Malnutrition due to both chronic and acute diseases is a widespread, under-recognised and under-treated problem at hospital admission(Reference Norman, Pichard and Lochs1). The association between hyporexia/anorexia, reduced energy intake and disease-related malnutrition has been studied extensively, and most of the underlying pathophysiological mechanisms have been clarified(Reference Norman, Pichard and Lochs1, Reference Evans, Morley and Argilés2). Nutritional depletion can also worsen during hospital stay(Reference Norman, Pichard and Lochs1, Reference McWhirter and Pennington3, Reference Incalzi, Gemma and Capparella4), and clinicians are now aware that nutritional care should be managed continuously during this period. Accordingly, malnutrition screening procedures at referral are now enforced and treatment guidelines have been drawn up, aimed at reducing malnutrition prevalence, the rate of related complications, hospital costs and the length of hospital stay(Reference Norman, Pichard and Lochs1, Reference Kondrup, Allison and Elia5, Reference Lochs, Allison and Meier6). However, the relationship between nutritional risk and fluid intake has never been investigated. In a healthy subject, body water comprises about 60 % of the total body weight, and it is distributed in two major compartments, extracellular and intracellular. The balance between these compartments and whole body homeostasis strongly depends on ingestion, absorption and retention. During periods of starvation and/or critical illness, water and salt retention usually increases, and both over- and under-prescription of fluids can be detrimental. Thus, in the past, researchers focused their attention on the best way to correct fluid and electrolyte imbalances(Reference Lobo7, Reference Allison8), but baseline fluid intake has never been investigated either in the critically ill or in a non-intensive setting. However, it can be hypothesised that the pre-existing fluid balance could affect fluid management during the hospital stay.
Taking into consideration this background, the present study was undertaken to evaluate, on admission to hospital, the association between fluid intake and nutritional risk in non-critically ill patients, and to identify factors with potential impact on clinical practice and deserving further investigation.
Methods
The present study was performed according to the principles of the Declaration of Helsinki, and before any form of assessment, we obtained local Ethics Committee approval. The protocol was approved initially by the Institutional Review Board of the coordinating centre (Bolzano Regional General Hospital), and then by that of the other recruiting centres. We also obtained written informed consent for every patient (the patient itself, relatives or legal guardians). The data analysed in the study were obtained from a larger observational multicentric (thirteen large (>400 beds) multidisciplinary regional hospitals) study, aimed at evaluating the prevalence of malnutrition among hospitalised patients in Italy (Project Iatrogenic Malnutrition in Italy study – PIMAI)(Reference Lucchin, D'Amicis and Gentile9), and from a personal database of assessments (C. P.) performed at the Hospital of Trento in agreement with the PIMAI study protocol. Thus, the present study is a retrospective analysis of data initially collected for other purposes. All the evaluations were made within 36 h of admission. Adult patients (age >18 years) were recruited from all the wards, except from those of both medical and surgical acute emergencies, according to randomised selection from the daily list of new admissions. A random sample of patients was chosen using the following criteria: one of three patients admitted; no more than three patients/d per hospital; an equal number of males and females; an equal number of subjects aged < 65 and ≥ 65 years. Patients whose fluid intake might be altered because of possible disease-related conditions (chronic heart or renal failure, vomiting, diarrhoea, not compensated diabetes, diabetes insipidus and compulsive water drinking) were excluded(Reference Sung, Kuo and Guo10–Reference Allison and Lobo13). Also, subjects with dementia syndromes, diagnosed according to the International Classification of Disease, 10th Revision(14), those with a moderate to high alcohol intake (set to ≥ 36 g/d) and pregnant women were excluded.
Information was collected on sex, age, educational level, work, speciality of admission, main admission diagnosis, co-morbidities, drugs, overall nutritional risk, determinants of nutritional risk (weight status, recent weight loss and food intake) and fluid intake.
Diagnosis of nutritional risk
Nutritional risk was diagnosed using the Nutritional Risk Screening 2002 scoring system(Reference Kondrup, Allison and Elia5, Reference Kondrup, Rasmussen and Hamberg15). This tool, recommended by the European Society of Parenteral and Enteral Nutrition for nutrition screening, is based on the sum of three different subscores that take into account nutritional status (‘Nutritional score’), disease severity (‘Disease severity score’) and age (‘Age score’). The ‘Nutritional score’ is derived from information on actual body weight as BMI, recent weight loss and period of weight loss ( ≥ 5 % within the last 1, 2 or 3 months), and dietary intake during the last week before admission (categorised into quartiles of requirement) as assessed by well-trained dietitians. Energy requirement was calculated as BMR (calculated using the Harris–Benedict equation) corrected by an activity factor or by a stress factor. In estimating dietary intake, we also considered energy from liquid foods. ‘Nutritional scoring’ is as follows: a score of 3 is assigned to the patient when BMI is < 18·5 kg/m2, or there has been a weight loss ≥ 5 % in the last month or only 0–25 % of the intake requirement. A score of 2 is given to the patient with 18·5 kg/m2 ≤ BMI ≤ 20·5 kg/m2, recent weight loss ≥ 5 % in the last 2 months or an intake of 25–50 % of the requirement. A score of 1 is assigned to the patient when recent weight loss is ≥ 5 % in the last 3 months or the dietary intake is 50–75 % of the requirement.
The ‘Disease severity score’ categorises the patient according to disease-related metabolic stress ( ≈ increased requirements); there are three classes: slight (score = 1), moderate (score = 2) or severe (score = 3). These scores are then added to give a total score to which an additional point is added when age is ≥ 70 years (‘Age score’). Any patient with a total score ≥ 3 is diagnosed as being ‘at nutritional risk’. A more detailed description of this scoring system is provided elsewhere(Reference Kondrup, Allison and Elia5, Reference Kondrup, Rasmussen and Hamberg15).
Fluid intake assessment
Fluid intake assessment was performed by well-trained dietitians, and consisted of the semi-quantitative evaluation of all the major fluid intakes (from sources such as water, juices, sweetened beverages, milk, wine, beer, tea and coffee) the week before admission; this was done by dietary recall and through the use of illustrated atlases. In some cases, additional information was obtained from caregivers. The level of acceptable intake was set at ≥ 5 cups/d (>1200 ml/d; 1 cup = 240 ml). This threshold value was chosen according to commonly accepted statements on fluid balance. In fact, given the normal daily water loss through urine, stools, skin and respiration, the average daily requirement is estimated to be 2500 ml/d, with approximately 1000–1500 ml coming from beverages and the remaining amount coming from substrate oxidation and foods(16, Reference Kleiner17).
Possible risk factors for altered fluid intake
Apart from age, the presence of malignancies (cancer), diabetes and the number of co-morbidities were investigated as possible independent risk factors(Reference Allison and Lobo13, Reference Sarhill, Mahmoud and Christie18, Reference DeFronzo19). The diagnosis of ‘being at nutritional risk’, the adequacy of dietary intake in the previous week (assessed as quartiles of requirement: < 25 %, 25– < 50 %, 50– < 75 % and ≥ 75 %) and recent body weight loss ( ≥ 5 % in 1, 2 or 3 months) were also considered. Finally, the use of blood pressure-lowering medications and diuretics, as well as the daily number of prescribed drugs (drugs/d; including antihypertensive medications and diuretics), were taken into account as potential determinants of fluid intake(Reference Kleiner17). Accordingly, the patients were stratified as follows: 0–2, 3–5 and ≥ 6 drugs/d.
Statistical analysis
All statistical analyses were performed using the SPSS 16·0 statistical package (SPSS for Windows; SPSS Inc., Chicago, IL, USA).
The normal distribution of the variables was examined using the Kolmogorov–Smirnov test. Continuous variables of the two groups were compared using unpaired Student's t test (normal distribution) or the Mann–Whitney U test (not normal distribution), whichever was appropriate. However, multiple-group comparison was performed using ANOVA or the Kruskal–Wallis test. Frequencies were compared using the χ2 test. Finally, we investigated the independent determinants of reduced fluid intake ( < 5 cups/d; dependent variable) using logistic regression models, and OR with 95 % CI were calculated. First, we ran univariate analyses adjusted for sex, height, educational level, work, admission speciality and recruiting centre. All the correlates with a P < 0·10 by these models were then analysed using multivariate analysis, basing the analysis on the crude model and making additional adjustments for diabetes and the use of diuretics and medication for lowering blood pressure. In all the models, included independent variables were treated categorically. Statistical significance for type I error was established by P < 0·05.
Results
From the initial population fulfilling the inclusion criteria (n 2136), we excluded patients due to the following reasons: n 252 (11·8 %), refusal to participate or terminal illness; n 301 (14·1 %), unavailability of complete data to perform risk assessment using the Nutritional Risk Screening 2002 tool; n 854 (40 %), lack of fluid intake assessment (not performed since the beginning of the recruitment phase because it was not included in the initial study protocol); n 170 (8·0 %), fluid intake potentially altered because of disease-related conditions.
Final analysis included a study sample of 559 subjects. Patients were recruited from all the regular wards (Table 1), but most of them were from internal medicine (22·9 %) and general surgery (13·2 %). Overall prevalence of nutritional risk was 57·2 % (n 320), with significantly higher rates in the medical setting than in the surgical one (61·2 v. 50·5 %) (Table 2). The prevalence of patients ‘at risk’ and of those reporting low fluid intake ( < 5 cups/d) was markedly heterogeneous among the investigated specialities, and it ranged from 26·7 % (psychiatry) to 78·9 % (gastroenterology) for nutritional risk, and from 25·0 % (endocrinology) to 88·5 % (geriatrics) for fluid intake. A weight loss ≥ 5 % and an energy intake < 75 % of the estimated requirement were recorded in 62·3 and 36·4 % of the cases, respectively. Both these features were reported by 24·2 % of the patients. ‘At-risk’ patients showed lower BMI, albumin and prealbumin serum levels, and a higher number of prescribed daily drugs taken, and were more likely to suffer malignancies and report a higher fluid intake (Table 2).
Table 1 Study sample distribution according to setting, speciality, nutritional risk and low fluid intake (<5 cups/d)

Table 2 Features of the population according to nutritional risk*
(Mean values and standard deviations)

M, male; F, female; WL, weight loss before hospital admission.
* Data are presented as prevalence/frequency as indicated.
† Percentages refer to the whole group (within a single column).
‡ According to unpaired t test or Mann–Whitney U test and χ2 test, where appropriate.
Therefore, the patients were divided into four groups according to fluid intake and nutritional risk (Table 3). The differences in BMI, albumin, prealbumin and lymphocytes between ‘at-risk’ and ‘not at-risk’ patients appeared to be independent of fluid intake. Patients ‘at risk’ and characterised by poor fluid intake were significantly older than those in the other groups. We also found a significant association with the entity of weight loss, food intake and the number of medications taken. It was noted that the ‘not at-risk’ patients with poor fluid intake and the patients ‘at risk’ with acceptable fluid intake, respectively, took the lowest and highest average number of drugs.
Table 3 Features of the population according to fluid intake and nutritional risk (Nutritional Risk Screening 2002 score ≥3)†
(Mean values and standard deviations)

M, male; F, female; WL, weight loss before hospital admission.
* Mean values were significantly different, post hoc comparison by Scheffe's test (P < 0·05).
† Data are presented as prevalence/frequency as indicated.
‡ Percentages refer to the whole group.
§ P values according to ANOVA or Kruskal–Wallis test and χ2 test, where appropriate.
Finally, we investigated the independent predictors of low fluid intake ( < 5 cups/d; dependent variable) at hospital admission using multivariate logistic regression models (Table 4). Multiple-adjusted univariate analyses revealed that patients aged ≥ 65 years, presenting with reduced energy intake and a lower number of drugs taken, were more likely to have a lower fluid intake. Moreover, higher fluid intake was detected in patients with decreasing BMI and those ‘at nutritional risk’. The multivariate model also confirmed similar associations, the exception being that with weight status.
Table 4 Logistic regression models of independent risk factors for low fluid intake (<5 cups/d)
(Odds ratios and 95 % confidence intervals)
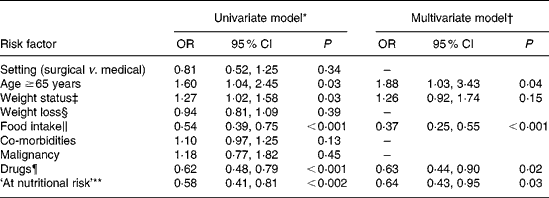
* Adjusted for sex, height, educational level, work, recruiting centre and admission speciality.
† Further adjusted for diabetes, use of diuretics and blood pressure-lowering medications.
‡ Entered as categorical variable: underweight, 0; normal weight, 1; overweight, 2; obesity, 3.
§ Entered as categorical variable: ≥ 5 % in 1 month, 0; ≥ 5 % in 2 months, 1; ≥ 5 % in 3 months, 2.
∥ Entered as categorical variable: < 25 % requirement, 0; 25– < 50 % requirement, 1; 50– < 75 % requirement, 2; ≥ 75 % requirement, 3.
¶ Entered as categorical variable: 0–2 drugs/d, 0; 3–5 drugs/d, 1; 6 or more drugs/d, 2.
** As defined by Nutritional Risk Screening 2002 score ≥ 3.
Discussion
Disease-related malnutrition has been proven to be frequently associated with reduced food/energy intake, and this, in turn, significantly contributes to body mass wasting and impairment of functional status(Reference Norman, Pichard and Lochs1, Reference Evans, Morley and Argilés2, Reference Incalzi, Gemma and Capparella4). Even dehydration of as little as 2 % leading to loss of body weight can result in impaired physiological responses and functional change(Reference Allison8, Reference Kleiner17). However, the failure to prescribe adequate fluids also, resulting in overload, might have consequences in terms of complications or recovery rate after surgery or a critical illness(Reference Lobo7, Reference Allison8). These pathological conditions, as well as many other diseases, are responsible for higher nutritional risk(Reference Norman, Pichard and Lochs1, Reference Kondrup, Allison and Elia5), but the relationship between nutritional status or risk and oral fluid consumption on admission to hospital has been never explored.
In the present study, we observed for the first time that non-critically ill patients at nutritional risk are more likely to present with an acceptable fluid intake. We also detected an independent association between fluid intake and age, food intake and multiple pharmacological treatment.
It has been well demonstrated that levels of thirst reduce progressively during ageing due to a decrease in the sensitivity of volume- and osmoreceptors(Reference Allison and Lobo13, Reference Farrell, Zamarripa and Shade20). Along with this, a role for mental (e.g. decline of cognitive function) and physical dependency, as well as for behavioural changes, should be recognised(Reference Allison and Lobo13).
We observed a direct relationship between the energy and fluid intakes. It is reasonable to argue that subjects eating to a normal extent drink more to accompany food. Food per se is an important source of water, but some energy can also be introduced in liquid form (wine, milk, beverages, etc.).
Also, the positive association between polypharmacy and fluid intake may be simple to explain as the ingestion of medication frequently takes place during meals, and the patients usually have a cup of water or other liquids everytime they need to swallow a pill.
However, some liquid preloads are able to reduce appetite if they provide energy, particularly in the elderly(Reference Sturm, Parker and Wishart21), and drugs are known to negatively affect energy intake(Reference Norman, Pichard and Lochs1, Reference Sturm, Parker and Wishart21). Malnourished patients are more likely to be older, and frequently present reduced energy intake and multiple drug prescriptions, which are interacting factors that result in detrimental consequences on nutritional status(Reference Norman, Pichard and Lochs1, Reference Kondrup, Allison and Elia5, Reference Omran and Morley22, Reference Pirlich, Schütz and Norman23). However, the association between nutritional risk and fluid intake was found to be independent. Thus, the mechanisms beyond this relationship are not clear, and some speculations would be in order.
When disease is present, particularly acute injury or critical illness, the inflammatory condition induces a generalised increase in capillary permeability with a leakage of albumin, and so of fluids, to the extravascular space(Reference Lobo7, Reference Allison8, Reference Anderson, Lawes and Lobo24–Reference Stanga, Brunner and Leuenberger26). Albumin levels fall also for an impairment in protein turnover(Reference Ballmer25). Moreover, the alteration electrolyte fluxes across cellular membrane due to ATP depletion should be taken into consideration. Accordingly, low plasma flow and electrolyte alterations can lead to the activation of volume- and osmoreceptors, resulting in an increase in water and Na retention and in the sense of thirst(Reference Lobo7, Reference Allison8, Reference Farrell, Zamarripa and Shade20).
The aforementioned pathophysiological mechanisms apparently refer to the two main types of malnutrition, kwashiorkor-like and marasmatic, and they could be useful to distinguish between them. Indeed, it has been demonstrated that the two conditions frequently coexist, and that both contribute to nutritional risk(Reference Norman, Pichard and Lochs1). Perhaps, the availability of data concerning inflammatory markers (e.g. C-reactive protein) would better sustain this hypothesis, and an evaluation of body fluid compartments (intracellular and extracellular) and natraemia would help explain this relationship. Nonetheless, part of the independent association found with nutritional risk could be explained by the tool used for risk assessment. The Nutritional Risk Screening 2002 tool was developed and validated in the acute care setting, and focusing much more on the effect of a disease, it seems to better reflect the inflammatory background associated with the pathological process leading to hospitalisation(Reference Kondrup, Rasmussen and Hamberg15).
We do not know the consequences of the present results on outcome and clinical practice. Indeed, fluid intakes are different from fluid status. Water and salt retention is usually increased in starved subjects, and both over- and under-prescription of fluids can be detrimental. Accordingly, a cautious correction of even modest deficits or excesses of fluid and electrolyte imbalances is recommended to avoid excess fluid retention and to optimise physiological functions, particularly in those patients suffering from critical illness or acute injury (e.g. burn or brain injury) or who have undergone surgical procedures(Reference Lobo7, Reference Allison8). In our study, the evaluation was performed at admission, thus before surgery, and most critically ill patients were excluded.
It is not clear if this attitude to a better fluid intake affects patient prognosis, and we are unable to set a threshold fluid intake level, over or under which the rates of complications might change significantly. It should be recognised that no real estimation of fluid balance was performed, as we did not consider output, loss or water coming from solid foods.
We cannot suggest that ‘at-risk’ patients be advised to change their behaviour. Indeed, in the initial phase of refeeding, water replacement by oral energy-dense formula could be advantageous for ‘at-risk’ patients, as the increase in both fluid and energy needs by this route seems feasible and effective(Reference Eneroth, Olsson and Thorngren27), and this practice would also be in agreement with the need to avoid fluid overload during the refeeding process(Reference Stanga, Brunner and Leuenberger26, Reference Mehanna, Moledina and Travis28).
The study has limitations other than those presented earlier. Indeed, the method used for assessing fluid intake (recall) is useful in population studies, and similarly to food intake, it is well applicable in this type of setting. The major disadvantage is its heavy reliance on memory, as well as that it also overlooks day-to-day food variation. Unfortunately, the use of quantitative tools of assessment, such as diaries, cannot be considered in such a study design. Limits should also be recognised in the lack of distinction between the different fluid sources (energetic and non-energetic) and in the threshold value chosen to define an acceptable intake. The one reported is suggested in the literature, but it refers to the real fluid balance.
The exclusion of some patient categories is also recognised as a limit, as well as the type of study design (retrospective rather than prospective). Accordingly, our findings should be interpreted cautiously, and they could not be generalised to the whole population of patients at risk of malnutrition at hospital referral. Some of the excluded pathological conditions are related to both nutritional risk and alteration of fluid intake (e.g. chronic heart or renal failure, persistent vomiting or diarrhoea, dementia and total dependence in the activities of daily living), but the inclusion would have been confounding. However, an extensive evaluation of cognitive function was not performed, and only patients with a reported diagnosis of dementia were excluded.
In conclusion, non-acute patients at risk of malnutrition or with overt malnutrition at hospital admission frequently present with better fluid intake. In critically ill patients, fluid overload must be avoided. Thus, our observations in the non-critically ill patients suggest that the evaluation of fluid balance at hospital admission is of potential interest and deserves investigation. It cannot be excluded that a better focus on this issue might be helpful in improving the nutritional management of hospitalised patients.
Acknowledgements
The present study was partially supported by grants of Nutricia (Milan, Italy). All authors certify that there are no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter or materials discussed in the manuscript. All the authors significantly contributed to the work, read and approved the final manuscript. E. C. designed the study, analysed data and wrote the manuscript. L. L., A. D'. A., M. G. G., N. C. B. and M. A. F. designed the PIMAI study. C. P. collected part of the data and contributed to their interpretation and manuscript drafting. All the authors belonging to the PIMAI group contributed to data collection and interpretation. We are particularly grateful to all the contributing centres (the PIMAI group) and the relative personnel (nutritional scientists, dietitians and nurses) involved in data collection:
1 Dietetic and Clinical Nutrition Unit, Regional General Hospital Bolzano: Lucchin L, Lando L, Borgo S, Saffiotti GL.
2 Dietetic and Clinical Nutrition Unit, ‘Niguarda-Ca Granda’ Hospital, Milano: Gentile MG, Rodeschini E, Sandri LG.
3 Dietetic and Clinical Nutrition Unit, ‘Maggiore della Carità’ Hospital, Novara: D'Andrea F, Brugnani M, Barbero B, Passera S.
4 Dietetic and Clinical Nutrition Unit, University Hospital, Padova: Caregaro L, Nardi MT.
5 Dietetic and Clinical Nutrition Unit, ‘S.Martino’ Hospital, Genova: Sukkar GS, Ferrari C.
6 Dietetic and Clinical Nutrition Unit, ‘Le Scotte’ University Hospital, Siena: Mattei R, Grosso A, Francalanzi C, Cardinali F, Borsi E.
7 Dietetic and Clinical Nutrition Unit, ‘Casa Sollievo della Sofferenza’ Hospital, S.Giovanni Rotondo, Foggia: Orban A, Cianti L.
8 Endocrinology, Diabetology and Clinical Nutrition Unit, ‘S.Sebastiano’ Hospital, Caserta: Prilli M, Capriello R, Sorrentino S, Pennino MR.
9 Dietetic and Clinical Nutrition Unit, ‘Canizzarro’ Hospital, Catania: Leonardi F, Bellino AME, Massimino EA.
10 Dietetic and Clinical Nutrition Unit, ‘Umberto I’ Hospital, Ancona: Nicolai A, Petrelli M, Taus M, Busni D, Borri MG, Vitrini S.
11 Dietetic and Clinical Nutrition Unit, ‘A.Cardarelli’ Hospital, Campobasso: Pastò S, Di Biase P, D'Onofrio R, Di Brino AM, Celi C, Mastronuzzi V.
12 Department of Internal Medicine, University ‘La Sapienza’, Roma: Muscaritoli M, Preziosa I, Canali A.
13 Dietetic and Clinical Nutrition Unit, ‘S.Maria’ Hospital, Terni: Fatati G, Mirri E, Palazzi M, Vendetti AL, Sette S, Panetta V.
14 National Institute for Research on Food and Nutrition (INRAN), Rome.






