The prevalence of dementia increases sharply with age(Reference Ott, Stolk and van Harskamp1) and a rise in the number of individuals with dementia is expected worldwide(Reference Ferri, Prince and Brayne2). This will impose a major burden on society in general and the health-care system in particular.
Decline in cognitive function is one of the major symptoms of dementia. A preclinical phase of reduced cognitive function precedes the appearance of diagnosed dementia by at least 10 years(Reference Geschwind, Robidoux and Alarcon3). Based on a continuous scale from normal ageing to dementia, individuals with a worse cognitive function at baseline and/or a more rapid cognitive decline over time have a higher risk of development of dementia(Reference Petersen, Doody and Kurz4).
The pathophysiology of dementia is complex and still largely unclear, but vascular factors, inflammation and oxidative processes appear to be involved. There is no treatment known that can stop or cure the progress of dementia. Age and genetics are risk factors that are associated with the development of dementia, but cannot be controlled. Therefore, for an individual who is already genetically predisposed, it is important that modifiable risk factors of cognitive decline are discovered which could lead to a more favourable risk profile.
An important modifiable determinant of human mental performance is nutrition(Reference Bourre5). In recent articles, it has been shown that adherence to a Mediterranean-type diet was associated with slower cognitive decline(Reference Feart, Samieri and Rondeau6) and a reduced risk for Alzheimer's disease(Reference Scarmeas, Luchsinger and Schupf7) in elderly individuals. Fruit and vegetables are important components of a Mediterranean diet and contain a variety of substances (for example, vitamins, antioxidants, minerals and phytochemicals) that could be beneficial for cognitive function, either alone or in combination(Reference Padayatty and Levine8).
Only a few human studies, in elderly individuals only, have been published on fruit and vegetable consumption in relation to cognitive function(Reference Kang, Ascherio and Grodstein9). In two studies, higher vegetable intake, but not fruit intake, was associated with less cognitive decline(Reference Kang, Ascherio and Grodstein9, Reference Morris, Evans and Tangney10). In two other studies, the consumption of fruits and vegetables (juices) has been associated with decreased risk of dementia and Alzheimer's disease(Reference Dai, Borenstein and Wu11, Reference Barberger-Gateau, Raffaitin and Letenneur12).
In order to postpone or prevent cognitive decline and eventually dementia at old age, intervention is required earlier in life during, or before, middle age(Reference Luchsinger, Tang and Shea13, Reference Staehelin14). However, the relationship between fruit and vegetable consumption and cognitive change has, as far as we know, never been investigated in middle-aged individuals. In the present observational study we analysed the effects of (subgroups of) fruit and vegetable consumption on cognitive function and cognitive decline in a middle-aged population.
Subjects and methods
Population
The Doetinchem Cohort Study(Reference Verschuren, Blokstra and Picavet15) is an ongoing prospective study that included a general population sample of 7769 men and women aged 20–59 years during the first examination (1987–91). Second (1993–7), third (1998–2002) and fourth (2003–7) examination rounds have been completed. The present study was conducted according to the guidelines laid down in the Helsinki Declaration and all procedures involving human subjects were approved by the external Medical Ethics Committee of The Netherlands Organization for Applied Scientific Research (Nederlandse Organisatie voor Toegepast Natuurwetenschappelijk Onderzoek; TNO). Written informed consent was obtained from all subjects. Details on the Doetinchem Cohort Study are described elsewhere(Reference Verschuren, Blokstra and Picavet15).
From 1995 onwards, cognitive testing for Doetinchem Cohort Study participants aged 45 years and older was introduced. In the years 1995–7, a random sample of one-third of participants aged 45 years and older was enrolled in the study on cognitive functioning, since a random sample of two-thirds was enrolled in an additional dietary study. Those participating in the dietary study during 1995–7 had their baseline measurement of cognitive testing during 2000–2. Between 1995 and 2002, 3350 respondents aged 43–70 years, 96 % of all respondents invited, participated in cognitive testing for the first time. Then, 5 years later, 2690 of them (80 %) participated in cognitive testing again. Participants who reported having experienced a stroke (n 77) were excluded from the analyses, since stroke has direct effects on brain functions and cognition. A total of 2613 participants (1288 men and 1325 women) who participated in two cognitive measurements were included in the present study.
Cognitive tests
The neuropsychological test battery included four tests: the 15 Words Verbal Learning Test (VLT), the Stroop Colour–Word Test (SCWT), the Word Fluency Test and the Letter Digit Substitution Test (LDST). It measures global cognitive function (all cognitive tests combined) and specific cognitive domains, i.e. memory (VLT) function, information processing speed (SCWT and LDST) and cognitive flexibility (i.e. higher-order information processing; SCWT). In the 15 Words VLT(Reference Van der Elst, van Boxtel and van Breukelen16) fifteen monosyllabic words printed on paper are displayed, one by one, in three subsequent trials, with a free recall procedure immediately following each presentation (immediate recall). After a delay of about 15 min, there is an additional free recall trial (delayed recall). The VLTtotal is calculated by summation of the words recalled correctly on the three immediate recalls. The VLTmaximal represents the highest score on one of the three immediate recalls. In the SCWT(Reference Van der Elst, Van Boxtel and Van Breukelen17) three skills are tested: (1) to read forty written colour names; (2) to name the colour of forty coloured patches; and (3) to name the colour of the ink in which forty incongruously named colour words are printed (so, for example, the word ‘blue’ is printed in red). In the Fluency Test(Reference Van der Elst, Van Boxtel and Van Breukelen18) the participant is asked to name as many animals as possible within 1 min. In the LDST(Reference Van der Elst, van Boxtel and van Breukelen19) nine letters of the alphabet are given a unique digit code (1 to 9) in a key displayed on the same sheet of paper. The participant is asked to fill in the correct digits corresponding to the letters, as fast as possible. The tests are sensitive to age, also the middle-age range. Cognitive tests were carried out by trained investigators and took about 20 min to complete. The tests have also been used in other large-scale studies on cognitive function(Reference Van Boxtel, Buntinx and Houx20–Reference Durga, van Boxtel and Schouten23).
Distributions of scores of the SCWT were normalised. For each cognitive test, a standardised z-score was computed for each participant at baseline and at follow-up, based on the means and standard deviations of the test scores at baseline. In this way we were able to examine changes over time. Standardised scores of the SCWT were inverted, so that higher scores represent better cognition. All (inverted) standardised scores were then combined to form scores for specific cognitive domains, i.e. scores for memory function, information processing speed and cognitive flexibility, and a score for global cognitive function, based on clustering used in former studies(Reference Van Boxtel, Buntinx and Houx20, Reference Durga, van Boxtel and Schouten23, Reference Kalmijn, van Boxtel and Ocké24):
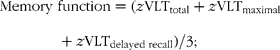


To calculate change in cognitive function, domain scores at baseline were subtracted from domain scores at follow-up.
Fruit and vegetable intake
A validated self-administered semi-quantitative FFQ was used to assess the habitual consumption of 178 food items during the previous year. This questionnaire was developed for the European Prospective Investigation into Cancer and Nutrition (EPIC) for which vegetable and fruit consumption in relation to cancer was one of the main research questions(Reference Ocké, Bueno-de-Mesquita and Goddijn25). For fruits and vegetables, consumption frequencies were assessed. In addition, portion sizes of vegetables were assessed by use of photographs. Spearman correlation coefficients for relative validity for vegetable intake were 0·38 and 0·31, and for fruit 0·68 and 0·56 for men and women, respectively(Reference Ocké, Bueno-de-Mesquita and Goddijn25). Subgroups of fruits and vegetables were classified (Fig. 1) based on the classification in EPIC(Reference Slimani, Ferrari and Ocké26).

Fig. 1 Overview of the classification of fruits, vegetables, legumes and juices.
The total intake of four main groups (i.e. fruits, vegetables, legumes and juices) was calculated. Fruits were subdivided in fruits and nuts (including nut spread). Vegetables were subdivided into leafy vegetables (chicory, endive, lettuce and spinach, except cabbage), fruiting vegetables (cucumber, sweet pepper and tomato), root vegetables (carrots and red beets), cabbages, mushrooms and allium (garlic, onion and leek). Legumes consist of green beans, green peas and other legumes. Apart from (solid) fruits, vegetables and legumes, the intake of vegetable and fruit juices was computed. In addition to all former main and subgroups, fruits and vegetables were also studied combined (‘total fruits and vegetables’). In order to obtain a measure for long-term (habitual) fruit and vegetable intake, the reported intakes at baseline and follow-up were averaged. For computing quintiles of intake cohort-wide cut-points were used. Subclasses of fruits and vegetables were studied separately in order to study whether specific subgroups of fruits and vegetables (with different important biochemical components) are associated with cognitive function or cognitive decline.
Other measures
During a physical examination at the research centre, height, weight, waist circumference and blood pressure were measured, and non-fasting blood samples were obtained(Reference Verschuren, Blokstra and Picavet15), to determine serum total and HDL-cholesterol levels.
Information on demographic characteristics (for example, age, education and marital status), lifestyle factors (for example, smoking and physical activity), medical history of chronic diseases and medication use was collected using standardised questionnaires. Educational level was assessed as the highest level reached and classified into five categories. Smoking status was defined as being a non-smoker or current smoker (of cigarettes) at baseline and the number of lifelong pack years was calculated for ever-smokers. One pack year corresponds to smoking twenty cigarettes per d for 1 year (or for example, smoking one cigarette per d for 20 years). Physical activity level was assessed by the use of the validated EPIC questionnaire on physical activity(Reference Pols, Peeters and Ocké27) and classified into four categories: inactive, moderately inactive, moderately active, and active(Reference Wareham, Jakes and Rennie28).
Based on the FFQ, energy intakes from fats, alcohol and other sources (protein and carbohydrates) were computed based on nutritional values in the Dutch Food Composition Database(29).
Depressive symptoms were questioned in the Dutch version(Reference Van der Zee and Sanderman30) of the MOS 36-Item Short-Form Health Survey (SF-36)(Reference Ware and Sherbourne31). The scales ‘mental health’ and ‘vitality’ denote symptoms of depression. Scores on both scales range from 0–100, in which higher scores represent better (mental) health.
Statistical analyses
In multivariate linear regression analyses, (subgroups of) fruit and vegetable consumption were associated with cognitive function at baseline and cognitive decline over follow-up. Cognitive function was analysed as a continuous outcome measure.
Two models were tested. First, we tested a basic model, adjusting for age, sex, level of education, total energy intake (separate for energy from fat, energy from alcohol and energy from other sources), intake of other fruits, vegetables, legumes and juices, and the baseline level of cognitive function (when studying cognitive change). Second, to find out whether associations could be clarified by other factors, we tested the basic model, additionally adjusted for physical activity, smoking (status and number of pack years), systolic blood pressure, use of blood pressure-lowering medication, serum HDL-cholesterol, waist circumference, coffee consumption, vitality and mental health.
Other potential confounders (marital status, oestrogen use, hypertension, heart disease, diabetes, BMI and total cholesterol) were not associated with total fruit and vegetable intake (P>0·20) after adjustment for age, sex and level of education. Therefore these variables were not taken along in the analyses.
P for trend was calculated using linear regression analyses on median values of intake in the quintiles(Reference Willett32) in relation to baseline or change in cognitive function, with adjustments as described in the models above. Standardised β coefficients were presented, which can be read as the amount of difference or change in cognitive function that is associated with 1 sd difference in the amount of fruit or vegetable consumption.
To test whether under- or over-reporting dietary intake affected the results, we also performed our analyses excluding data from participants with extreme (lowest and highest 10 %) sex-specific total energy intake. Since this did not essentially affect the results, results of all participants are reported. In addition, an interaction effect of fruit and vegetable consumption with age was tested but turned out to be not statistically significant.
In order to report effect size at baseline in age-equivalents, the difference in cognitive function between the highest and the lowest quintile of intake was divided by the parameter estimate for age (in years) in the same regression model. For relative rate of change in cognitive function over follow-up, the rate of change in cognitive function in the lowest quintile was divided by that in the highest quintile.
All analyses were performed using SAS statistical software (version 9.2; SAS Institute Inc., Cary, NC, USA).
Results
Individuals who reported a higher intake of fruits and vegetables were on average older, more often female or highly educated, and less often smokers, heavy alcohol drinkers, or inactive and had higher serum HDL-cholesterol levels compared with individuals who reported a lower intake of fruit and vegetables (Table 1). The total intake of solid fruits and vegetables ranged from 50 to 1131 g/d.
Table 1 Characteristics of the study population by quintiles of total (solid) fruit and vegetable intake
(Mean values and standard deviations or percentages)

*Significant (P < 0·05) trend over quintiles in regression analyses (for continuous variables) or χ2 analyses (for categorical variables).
† At least intermediate vocational education.
‡ Standardised cognitive scores are multiplied by 10.
§ Among ever smokers; one pack year is the equivalent of one full year of smoking twenty cigarettes per d.
∥ Three or more glasses per d for men; two or more glasses per d for women.
¶ Inactive was defined as the lowest two out of four categories in the physical activity index(Reference Ocké, Bueno-de-Mesquita and Goddijn25).
Average change in cognitive function domains over the 5-year follow-up was − 0·14 (sd 0·77) for memory, − 0·13 (sd 0·45) for information processing speed, − 0·08 (sd 0·66) for cognitive flexibility and − 0·10 (sd 0·42) for global cognitive function.
Main groups of fruits and vegetables
Higher reported intake of vegetables was associated with lower information processing speed (P-trend = 0·02) and worse cognitive flexibility (P-trend = 0·03) at baseline, and with smaller decline in information processing speed (P-trend < 0·01) and global cognitive functioning (P-trend = 0·02) at follow-up. At baseline, the difference in cognitive function between the lowest and the highest quintile of total vegetable consumption was equivalent to about 3·5 years' difference in age. At follow-up, individuals in the lowest quintile of total vegetable consumption showed about two times the cognitive decline of the individuals in the highest quintile (see also Fig. 2(e)). Reported intakes of total fruits and vegetables, fruits, legumes and juices were not associated with scores at any domain of cognitive functioning at baseline, nor with change in cognitive function over follow-up (Table 2).
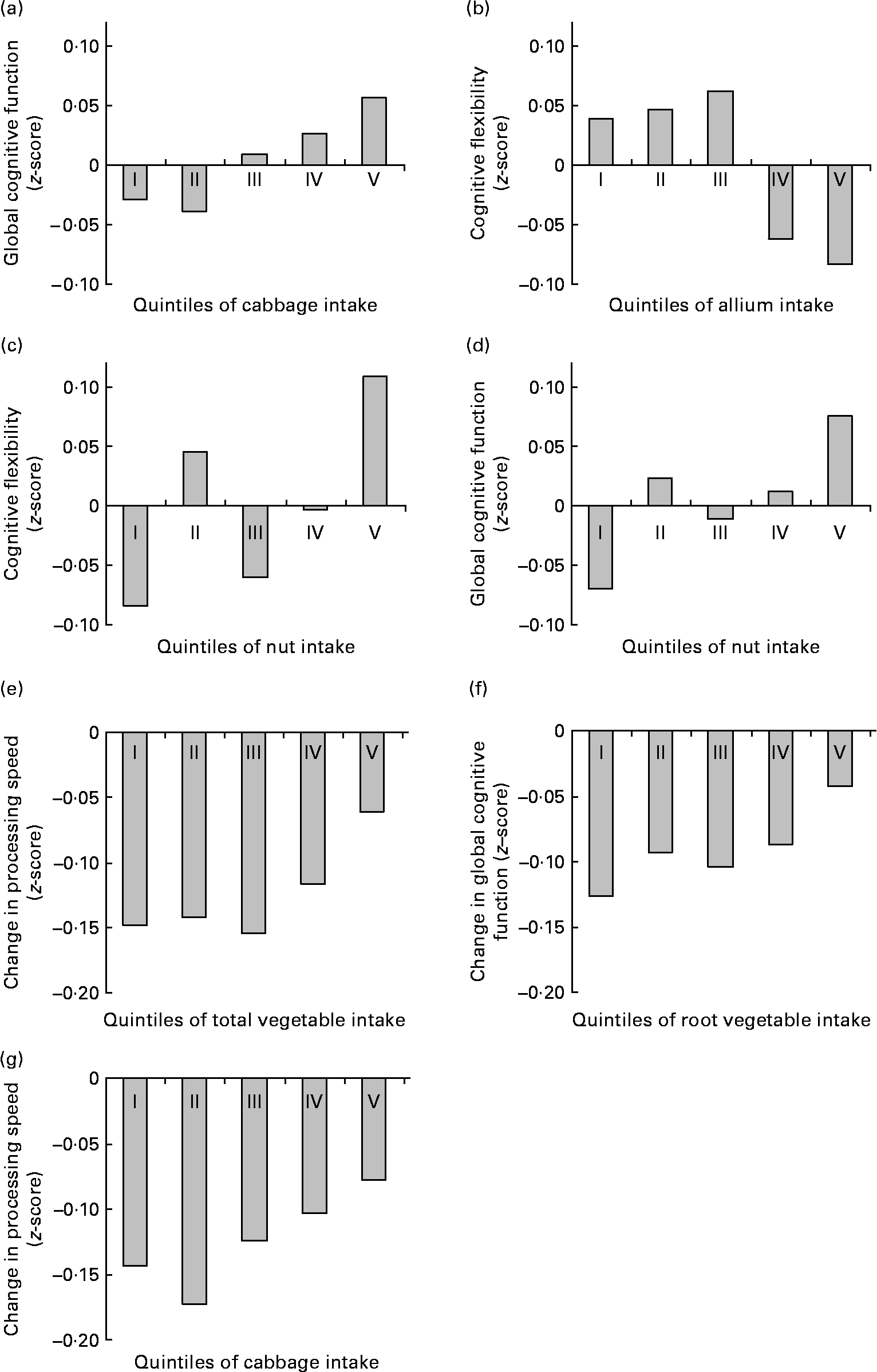
Fig. 2 Significant (P-trend < 0·01) associations between habitual fruit and vegetable consumption and baseline cognitive function ((a)–(d)) and change in cognitive function at follow-up ((e)–(g)) by quintile of intake. Values are z-scores.
Table 2 Associations between habitual vegetable, fruit, legume and juice intake and (change in) cognitive function: The Doetinchem Cohort Study, 1995–2007
(Standardised β coefficients and P for trend, calculated over median intakes in the quintiles)

NS, P ≥ 0·05.
* P < 0·05, ** P < 0·01.
† In model 1, associations are adjusted for age, sex, level of education, total energy intake (separate for energy from fat, energy from alcohol and energy from other sources), intake of other fruits, vegetables, legumes, and juices, and the baseline level of cognitive function (in the analyses on cognitive change).
‡ In model 2, associations in model 1 are additionally adjusted for serum HDL-cholesterol, systolic blood pressure, usage of blood pressure-lowering medication, waist circumference, coffee consumption, smoking, physical activity, vitality and mental health.
Subgroups of fruits
Higher intake of nuts was associated with better cognitive function at baseline in all domains (all P-trend ≤ 0·01; Table 3). The difference in cognitive function between the lowest and the highest quintile of nut consumption was equivalent to 5–8 years' difference in age.
Table 3 Associations between subgroups of vegetable and fruit intake and (change in) cognitive function: The Doetinchem Cohort Study, 1995–2007†
(Standardised β coefficients and P-for-trend, calculated over median intakes in the quintiles)
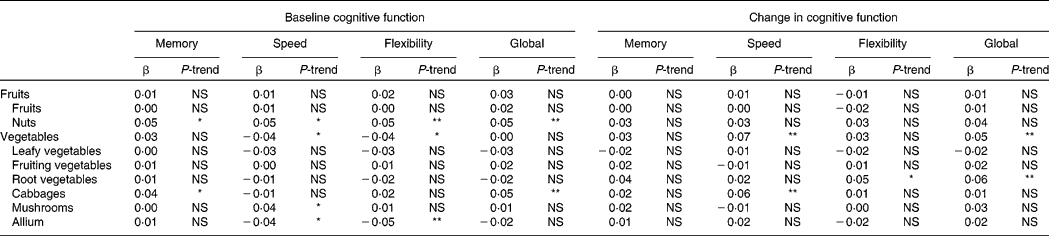
NS, P ≥ 0·05.
* P < 0·05, ** P < 0·01.
† Associations are adjusted for age, sex, level of education, total energy intake (separate for energy from fat, energy from alcohol and energy from other sources), intake of other fruits, vegetables, legumes, and juices, serum HDL-cholesterol, systolic blood pressure, usage of blood pressure-lowering medication, waist circumference, coffee consumption, smoking, physical activity, vitality, mental health and the baseline level of cognitive function (in the analyses on cognitive change).
Subgroups of vegetables
Higher intake of cabbage was associated with better memory function (P-trend = 0·02) and better global cognitive function (P-trend < 0·01) at baseline, and with smaller decline in information processing speed at follow-up (P-trend < 0·01). At baseline, the difference in cognitive function between the lowest and the highest quintile of cabbage consumption was equivalent to about 4 years' difference in age. At follow-up, individuals in the lowest quintiles of cabbage consumption showed about two times the decline in information processing speed of the individuals in the highest quintile (see also Fig. 2(g)).
Higher intake of mushrooms was associated with higher information processing speed at baseline (P-trend = 0·02; the difference between the lowest and the highest quintile was equivalent to about 2 years' difference in age). However, higher intake of allium was associated with lower information processing speed and worse cognitive flexibility at baseline (both P-trend ≤ 0·01; the difference between the lowest and the highest quintile was equivalent to about 3·5 years' difference in age).
Higher reported intake of root vegetables was associated with smaller declines in cognitive flexibility and global cognitive function at follow-up (both P-trend ≤ 0·01; Table 3). Individuals in the lowest quintile of root vegetable consumption showed over three times the cognitive decline of the individuals in the highest quintile (see also Fig. 2(f)).
To illustrate the associations between habitual fruit and vegetable intake and (change in) cognitive function, significant associations (P-trend < 0·01) are presented in Fig. 2.
Discussion
In the present study, self-reported habitual consumption of fruits, vegetables, legumes and juices was studied in relation to (change in) cognitive function in middle-aged individuals. After adjustment for several potential confounders, higher intake of total vegetables was associated with worse cognitive function at baseline. Higher intakes of nuts, cabbage and mushrooms were associated with better cognitive function at baseline, and higher intake of allium was associated with worse cognitive function. At follow-up, higher intake of total vegetables, cabbage or root vegetables was associated with smaller decline in cognitive function. Elevated habitual consumption of total vegetables and some specific fruits and vegetables (i.e. nuts, root vegetables and cabbage) may diminish age-related cognitive decline in middle-aged individuals.
The major strengths of the present study were the prospective design, relatively young participants and the use of a sensitive cognitive test battery without a ceiling effect. In addition, dietary habits were measured extensively in a large sample twice and lifestyle (for example, diet) was assessed at a stage of life before cognitive impairment is present, so lifestyle was not altered as a result of cognitive function. Although the participants were still relatively young and healthy when they entered the study, a clear decline in cognitive function, with a wide range, was detected. In addition, the Doetinchem Cohort Study has an extensive spectrum of variables assessed during the measurements. Therefore, we were able to adjust for a wide range of potential confounders.
One of the potential drawbacks of the present study was the drop-out of initial participants, although 80 % response was still high. At baseline, the groups of subjects who did not participate again at follow-up were 1·5 years older and scored on average 0·2 standardised points (equal to a difference in age of about 5–10 years) lower on all cognitive domain scores in comparison with the group that participated twice. In addition, average consumption of fruit and vegetables was higher in the group that participated twice (307 v. 283 g/d; P < 0·01). Although baseline characteristics were different, cross-sectional associations between fruit and vegetable intake and cognitive function were comparable between the groups that did and did not participate at follow-up.
Further, it can be argued that the observed associations were the result of multiple testing and form the 5 % of associations that would have been found based on statistical coincidence. We cannot explain the opposed directed associations of total vegetable consumption and information processing speed v. change in information processing speed. In our analyses, we adjusted for level of education, since level of education was positively associated with fruit and vegetable consumption and level of education was positively associated with cognitive function. However, in the case of total vegetables, this adjustment resulted in a negative association between total vegetable intake and cognitive function at baseline: within groups of individuals with the same level of education, individuals with a higher vegetable consumption tended to have a lower cognitive function. Furthermore, adjusting for educational level is standard in cognitive studies. By adjusting for educational level we made the present results more comparable with results in other studies.
Also the unfavourable effect of allium is not readily explained. All fruits and vegetables contain a variety of substances that could be beneficial for cognitive function(Reference Padayatty and Levine8). For instance, fruits and vegetables are high in antioxidants (for example, vitamins C and E, β-carotene, polyphenols) that can decrease the enhanced vulnerability to oxidative stress that occurs in ageing(Reference Joseph, Shukitt-Hale and Willis33). Therefore, we did not expect any negative associations between fruits and vegetables and (change in) cognitive function. For nuts and cabbage, we found overall beneficial (but not always significant) associations with baseline cognitive function and change in cognitive function.
Nuts are dense in a variety of nutrients and provide protein, fat (mostly unsaturated fatty acids), dietary fibre, vitamins (for example, folic acid, niacin, vitamin E, vitamin B6), minerals (for example, Cu, Mg, K, Zn), antioxidants, phyto-oestrogens and other phytochemicals(Reference Dreher, Maher and Kearney34). Nuts have been shown to lower the risk of CVD(Reference Kris-Etherton, Zhao and Binkoski35–Reference Hu, Stampfer and Manson39) and type 2 diabetes(Reference Jiang, Manson and Stampfer40), and they can lower serum total and LDL-cholesterol levels(Reference Kris-Etherton, Yu-Poth and Sabate41, Reference Mukuddem-Petersen, Oosthuizen and Jerling42). In the present study, nut consumption was associated with better cognitive function at baseline. In addition, nut consumption was also statistically significantly associated with less cognitive decline for the domains memory (P = 0·03) and global cognitive function (P = 0·02) in the basic model (data not shown). These associations weakened after additional adjustment for cardiovascular risk factors. Therefore, nut consumption may partly reduce cognitive decline through lowering the cardiovascular risk profile. In addition, a high midlife cholesterol level has been associated with higher risk of dementia(Reference Anstey, Lipnicki and Low43) and in aged rats, walnut supplementation has been shown to improve working memory(Reference Willis, Shukitt-Hale and Cheng44), possibly through the anti-inflammation effect of n-3 PUFA in walnuts.
In line with results of the studies by Kang et al. (Reference Kang, Ascherio and Grodstein9) and Morris et al. (Reference Morris, Evans and Tangney10) we observed a beneficial effect of cabbage consumption on cognitive decline. Cabbage contains a large number of bioactive compounds such as glucosinolates, which previously have been associated with reduced cancer risk(Reference Higdon, Delage and Williams45). In the studies by Kang et al. (Reference Kang, Ascherio and Grodstein9) and Morris et al. (Reference Morris, Evans and Tangney10) higher intake of green leafy vegetables was also associated with better episodic memory(Reference Kang, Ascherio and Grodstein9) at baseline and less cognitive decline(Reference Kang, Ascherio and Grodstein9, Reference Morris, Evans and Tangney10). Legumes were associated with worse cognitive function at baseline, but with less decline in cognitive function at follow-up(Reference Kang, Ascherio and Grodstein9). These results were not confirmed in the present study. However, Kang et al. (Reference Kang, Ascherio and Grodstein9) studied only women who were on average 75 years of age at the first cognitive measurement, with only a 2-year change in cognitive function. Also Morris et al. (Reference Morris, Evans and Tangney10) studied elderly individuals only. In addition, they used different cognitive tests. It is possible that some associations between fruits and vegetable consumption and cognitive function do not show during middle age.
The observed beneficial effect of root vegetables on cognitive decline may be found in the β-carotene content, especially from carrots which make by far the largest contribution to the consumption of root vegetables in The Netherlands(Reference Hulshof, Ocké and van Rossum46). β-Carotene is a potent antioxidant and may therefore protect the central nervous system from damage through oxidation of tissue with ageing. Short-term β-carotene supplementation has not been shown to affect cognitive function, but it is very well possible that longer-term elevated β-carotene intake through habitual diet may have beneficial effects on cognitive function(Reference Grodstein, Kang and Glynn47).
Except for nuts, no statistically significant association between fruit consumption and cognitive decline was observed in the present study. In animal studies, supplementation of high-antioxidant fruits retarded age-related cognitive decline(Reference Joseph, Shukitt-Hale and Casadesus48). The effect of fruits on cognitive decline may be too small to be discovered in a 5-year follow-up period in a human population demonstrating more heterogeneous lifestyles. Also in both epidemiological studies in older human populations, no association between fruit consumption and cognitive decline was observed over 2 and 6 years of follow-up(Reference Kang, Ascherio and Grodstein9, Reference Morris, Evans and Tangney10).
From a perspective of public health nutrition, consumer recommendations on nutrition should be based on foods rather than on nutrients. We identified some food groups that were associated with less cognitive decline. Replication of the present results in other studies is required before recommendations should be implemented in public health strategies and further research is needed to understand which components of these foods can explain their protective characteristics.
In conclusion, based on results of the present study, habitual consumption of vegetables and some specific fruits and vegetables (nuts and cabbage) may have favourable effects on age-related cognitive decline in middle-aged individuals. These findings need to be verified in other prospective studies before recommendations for lifestyle interventions to prevent cognitive decline or to delay the onset of dementia can be made.
Acknowledgements
The Doetinchem Cohort Study is financially supported by the Ministry of Public Health, Welfare and Sport of The Netherlands and the National Institute for Public Health and the Environment. The data up to and including 1997, including the dietary assessment method, were additionally financially supported by the Europe against Cancer programme of the European Commission (DG SANCO). The work of H. B. B.-de-M. was supported by the European Commission: Public Health and Consumer Protection Directorate 1993–2004 and the Ministry of Public Health, Welfare and Sport of The Netherlands. The authors thank the respondents and the epidemiologists and fieldworkers of the Municipal Health Service in Doetinchem for their contribution to the data collection for the present study.
W. M. M. V. was the principal investigator. Logistic management was provided by J. Steenbrink and P. Vissink, and administrative support by E. P. van der Wolf. Data management was provided by A. Blokstra, A. W. D. van Kessel and P. E. Steinberger.
A. C. J. N. performed the analyses of the data and led the writing of the article. W. M. M. V. supervised the data collection, analyses and preparation of the article. H. B. B.-de-M. supervised the development of the dietary assessment method and co-supervised the dietary data collection. All authors were involved in interpreting the findings and reviewed drafts of the manuscript.
None of the authors had any conflict of interest.







