Investigation of the role of diet in CHD may focus on individual nutrients, food groups or overall dietary patterns. Disentangling the effects of correlated nutrient intakes such as MUFA and PUFA is problematic, likewise, those of food groups such as wholegrain cereals, fruit and vegetables(Reference Ness and Powles1). Analysis of the epidemiological effects of overall dietary patterns offers an alternative approach(Reference Newby and Tucker2). Several a posteriori statistical methods have been used to determine dietary patterns(Reference Newby and Tucker2, Reference Brunner, Mosdøl and Witte3). Reduced rank regression (RRR) is particularly useful as a hypothesis-driven method for confirming pathways through which diet may act to alter chronic disease risk(Reference Schulze and Hoffmann4). RRR combines multivariate dietary pattern analysis with existing knowledge of diet–disease relationships(Reference Hoffmann, Schulze and Schienkiewitz5). In the first step, factor loadings for food groups are derived in an RRR model with intermediate disease markers, such as biomarkers of disease process, as one or more dependent variables. In the second step, the relationship between dietary pattern scores and disease outcome is tested. This methodology has been used to study dietary influences on obesity(Reference Schulz, Nothlings and Hoffmann6), CVD(Reference Hoffmann, Zyriac and Boeing7–Reference Drogan, Hoffmann and Schulz10), diabetes(Reference Schulze, Hoffmann and Manson11, Reference Heidemann, Hoffman and Spranger12) and all-cause mortality(Reference Hoffmann, Boeing and Boffetta13).
Studies of diet and CHD using RRR methods have focused on pathways based on the fat, carbohydrate and fibre density of the diet(Reference Drogan, Hoffmann and Schulz10), plasma homocysteine, folate and vitamin B12(Reference Weikert, Hoffmann and Dierkes8) and HDL-cholesterol, LDL-cholesterol, lipoprotein(a), C-peptide and C-reactive protein(Reference Hoffmann, Zyriac and Boeing7). While Hoffmann et al. (Reference Hoffmann, Zyriac and Boeing7) utilised HDL- and LDL-cholesterol as dependent variables, their study used a case–control design with potential measurement bias relating to the recall of dietary intake and the timing of the biomedical risk factor measurement. In addition, they incorporated markers of other risk-factor pathways and did not focus on specific lipid-related dietary patterns. Research exploring the relationship between dietary patterns and lipids using RRR methods focuses on the pathways through which diet may influence CHD.
The aim of the present study was to explore the link between dietary patterns associated specifically with blood lipid levels – serum total and HDL-cholesterol and TAG – and risk of verified incident coronary events over 15 years of follow-up in a large prospective cohort of men and women.
Materials and methods
Study population
Men and women aged 35–55 years were recruited to the Whitehall II study in 1985–8 (phase 1) from twenty Civil Service departments in London with a 73 % response rate (n 10 308). Full details of the study are reported elsewhere(Reference Brunner, Marmot and Nanchahal14, Reference Marmot and Brunner15). Medical examinations and self-report questionnaires were repeated at 5-yearly intervals and the cohort completed postal questionnaires between each clinic visit. At phase 3 (1991–3), 8104 participants attended the screening clinic. Ethical approval was obtained from the University College London Medical School Committee on the Ethics of Human Research. Informed consent was obtained at phase 1 and renewed at each contact. This analysis is based on data from 7314 participants that completed an FFQ at phase 3, who were fasted (duration of fasting >5 h) at the time of blood sample collection, had no history of CHD at the phase 3 screening and had complete data for all covariates.
Outcome ascertainment
Participants were flagged by the National Health Service Central Registry, which notified us of the date and cause of all deaths up to 31 July 2006. Deaths were classified as coronary if International Classification of Diseases (ICD)-9 codes 410–414 or ICD10 codes I20–I25 were present on the death certificate. Cases of non-fatal myocardial infarction were identified from twelve-lead electrocardiograms obtained at study phases 3, 5 and 7 and questionnaire items on chest pain and doctor's diagnosis. Details of physician investigations, diagnoses and interventions were sought from medical records for all potential cases for final ascertainment. Classification, following WHO Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) methods, was carried out independently by two trained coders, with adjudication in the event of disagreement. Self-report in the absence of verification was not classified as myocardial infarction. Prevalent cases of myocardial infarction were excluded from the analysis. Participants' date of censoring depended on the last attended phase: 30 September 2004 for those who attended phase 7.
Dietary intake
Dietary intake was assessed using a 127-item FFQ. Participants were asked to report the frequency of consumption of each food during the previous year. Standard portion sizes were specified for each food with response options ranging from ‘never or less than once per month’ to ‘six or more times per day’. Responses were converted to food intake in g/d for analysis. The questionnaire was previously validated in this cohort among 457 males and 403 females aged 39–61 years using 7 d food diaries(Reference Brunner, Wunsch and Marmot16). Spearman correlation coefficients for energy-adjusted nutrient intakes ranged from 0·35 (β-carotene) to 0·78 (alcohol) for men and 0·33 (vitamin E) and 0·83 (alcohol) for women.
Total energy intake (EI) was evaluated by examining the ratio EI:estimated energy expenditure (EE), reflecting energy misreporting. The ratio EI:EE will be 1 if there is no energy misreporting and less than 1 if there is underreporting(Reference Black17). EE was based on estimated BMR(Reference Schofield, Schofield and James18) and a physical activity level of 1·55 (sedentary) was assumed for this cohort of office workers. The energy cost of reported leisure time physical activity was added using metabolic equivalent (MET) values for EE per kg body weight (3 MET/h moderate activity, 5 MET/h vigorous activity)(Reference Ainsworth, Haskell and Whitt19). Fifty-five participants (0·75 %) with a log EI:EE value outside 3 sd of the log mean were excluded from the analysis.
The food and beverage items on the FFQ were aggregated into seventy-one food groups on the basis of nutrient content, cooking and preparation methods and consistency with groupings used in other studies of dietary patterns and CVD(Reference Newby and Tucker2, Reference Schulze and Hoffmann4, Reference Nettleton, Steffen and Mayer-Davis20, Reference Van Dam, Grievink and Ocke21) and the UK National Diet and Nutrition Survey(Reference Henderson, Gregory and Swan22). In addition, single food items that were hypothesised to represent specific eating behaviours were retained(Reference Williams, Prevost and Whichelow23–Reference Esmaillzadeh, Kimiagar and Mehrabi25). As alcohol intake has a distinctive U-shaped relationship with CVD and all-cause mortality(Reference Emberson, Shaper and Wannamethee26), we excluded alcoholic beverages from the dietary pattern analysis and treated them as a separate covariate.
Blood collection
Blood samples were collected following an overnight fast or in the afternoon after no more than a light fat-free breakfast eaten before 08.00 hours(Reference Brunner, Marmot and Nanchahal14). Cholesterol and TAG were measured in a centrifugal analyser by enzymic colorimetric methods. HDL-cholesterol was determined after dextran sulfate-magnesium chloride precipitation of non-HDL-cholesterol(Reference Brunner, Marmot and Nanchahal14).
Covariates
Employment grade within the Civil Service was used as the measure of adult socio-economic position(Reference Martikainen, Brunner and Marmot27). Weight and height were measured in standardised fashion with participants dressed in a cloth gown and underclothes. Smoking habit (never/former/current) and leisure time physical activity (hours of moderate/hours of vigorous activity per week) was self-reported. Alcohol intake (in g/d) was calculated from the FFQ. Blood pressure was measured twice after 5 min rest with the Hawksley random-zero sphygmomanometer at the phase 3 research clinic and the mean value taken (mmHg).
Statistical analysis
Dietary patterns were determined using RRR techniques as previously described by Hoffmann et al. (Reference Hoffmann, Schulze and Schienkiewitz5) and applied in a number of recent studies of dietary patterns and chronic disease(Reference Hoffmann, Schulze and Schienkiewitz5–Reference Nettleton, Steffen and Schulze9, Reference Schulze, Hoffmann and Manson11, Reference Hoffmann, Boeing and Boffetta13). RRR determines factors from food intake data that maximise the explained variation in the intermediate markers that are hypothesised to be related to the health outcome. The resulting dietary pattern scores are used in epidemiological analysis.
Total cholesterol, HDL-cholesterol and TAG were used as intermediate markers or response variables based on the established relationships with CHD(Reference Lewington, Whitlock and Clarke28–Reference Wittrup, Andersen and Tybjaerg-Hansen31). The number of dietary patterns extracted using RRR analysis is determined by the number of response variables (n 3)(Reference Hoffmann, Schulze and Schienkiewitz5). Foods with absolute factor loadings >0·20 were used to describe the dietary pattern but all foods contributed to calculation of the dietary pattern score(Reference Hoffmann, Schulze and Schienkiewitz5, Reference Nettleton, Steffen and Schulze9, Reference Heidemann, Hoffman and Spranger12, Reference Hoffmann, Boeing and Boffetta13). To investigate robustness of the dietary patterns, we randomly split the cohort five times and repeated the RRR analysis in half of the cohort (i.e. the analysis was repeated five times). The mean Spearman correlation coefficient between these dietary pattern factor loadings and the original factor loadings was 0·98, 0·93 and 0·90 for patterns 1, 2 and 3, respectively. Dietary patterns were analysed using food intakes adjusted for EI, sex and employment grade using the residual method(Reference Willett, Stampfer and Willett32). The resulting dietary patterns were similar (data not shown), and the unadjusted patterns were used in the analysis with incidence of CHD. Sex-specific quartiles of the dietary pattern scores were calculated.
Cox proportional hazards regression was conducted using follow-up time as the time variable using Stata 9.0 (StataCorp LP, College Station, TX, USA). Males and females were combined for analysis, as models including an interaction term indicated that there was no sex interaction. Initial models were adjusted for age, sex, energy misreporting and ethnicity(Reference Brunner, Mosdøl and Witte3). Further adjustments were made for employment grade, health behaviours (smoking, alcohol and physical activity), blood pressure and BMI. P values < 0·05 were considered to be significant.
Results
Three dietary patterns were extracted explaining 4·2, 1·7 and 0·3 % of the variation in response variables (Table 1). Dietary pattern 1 explained 7·14 % of variation in HDL-cholesterol and 5·3 % of variation in TAG while dietary pattern 2 explained 3·5 % of variation in total cholesterol. As dietary pattern 3 explained little variation in responses, further results are not presented. Dietary pattern 1 was characterised by higher consumption of white bread, fried potatoes, sugar in tea and coffee, burgers and sausages, soft drinks, and lower consumption of French dressing, vinaigrette and other vegetables (Table 2). A higher score on dietary pattern 1 was associated with higher total cholesterol, lower HDL-cholesterol and higher TAG. Dietary pattern 2 was characterised by higher consumption of red meat, cabbage, Brussels sprouts and cauliflower, and lower consumption of wholemeal bread, jam, marmalade and honey, tofu and soya, buns, cakes, pastries, fruit pies and polyunsaturated margarine. A higher score on dietary pattern 2 was associated with higher total cholesterol and higher TAG.
Table 1 Explained variation (%) in food intakes and responses of each dietary pattern as assessed using reduced rank regression and correlations between the dietary pattern scores and responses
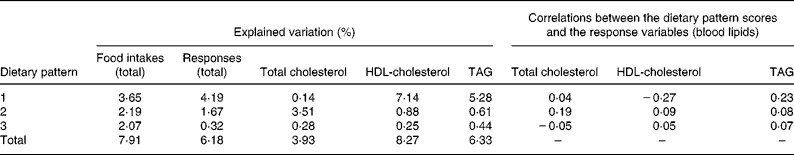
Table 2 Intakes of the key foods and response variables across sex-specific quartiles of dietary pattern scores
(Mean values with their standard errors for 7314 subjects)

* Adjusted for age and ethnicity.
After 83 536 person-years of follow-up, 243 incident coronary events were identified (Table 3). For dietary pattern 1, a higher dietary pattern score was associated with increased risk of CHD (model 1, adjusted for age, sex and energy misreporting, ethnicity; P for trend < 0·0001). This relationship was attenuated after adjustment for employment grade (model 2) and health behaviours (model 3, smoking, alcohol and physical activity), but remained significant even after further adjustment for blood pressure and BMI (model 6, hazard ratio for top quartile: 1·57; 95 % CI 1·08, 2·27). Further adjustment for dietary patterns 2 and 3 did not affect the findings (hazard ratio for top quartile: 1·57; 95 % CI 1·08, 2·27; data not shown). Dietary pattern 2 showed a significant linear trend across quartiles with a higher dietary pattern score also associated with increased risk of CHD (model 3, adjusted for age, sex and energy misreporting, ethnicity, employment grade, smoking, alcohol and physical activity; P < 0·0001). However, this relationship was no longer significant after further adjustment for BMI and blood pressure. Dietary pattern 3 was not associated with CHD (hazard ratio for top quartile: 0·90; 95 % CI 0·61, 1·31, adjusted for age, sex, energy misreporting, ethnicity and employment grade; data not shown).
Table 3 CHD across quartiles of dietary pattern score
(Hazard ratios and 95 % confidence intervals for 7314 subjects)

HR, hazard ratio.
Discussion
We identified a dietary pattern, characterised by high consumption of white bread, fried potatoes, sugar in tea and coffee, burgers and sausages, and soft drinks, and lower consumption of French dressing/vinaigrette and other vegetables, that was associated with higher total cholesterol, lower HDL-cholesterol and higher TAG and was predictive of increased risk of CHD. A second dietary pattern, characterised by higher consumption of red meat, cabbage, Brussels sprouts and cauliflower, and lower consumption of wholemeal bread, jam, marmalade and honey, tofu and soya, buns, cakes, pastries, fruit pies and polyunsaturated margarine, also remained associated with increased risk of CHD after adjustment for non-dietary health behaviours and socio-economic status.
We selected serum lipids to serve as intermediate biomarkers of risk in the dietary pattern–CHD analysis. There is strong evidence from observational studies(Reference Lewington, Whitlock and Clarke28), cholesterol-lowering trials(Reference Baigent, Keech and Kearney29) and genetic association studies(Reference Wittrup, Tybjaerg-Hansen and Nordestgaard30, Reference Wittrup, Andersen and Tybjaerg-Hansen31) that blood lipids are causal factors for disease. We found mean differences in total cholesterol, HDL-cholesterol and TAG of 0·15, 0·18 and 0·37 mmol/l across quartiles of dietary pattern score 1, corresponding to a two-fold higher disease rate in the base model. The impact of diet appeared little confounded by associated health behaviours. Adjustments for employment grade, a powerful indicator of adult socio-economic status, as well as smoking, alcohol and physical activity reduced the observed effect by 15 %.
Dietary pattern 1 was strongly associated with lower HDL-cholesterol and higher TAG levels. The relationship between dietary pattern 1 and CHD risk was attenuated by inclusion of BMI in the model. This suggests that this dietary pattern is a determinant of the metabolic syndrome risk profile. Additional attenuation by the inclusion of blood pressure supports this interpretation. Further work is required to directly investigate the impact of this dietary pattern on risk of the metabolic syndrome.
Previous RRR studies of diet and CHD have used a range of intermediate markers including HDL-cholesterol, LDL-cholesterol, lipoprotein(a), C-peptide, C-reactive protein(Reference Hoffmann, Zyriac and Boeing7), plasma homocysteine, folate, vitamin B12(Reference Weikert, Hoffmann and Dierkes8) and fat, carbohydrate and fibre density(Reference Drogan, Hoffmann and Schulz10). Despite differences in the intermediate markers, there are some similarities in the foods identified which were associated with increased risk of CVD such as potatoes and fried potatoes(Reference Weikert, Hoffmann and Dierkes8, Reference Nettleton, Steffen and Schulze9) and processed meat(Reference Nettleton, Steffen and Schulze9) and in the foods that were shown to be associated with decreased risk of CVD, namely, whole grains(Reference Drogan, Hoffmann and Schulz10) and vegetables(Reference Hoffmann, Zyriac and Boeing7–Reference Drogan, Hoffmann and Schulz10). Similarly, using cluster analysis to identify dietary patterns in the Whitehall II study, Brunner et al. (Reference Brunner, Mosdøl and Witte3) identified a healthy dietary cluster that was characterised by higher intakes of wholemeal bread, fruits, vegetables and polyunsaturated margarine and lower intakes of red meat which was associated with a lower risk of CHD. Previous RRR studies have not identified soft drinks in their dietary patterns although recent research has identified associations between soft drink consumption and risk factors for CVD such as obesity, high blood pressure, abnormal glucose and low HDL-cholesterol, which is consistent with our findings(Reference Dhingra, Sullivan and Jacques33).
Strengths of the present study include the large sample size, its prospective nature and the rigorous methods of outcome ascertainment. While Hoffmann et al. (Reference Hoffmann, Zyriac and Boeing7) also incorporated HDL- and LDL-cholesterol as dependent variables, as well as a range of intermediate markers, the study was based on a case–control design which has potential for recall bias and the biomarker measurements used as the intermediate markers were measured after the diagnosis of disease. In addition, their study only included women. Nettleton et al. (Reference Nettleton, Steffen and Schulze9) conducted a cross-sectional study investigating carotid intima media thickness rather than coronary events.
Foods identified in the dietary pattern may be indicators of other foods with which they are consumed. For example, salad dressing is not consumed alone and was correlated with salad vegetables in the present study (data not shown); its presence in the dietary pattern is likely to reflect consumption with salad vegetables. When investigated separately, salad dressing was not associated with CHD risk; however, as the effects of a total dietary pattern may be additive, a causal role for salad dressing cannot be ruled out. Similarly, a dietary pattern containing cruciferous vegetables (such as Brussels sprouts and cauliflower) was associated with an increased risk of CHD in this analysis. Importantly, when the individual foods were investigated separately, none of the foods showed significant associations with risk of CHD (data not shown).
Research into dietary patterns aims to characterise the whole diet in combination, providing a summary measure of dietary exposure and capture complex behaviours and potentially interactive and antagonistic effects among nutrients that might impact upon health outcomes. RRR analysis is a new statistical method which has been applied to dietary pattern research. It combines data-driven methods with use of a priori knowledge of diet–disease relationships. RRR determines dietary patterns that explain the greatest variation in the intermediate markers and responses rather than explaining variation in food intakes and, therefore, is particularly suited to identifying and confirming pathways through which dietary factors influence disease development in studies where intermediate biomarkers of exposure or disease are available(Reference Heidemann, Hoffman and Spranger12).
Additional work with the RRR techniques is required to determine the importance of dietary patterns extracted subsequent to the first pattern, which by definition explains the greatest variation in biomarkers. In the present study, the third dietary pattern explained very little additional variation in any of the response variables, and was not predictive of CHD. However, previous work by Hoffmann et al. (Reference Hoffmann, Schulze and Schienkiewitz5) showed that only the fourth dietary pattern extracted was associated with the risk of type 2 diabetes, despite explaining a small proportion of the variation in comparison with the first three dietary patterns that were extracted. Further work is required is determine the importance of extracting and investigating these subsequent patterns. A potential weakness of the RRR approach is the cross-sectional nature of underlying RRR dietary pattern analysis; however, in our analysis we excluded participants who had a previous history of CHD in order to reduce the impact of changes in dietary behaviour due to pre-existing disease. In addition, further work is also required to determine whether the optimal use of the RRR method involves using biomarkers that reflect one disease progression pathway, with individual pathways modelled separately or whether there is an advantage to using multiple biomarker pathways in the same regression modelling(Reference Schulze, Hoffmann and Manson11, Reference Heidemann, Hoffman and Spranger12).
The dietary patterns explained 3·9 % of the variation in total cholesterol, 7·1 % of the variation in HDL-cholesterol and 5·3 % of the variation in TAG. These results are comparable with other studies using RRR methods where biomedical risk factors have been used as the response variable(Reference Hoffmann, Zyriac and Boeing7, Reference Schulze, Hoffmann and Manson11). Studies using intakes of nutrient have tended to explain higher variation in those responses(Reference Hoffmann, Schulze and Schienkiewitz5, Reference Schulz, Nothlings and Hoffmann6, Reference Hoffmann, Boeing and Boffetta13). However, this is unsurprising as bloods lipids are a more remote response variable than nutrient intakes.
A range of socio-demographic factors (age, sex, ethnicity, employment grade), health behaviours (smoking, physical activity, alcohol) and other risk factors (blood pressure and BMI) were investigated as confounders and shown to attenuate the relationship between the dietary pattern and CHD, although the relationship remained significant. However, residual confounding cannot be ruled out due to the potential for measurement error among the covariates. BMI and blood pressure were included in the final regression models and shown to attenuate the relationship between dietary patterns and risk of CHD. This finding is consistent with the mediating role of these risk factors. The fully adjusted models may therefore underestimate the effect of diet(Reference Srinath Reddy and Katan34), while model 3, adjusted for energy misreporting, socio-demographic factors and non-dietary health behaviours, may reflect the role of diet in CHD causation operating through multiple pathways.
This research highlights that certain dietary patterns are strongly associated with blood lipids and risk of CHD, and adds to the evidence that influences on HDL-cholesterol and TAG levels are important in the dietary prevention of CHD. While the dietary pattern we identified may in part act though obesity and increased blood pressure, it appeared to have independent effects on the risk of CHD. Our findings are consistent with the operation of multiple dietary pathways influencing CHD risk. The RRR method is an advance in methodology, able to identify and confirm mechanisms through which diet may influence disease risk.
Acknowledgements
The Whitehall II study has been supported by grants from the UK Medical Research Council, British Heart Foundation, Health and Safety Executive, Department of Health, National Heart Lung and Blood Institute (HL36310), National Institute on Aging (AG13196), Agency for Health Care Policy Research (HS06516) and the MacArthur Foundation Research Network on Socio-economic Status and Health. No funding source had direct influence over the design, conduct or reporting of the present study. S. A. M. is supported by a National Health & Medical Research Council (NHMRC) Public Health Postdoctoral Fellowship and an NHMRC Travelling Award for Research Training.
S. A. M. contributed to the development of the study hypothesis, reviewed the literature, conducted the statistical analysis, provided interpretation of results, and drafted and edited the manuscript. G. D. M. contributed to the development of the study hypothesis, guided the statistical analysis, provided advice regarding interpretation of the results, and assisted with drafting and editing of the manuscript. E. J. B. was responsible for the study design, data collection, provided advice regarding interpretation of the results and edited the manuscript.
None of the contributing authors had a conflict of interest.





