalsis and gastric emptying rates in vivo.
Due to rapid gastric emptying (determined to occur within approximately 30 min), it was not possible to fully determine the percentage of native protein entering the pH-neutral conditions of the duodenum. Although final gastric emptying occurs in the form of ‘dumping’ (when the pyloric sphincter opens allowing all the gastric content present within the antrum to enter the duodenum), given the liquid nature of the drinks, it is possible that the liquid containing the intact protein enters the duodenum and thus more amounts of native protein may remain.
α-LA is the most abundant protein and OA is the most abundant fatty acid in human milk( Reference Jensen 2 ); thus, the potential for association with OA and the subsequent production of a HAMLET-like complex in vivo within the gastrointestinal tract of infants are of clinical interest to researchers. Under simulated infant gastric digestion, a BAMLET-like complex can form( Reference Sullivan, Mok and Brodkorb 20 ). However, the present study demonstrates that the rate of digestion in the stomach of healthy adults is higher than that observed in both in vitro ( Reference Rudloff and Lönnerdal 7 ) and in vivo ( Reference Agunod, Yamaguch and Lopez 33 ) digestion studies in infants. Different pH profiles also exist within the gastric environment of infants( Reference Mitchell, McClure and Tubman 24 , Reference Omari and Davidson 34 ) when compared with those within the gastric environment of adults( Reference Troost, Steijns and Saris 35 ). Throughout the in vivo monitoring of gastric pH after the ingestion of the test drinks, a pH gradient was found to exist, whereby the pH gradually decreased as a function of time; however, it has been reported that the pH in adults decreases over a shorter time due to higher rates of acid secretion( Reference Troost, Steijns and Saris 35 ).
Although pepsin activity levels are similar in infants and adults, the optimum pH for pepsin activity is 1·8, with no activity occurring above pH 4( Reference Agunod, Yamaguch and Lopez 33 ). Both in the present study and in previous studies( Reference Troost, Steijns and Saris 35 ), the pH of the stomach was found to decrease at a faster rate in adults than in infants, with a higher rate of proteolysis occurring subsequently over a 30 min period.
A recent study highlights both the prevention and treatment of colon cancer after the ingestion of HAMLET in mice( Reference Puthia, Storm and Nadeem 19 ). Although this study is of clinical significance, conclusions cannot be drawn that should HAMLET be ingested, it would result in an activity against colon cancer cells in vivo in humans, in part due to the differences in the anatomy of mice and humans. Second, HAMLET was pre-formed and fed to mice as a therapeutic agent. Although the presence of OA does reduce the digestibility of α-LA( Reference Casbarra, Birolo and Infusini 36 ), it has been demonstrated in the present study that the harsh conditions within the stomach are not conducive to α-LA polypeptide chain stability and the protein would not survive transit to its active site within the intestines.
Antimicrobial peptides and peptides with opioid activity have been derived from α-LA by hydrolysis. It is still unclear whether peptides with any biological activity can be derived from the in vivo digestion of α-LA. Currently, few studies exist on the in vivo digestion of food proteins. However, studies on the digestion of lactoferrin, the Fe-binding and transport protein, exist( Reference Troost, Steijns and Saris 35 , Reference Troost, Saris and Brummer 37 ). Lactoferrin has been found to have pH curve and gastric emptying rate similar to those of α-LA. However, one striking difference is with regard to the proportion of native protein remaining after gastric digestion: in the case of lactoferrin, depending on the molecular state of the protein, between 60 and 80 % of native protein remains intact, which is significantly higher than that observed after the gastric digestion of α-LA in the present study( Reference Troost, Steijns and Saris 35 , Reference Troost, Saris and Brummer 37 ). A recent study on the in vivo digestion of proteins has reported the formation of bioactive peptides within the jejunum of healthy adult volunteers. In this study, triple-port nasogastric tubes were used, which allowed simultaneous sampling from the stomach, duodenum and jejunum( Reference Boutrou, Gaudichon and Dupont 38 ); however, as peptides were formed from casein in the jejunum, it is difficult to make comparisons with the results of the present study.
The standardisation of digestion within the pharmaceutical industry has been proposed; however, to date, no such standardisation has been found to exist for food digestion( Reference Hur, Lim and Decker 39 ). COST Action FA 1005 Infogest( Reference Dupont, Bordoni and Brodkorb 30 ) is a group recently set up with the aim of standardising food digestion across the discipline. One initial proposal of the action was the standardisation of mixing rates in in vitro digestion and 150 rpm was proposed as the mixing rate. This mixing speed was employed in a previous study while mixing the in vitro digestion mixture, and on visually comparing this mixture with the in vivo digestion mixture, it was found that the mixing within the stomach does not produce a homogeneous mixture similar to that produced in vitro. One outcome of the present study indicates that the rate of mixing within the stomach is overestimated in many in vitro food digestion studies; for example, the rates of 80 rpm( Reference Sullivan, Mok and Brodkorb 20 ), 95 rpm( Reference Granado-Lorencio, Herrero-Barbudo and Acien-Fernandez 40 ) and 1000 rpm( Reference Golding, Wooster and Day 41 ) have been employed previously.
In conclusion, despite (1) the occurrence of protein unfolding, (2) the presence of OA and (3) the presence of native protein, the complex produced in vivo was not toxic to cancer cells. However, detailed studies of protein unfolding have been completed. There is also a detailed mass spectrometry study on peptide profiling during the in vivo gastric digestion of α-LA ongoing. Capsule endoscopy technology allowed in situ visualisation of the mixture through the capture of images and videos (online supplementary material) in vivo and revealed that mixing was not adequate for the formation of a complex.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514001196
Acknowledgements
The authors thank Ian O'Loughlin, Solène LeMaux, Noelle O'Riordan and Zhanmei Zhang for their assistance in the processing of gastric aspirates.
The present study was funded under the Food Institute Management Research project no. 08RDTMFRC650. L. M. S. was funded under the Teagasc Walsh Fellowship scheme. The present study was carried out in conjunction with COST Action FA 1005 Infogest. L. M. S. was sponsored with a travel grant by this action. Mirocam® kindly sponsored the capsule endoscopy camera.
The authors' contributions are as follows: L. M. S., J. J. K., L. B. and A. B. were involved in the acquisition of samples; L. M. S., J. J. K., K. H. M. and A. B. interpreted the results. All authors had a role in the conception and design of the study, had responsibilities in drafting the article, and gave final approval before the submission of the article for publication.
None of the authors has any conflicts of interest to declare.









