Flaxseed (Linum usitatissimum) is an excellent source of α-linoleic acid, a polyunsaturated n-3 fatty acid (FA), and dietary fibre; it is also the richest source of plant lignan( Reference Thompson, Boucher and Liu 1 ). Secoisolaricirescinol diglucoside (SDG) is the predominant lignan present in flaxseed( Reference Axelson, Sjovall and Gustafsson 2 ). Flaxseed is known to exhibit protective effects against a multitude of chronic ailments, including CVD, stroke and cancer. Investigations have shown that flaxseed reduces the risk of breast and colon cancers, atherosclerosis, insulin-dependent diabetes mellitus and hyperlipoproteinaemia( Reference Prasad 3 – Reference Thompson, Cunnane, Thompson and Cunnane 5 ).
Using the chicken model, we have demonstrated in an earlier study that flaxseed is beneficial in mitigating the severity and reducing the incidence of ovarian cancer( Reference Eilati, Bahr and Hales 6 ). The anti-oncogenic properties attributed to flaxseed are mainly a result of the n-3 FA and the lignan component. n-3 FA inhibit the synthesis of certain arachidonic acid-derived PG, such as PGE2, which are known to be elevated in many cancers. PGE2 can initiate a tumour's growth by binding to its receptors on target tissues and activating signalling pathways which control processes such as cell proliferation, migration, apoptosis and angiogenesis( Reference Ambros and Chen 7 ). Cyclo-oxygenase (COX) enzymes catalyse the synthesis of PG from arachidonic acid. The COX-1 enzyme is expressed constitutively and imparts its homeostatic effects by synthesising PG that maintain the integrity of the stomach lining. COX-1 is elevated with ovarian cancer in humans( Reference Gupta, Tejada and Tong 8 ) as well as laying hens( Reference Eilati, Pan and Bahr 9 – Reference Giles, Olson and Johnson 11 ). COX-2 is an inducible enzyme and is mainly stimulated under inflammatory responses, commonly by inflammatory cytokines( Reference Vane, Bakhle and Botting 12 ). COX-2 is known to be up-regulated in a myriad of cancers, including gastric adenocarcinoma( Reference Ristimäki, Honkanen and Jänkälä 13 ), colorectal cancer( Reference Eberhart, Coffey and Radhika 14 ), breast cancer( Reference Parrett, Harris and Joarder 15 ), prostate carcinoma( Reference Gupta, Srivastava and Ahmad 16 ), cervical cancer( Reference Kulkarni, Rader and Zhang 17 ), pancreatic cancer( Reference Molina, Sitja-Arnau and Lemoine 18 ) and lung cancer( Reference Hida, Kozaki and Muramatsu 19 ).
SDG is a precursor to the phytoestrogens enterolactone (EL) and enterodiol (ED). The initial step includes the conversion of SDG to secoisolaricirescinol by hydrolysis. Gut flora demethylate and dehydroxylate secoisolaricirescinol form ED, which can be oxidised further to EL( Reference Wang, Meselhy and Li 20 ). ED and EL are known to have phytoestrogenic and antioxidant effects. Recent studies( Reference Buck, Zaineddin and Vrieling 21 , Reference Velentzis, Cantwell and Cardwell 22 ) have shown that dietary lignan reduces the risk of post-menopausal breast cancer. In other control studies( Reference McCann, Moysich and Freudenheim 23 , Reference McCann, Muti and Vito 24 ), women who consumed a lignan-rich diet also showed a decreased risk of breast cancer.
There is evidence to suggest that in response to oestradiol (E2), oestrogen receptor α (ERα) stimulates growth and invasion in ovarian cancer cells( Reference O'Donnell, Macleod and Burns 25 ). The anti-oestrogenic property of the phytoestrogen lignans, which can be attributed to their weak antagonism to ER( Reference Mueller, Simon and Chae 26 ), might mitigate the oestrogen-dependent aggressiveness in ovarian cancer cells.
Ovarian cancer is one of the deadliest gynaecological malignancies: it has a 5-year survival rate of 44 % in the United States( Reference Matsuda and Katanoda 27 ). This is a result of its late stage of detection, when the prognosis is poor and treatment options are limited. Although identifying early detection markers is the need of the day, developing a preventative approach is equally essential. Ovarian cancer in the laying hen (Gallus domesticus) develops spontaneously and significantly resembles the human disease with respect to histopathology as well as gross pathology( Reference Barua, Bitterman and Abramowicz 28 – Reference Johnson and Giles 31 ). We have previously shown that a 10 % flaxseed diet decreases the incidence and severity of ovarian cancer in chickens and decreases COX-2 and PGE2 levels in the ovaries( Reference Eilati, Bahr and Hales 6 , Reference Ansenberger, Richards and Zhuge 32 ).
The objective of the present study was to determine the effects of different doses of a flaxseed-supplemented diet on the levels of PGE2, ER and the E2 metabolising enzymes involved in the oestrogen pathway. An earlier study has shown that a 10 % flaxseed diet reduced the aggressiveness of ovarian cancer and was correlated with a decrease in PGE2 ( Reference Eilati, Bahr and Hales 6 ). The present study was therefore designed to determine the optimum dose of flaxseed in the diet to maximise the beneficial effects without being toxic. A maximum dose of 15 % was selected for the study, because an earlier study indicated that a 20 % dose of flaxseed could be hepatotoxic for chickens (DB Hales, K Ansenberger and JM Bahr, unpublished results).
Materials and methods
Reagents
The reagents were obtained from the following: Biotinylated Anti-Rabbit IgG (Vector Laboratories); AffiniPure Alexa 488 Conjugated Donkey Anti-Mouse IgG (Jackson ImmunoResearch); Streptavidin, Alexa Fluor® 488 Conjugate (Life Technologies); the iScript Complementary DNA Synthesis Kit and Ssofast EvaGreen Supermix (SYBR Green; Bio-Rad Laboratories, Inc.); the TUNEL Apoptosis Detection Kit (GenScript); Estramet ELISA Kits for 2-hydroxyestrone (2-OHE1) and 16-hydroxyestrone (16-OHE1) analysis (Immuna Care Corporation); ED and EL standards (Sigma-Aldrich); Genistein–d4 (4-hydroxyphenyl-2,3′,5′,6–d4, 98 % atom% D; C/D/N Isotopes, Inc.); and β-glucuronidase enzyme, ≥ 30 000 units/g solid and SDG standard (Sigma-Aldrich). The HPLC analysis was done on a Shimadzu LC-20A HPLC system (Shimadzu Company). The GC analysis was performed on a GC-2010 (Shimadzu Company). All of the other reagents were those used in previous studies( Reference Eilati, Pan and Bahr 9 ).
Animals
A total of 200 single comb White Leghorn hens (G. domesticus) aged 1·5 years were used for the present study. The hens were exposed to a photoperiod of 17 h light–7 h dark, with lights being turned on at 05.00 hours and turned off at 22.00 hours. Animal management procedures were reviewed and approved by the Institutional Animal Care and Use Committees at the University of Illinois at Urbana-Champaign and Southern Illinois University at Carbondale.
Each group of fifty chickens was fed a different percentage (0, 5, 10 or 15 %) of a flaxseed-supplemented diet for a period of 4 months. At the end of 4 months, the birds were euthanised, and their tissues were harvested.
Diet composition
The composition of the test diets is shown in Table 1. The hens consumed 110 g of food per day and were provided water ad libitum. Their levels of SDG and α-linoleic acid were routinely analysed to ensure that the diets were consistent.
Table 1 Composition (%) of diets* containing different levels of flaxseed

CP, crude protein; TME, true metabolisable energy.
* Each diet contains: limestone (8·75%); dicalcium phosphate (1·50%); iodised salt (0·30%); dl-methionine (0·10%); vitamin premix (0·20%); trace mineral premix (0·15%); and Solka Floc (cellulose; International Fiber Corporation) (0·30%). Vitamin premix provided per kg of diet: retinyl acetate, 4400 IU; cholecalciferol, 25 μg; dl-α-tocopheryl acetate, 11 IU; vitamin B12, 0·01 mg; riboflavin, 4·41 mg; d-Ca-pantothenate, 10 mg; niacin, 22 mg; and menadione sodium bisulfite, 2·33 mg. Trace mineral premix provided as mg/kg of diet: Mn, 75 from MnO; Fe, 75 from FeSO4·7H2O; Zn, 75 from ZnO; Cu, 5 from CuSO4·5H2O; I, 0·75 from ethylene diamine dihydroiodide; and Se, 0·1 from Na2SeO3.
Tissue collection
All of the birds included in the study were euthanised and their tissues were collected on necropsy as described in our earlier study( Reference Eilati, Bahr and Hales 6 ). Histology was performed to confirm that all of their ovaries were normal( Reference Lynch, Mellor, Spare and Inwood 33 ).
Histology and immunofluorescence
Formalin-fixed ovarian tissue was embedded in paraffin, and 5 μm thick sections were cut and mounted on SuperFrost Plus microscope slides. Following deparaffinisation, the slides were rehydrated by being run through xylene and graded ethanol solutions. Hematoxylin and eosin staining was performed on the slides as described by Sheehan & Hrapchak( Reference Sheehan and Hrapchak 34 ). The slides were also used for assessing COX-1 and COX-2 tissue expression as well as localisation by immunofluorescence. Antigen retrieval was performed by using a 0·9 % Antigen unmasking solution (Vector Laboratories) and pressure cooking the slides for 5 min at 20 psi. The slides were allowed to cool, and sections were blocked with a 5 % normal goat serum (COX-1 and COX-2) in 1 × Tris-buffered saline for 1 h at room temperature. Sections were incubated with either an anti-human COX-1 (1:200) or an anti-human COX-2 (1:200) antibody at 4°C overnight. For COX-1 and COX-2 staining, sections were washed with 1 × Tris-buffered saline, incubated with decanted antibody and then incubated with Streptavidin Alexa Fluor 488 conjugate for 30 m at room temperature. Sections were rinsed in 1 × Tris-buffered saline with 0·5 % Tween 20. Slides were mounted using Dapi Fluoromount G (Southern Biotech). All antibody dilutions were made in blocking solution. Control sections were incubated with non-immune anti-rabbit IgG (COX-1 and COX-2).
RNA extraction and analysis
Total RNA was extracted from the ovarian tissue using a Trizol reagent as described previously( Reference Eilati, Pan and Bahr 9 , Reference Eilati, Hales and Zhuge 35 ). Quantification was performed by determining absorbance at A 260, and RNA quality was assessed with the Experion RNA StdSens Analysis Kit. RNA samples were then treated with RQ1 Rnase-free Dnase before a reverse transcription reaction was performed. Complementary DNA was synthesised using the Bio-Rad iScript Kit.
Real-time PCR
Target gene mRNA levels were analysed with real-time quantitative PCR using the CFX384 Real-Time System. Gene-specific primers were used for target as well as reference gene amplification (Table 2). To normalise target gene expression, three housekeeping genes were used. The amplification conditions were: 95°C for 30 s; forty cycles for 10 s at 95°C and 15 s at 58°C with a melt curve measured at 65 to 95°C for 5 s every 0·5°C gradient. A non-template control was run for every target gene that was amplified.
Table 2 Primers used for real-time quantitative PCR

Cell apoptosis and proliferation staining
We assessed 5 μm paraffin-embedded histological sections of chicken ovarian tissue from the control and all of the dosage groups for apoptotic and proliferating cells using TdT-mediated dUTP nick end labelling and proliferating cell nuclear antigen staining, respectively, as described previously( Reference Eilati, Small and McGee 36 ).
PGE2 enzyme immunoassay
A total of six ovary tissue samples from each dietary group were analysed for PGE2 levels using the PGE2 Enzyme Immunoassay Kit (Cayman Chemicals). Snap-frozen ovarian tissues maintained at − 80°C were pulverised on dry ice, resuspended in homogenising buffer and transferred to clean tubes in preparation for solid-phase extraction. Extraction and analysis were carried out as described previously( Reference Eilati, Bahr and Hales 6 ).
2-Hydroxyestrone and 16-hydroxyestrone ELISA assay
Blood samples were collected from all of the chickens during necropsy. Serum samples from the control and each of the dosage groups were analysed for their levels of both the 2-OHE1 and the 16-OHE1 metabolites using the 2-hydroxyestrone and 16-hydroxyestrone Estramet Double ELISA Kit (Immuna Care Corporation). Measurement of the oestrone metabolites is proportional to the concentrations of the oestrone and E2 metabolites combined.
Western blot analysis
Snap-frozen ovary tissue samples (25 μg) from each of the groups were analysed for COX-1/COX-2 expression and normalised to β-actin, as described previously( Reference Eilati, Bahr and Hales 6 ).
GC for fatty acid analysis
The n-3 FA and n-6 FA levels in the chicken ovaries were analysed using a Shimadzu GC-2010 (Shimadzu Company). A solution containing 12·5 μg/ml 17 : 0 standard (Sigma-Aldrich) in methanol was added to the tissue sample (25 μg) as a recovery standard. Extraction and analysis was performed as described previously( Reference Eilati, Small and McGee 36 ).
HPLC for secoisolaricirescinol diglucoside analysis
Secoisolaricirescinol diglucoside extraction
We hydrolysed 1 g of flaxseed with 50 ml of 0·5 m-NaOH at 135 W, microwaving intermittently (30 s on–30 s off) for 3 min. Acidified hydrolysate (pH 3, 2·5 m-H2SO4) was added to 100 ml of methanol to precipitate the carbohydrates and proteins. After centrifugation for 10 min at 3000 rpm, the supernatants were filtered through a 0·22 μm filter and analysed by HPLC.
HPLC analysis
All extracts were analysed by a Shimadzu LC-20A HPLC system (Shimadzu Company) by measuring A 280 nm using a variable wavelength detector. SDG was separated on a Kinetex 2.6u C18 column (2·6 μm, 50 × 3·0 mm; Phenomenex) using a slightly modified version of the gradient elution method( Reference Muir and Westcott 37 ). The column thermostat was set to 40°C, and the injection volume was set to 10 μl. The mobile phase consisted of 1 % acetic acid (solvent A) and methanol (solvent B) mixed A/B (v/v) for 0 min (95:5), 12 min (40:60), 18 min (60:40) and 18·2 min (95:5) and used at a flow rate of 0·4 ml/min. The SDG peaks were identified and quantified by comparison to the SDG standard.
Liquid chromatography tandem MS for enterodiol and enterolactone analysis
Extraction
Tissues from all of the different diet groups were finely homogenised in citrate buffer (25 mm, pH 5·0) while the serum samples were being centrifuged before extraction. We then added four times the volume of methanol to the samples and treated them with ninety units of β-glucuronidase enzyme. The majority of the lipid was removed using n-hexane. A 1:4 solution of the EL and ED standards were prepared in methanol. Internal standard genistein–d 4 was added to all of the samples and standards. The supernatant was applied to a preconditioned solid phase extraction (SPE) cartridge, and bound compounds were then eluted in methanol. The eluted sample was concentrated, and the residue was then reconstituted with the loading solvent and acetonitrile–water (1:3).
Liquid chromatography MS/MS analysis
All of the extracts were analysed by a Shimadzu Prominence UFLC-8080 system (Shimadzu Company). The ED and EL were separated from the other compounds in the extract by chromatography on a Water XTerra MS C18 column (3·0 μm, 2·1 × 50 mm). The mobile phase consisted of 0·2 % formic acid in water (solvent A) and acetonitrile (solvent B) mixed A/B (v/v) for 0·3 min (80:20), 1·8 min (10:90), 1·81 min (80:20) and 2·50 min (end) and used at a flow rate of 0·6 ml/min and an injection volume of 5 μl. The compound peaks were identified and quantified by comparison with those of standard ED, EL and genistein–d 4. The molecules with an m/z ratio of 301, 296·9 and 273·1 corresponded to ED, EL and genistein–d 4, respectively.
Statistics
All of the experiments were performed in duplicate, and the target values were normalised to control. Statistical calculations were done using GraphPad Instat software by employing one-way ANOVA analysis. A P< 0·05 was considered significant, whereas a P< 0·01 was considered highly significant.
Results
ELISA analysis for PGE2 in ovaries
PGE2 levels in the ovarian tissue decreased with an increase in the percentage of flaxseed in the diet. PGE2 levels were significantly lower in the 15 % diet group as compared to the control and 5 % groups (Fig. 1(A)).
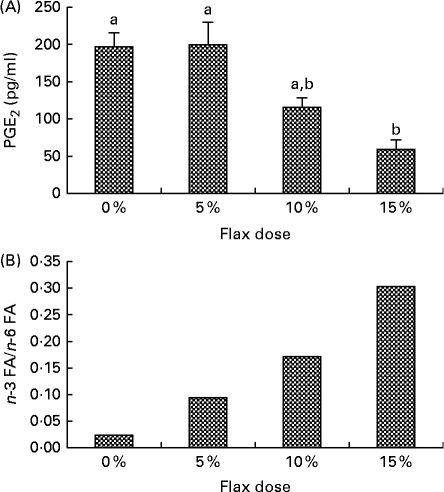
Fig. 1 Flaxseed dose-dependent alteration in the levels of n-3 fatty acids (FA) and PG. (A) PGE2 levels were assessed in the ovary tissue of the control and flaxseed-fed hens using an ELISA assay kit. Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·01; one-way ANOVA). (B) Ovary tissue levels of FA and n-6 FA measured using GC. Values are means (n 6).
Hematoxylin and eosin staining on ovary sections
Ovary tissues from all of the diet groups were assessed using hematoxylin and eosin staining. Ovaries from all of the diet groups appeared normal histologically (Fig. 2(A)).

Fig. 2 Hematoxylin and eosin (H&E) staining on ovarian section and immunofluorescence staining for cyclo-oxygenase (COX)-1 and COX-2 proteins. (A) Ovary tissue sections from the control (a), 5 % flaxseed (b), 10 % flaxseed (c) and 15 % flaxseed (d) groups were stained using H&E and assessed histologically under 100 × magnification, n 3. (B) and (C) Ovary sections from the control (a), 5 % flaxseed (b), 10 % flaxseed (c) and 15 % flaxseed (d) groups were stained for COX-1 and COX-2 proteins, respectively, and observed at 200 × magnification. (Insert: non-immune IgG, 200 × ), n 3.
Cyclo-oxygenase-1 and cyclo-oxygenase-2 enzyme expression
COX-1 expression was observed in the granulosa cells and also in some stromal cells, and COX-2 was predominantly expressed in the granulosa cells and the ovarian surface epithelium (Fig. 2(B) and (C)). COX-1 protein was expressed consistently across all of the groups (Fig. 2(B)). COX-2 protein was deceased in the 15 % flaxseed diet group (Fig. 2(C) and (D)). COX-1 mRNA and protein levels in the ovaries were not affected by the amount of flaxseed (Fig. 3(A) and 3(B)). COX-2 mRNA levels appeared to be consistent across all of the different diet groups (Fig. 3(C)), whereas COX-2 protein expression was significantly reduced in the ovaries of the chickens that were fed a 15 % flaxseed diet (Fig. 3(D)).
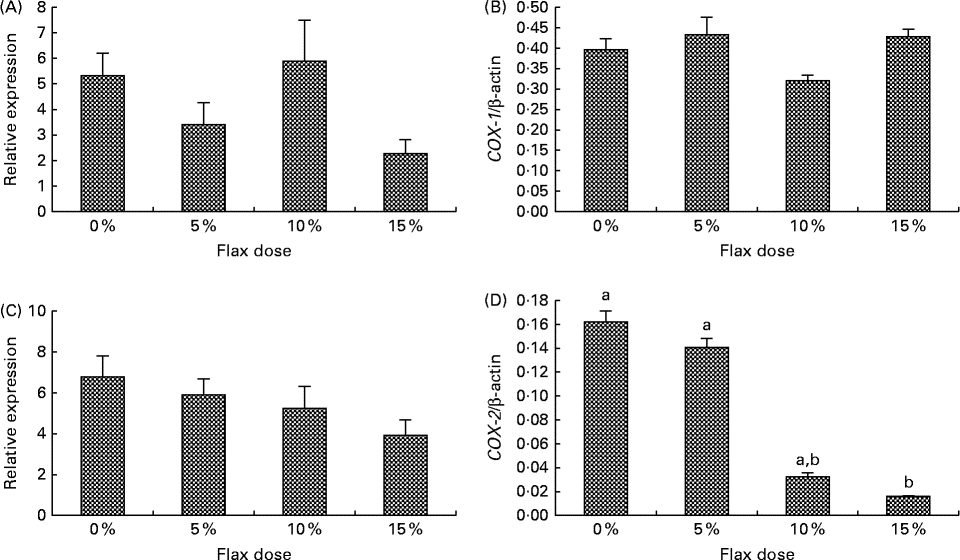
Fig. 3 Cyclo-oxygenase (COX)-1 and COX-2 protein and mRNA expression in the ovaries. (A) and (B) COX-1 enzyme mRNA expression was measured using real-time PCR, and protein levels were analysed using Western blotting in control and flaxseed-fed chickens, respectively. Values are means (n 6), with standard deviations represented by vertical bars. (C) and (D) COX-2 enzyme mRNA expression was measured using real-time PCR, and protein levels were analysed using Western blotting in control and flaxseed-fed chickens, respectively. Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA).
n-3 Fatty acid and n-6 fatty acid levels in ovaries
n-3 FA and n-6 FA analysis revealed that the higher the dose of flaxseed in the diet was, the higher the level of n-3 FA incorporation in the chicken ovaries. The n-3 FA:n-6 FA ratio increased with higher doses of flaxseed (Fig. 1(B)).
Cell proliferation and apoptosis assay
TdT-mediated dUTP nick end labelling staining for the detection of apoptotic cells indicated that there was no significant difference with respect to the number of apoptotic cells in the ovarian tissue between the birds in the different dietary groups (Fig. 4(B) and (D)). Similarly, proliferating cell nuclear antigen expression was not affected by the flaxseed diet (Fig. 4(A) and (C)). As a result, the ratio of proliferating to apoptotic cells in the ovaries was comparable across all of the diet groups (Fig. 4(E).
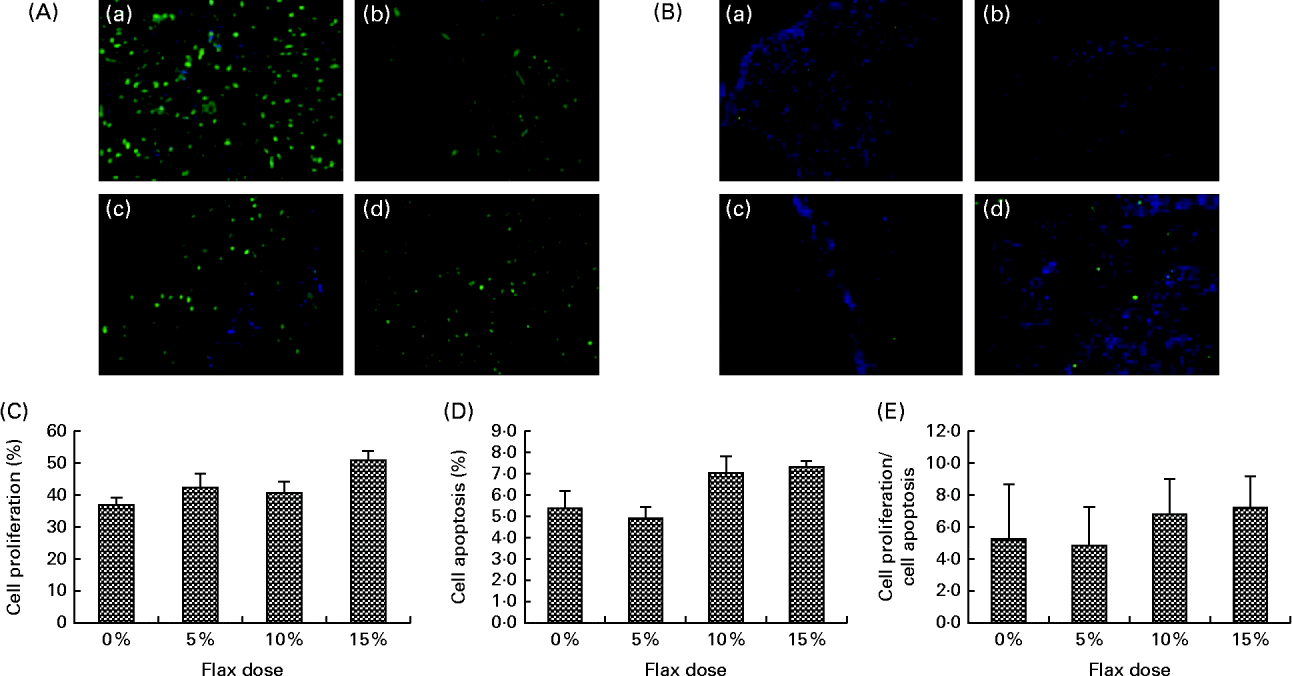
Fig. 4 Effect of 5, 10 and 15 % flaxseed-supplemented diet on cell proliferation and apoptosis in the ovaries. (A) Green fluorescent staining indicated proliferating cellular nuclear antigen (PCNA)-positive proliferating cells for ovary sections from the control (a), 5 % flaxseed (b), 10 % flaxseed (c) and 15 % flaxseed (d) groups. (B) Green fluorescent staining indicated TdT-mediated dUTP nick end labeling (TUNEL)-positive apoptotic cells for ovary sections from the control (a), 5 % flaxseed (b), 10 % flaxseed (c) and 15 % flaxseed (d) groups observed at 200 × magnification. (Insert: no primary control, 200 × magnification). (C) Graph indicating percentage of cell proliferation. (D) Graph indicating percentage of cell apoptosis. (E) Graph indicating ratio of cell proliferation to cell apoptosis in normal ovary tissue from chickens (n 3).
Secoisolaricirescinol diglucoside concentration in different diets
The level of SDG was measured in the 0, 5, 10 and 15 % flaxseed diet groups using liquid chromatography. As expected, the level of SDG increased proportionally with the percentage of flaxseed (Fig. 5(A)). The SDG concentrations were 0·56, 1·12 and 1·65 mg/g in the 5, 10 and 15 % flaxseed diets, respectively.
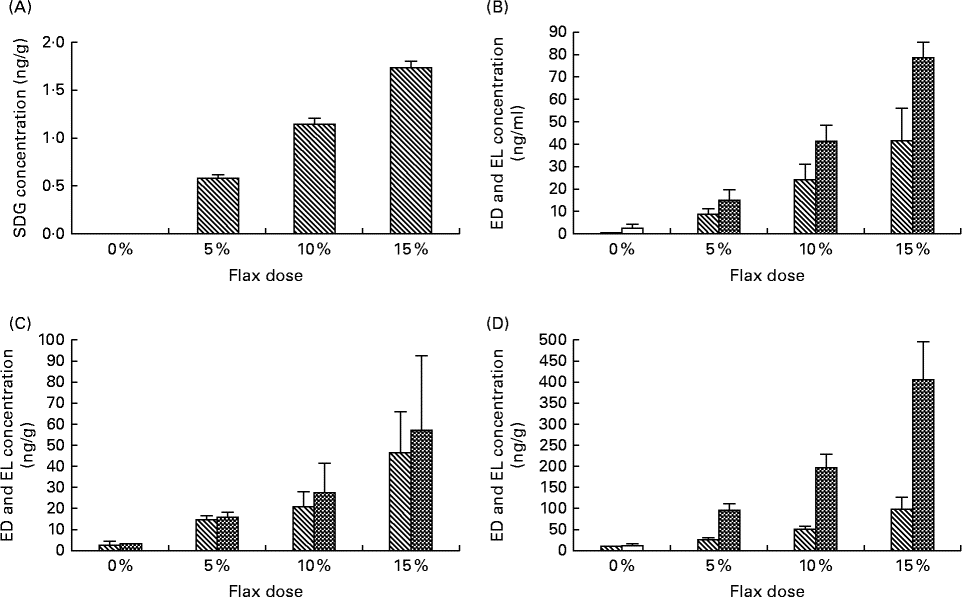
Fig. 5 Levels of secoisolaricirescinol diglucoside (SDG), enterodiol (ED) and enterolactone (EL) in the diets and tissues, respectively. SDG is hydrolysed and ultimately metabolised by the gut flora to EL and ED. (A) Levels of SDG were measured in the varying percentage of flaxseed-supplemented diets using liquid chromatography (LC). (B)–(D) Levels of ED (![]() ) and EL (
) and EL (![]() ) were analysed in the different flaxseed-supplemented diet groups using LC, MS and MS analysis in the serum, ovary and liver tissues, respectively (n 6).
) were analysed in the different flaxseed-supplemented diet groups using LC, MS and MS analysis in the serum, ovary and liver tissues, respectively (n 6).
Enterolactone and enterodiol levels in the tissue were proportional to the flaxseed content of the diet
The concentrations of the phytoestrogen lignans EL and ED were measured in the serum, ovary and liver samples from birds that were fed different doses of flaxseed in their diets. The levels of EL and ED in serum (Fig. 5(B)), ovary (Fig. 5(C)) and liver (Fig. 5(D)) tissues increased proportionally to the increase in flaxseed dose. EL levels were considerably higher than those of ED in the liver tissue for all of the dosage groups, whereas in the serum, EL levels were lower than ED for all of the doses. ED and EL levels were comparable in the ovarian tissues.
Flax diet influences levels of oestrogen metabolites
Serum samples from the different diet groups were analysed for their levels of certain oestrogen metabolites. The level of 16-OHE1 was considerably reduced in the flax-fed birds as compared to those in the control group, whereas the level of 2-OHE1 was substantially increased in the 15 % flax diet group (Fig. 6(A)). The clinically relevant 2-OHE1:16-OHE1 ratio was seen to increase significantly in the serum of the birds in the 15 % dosage group (Fig. 6(B)).
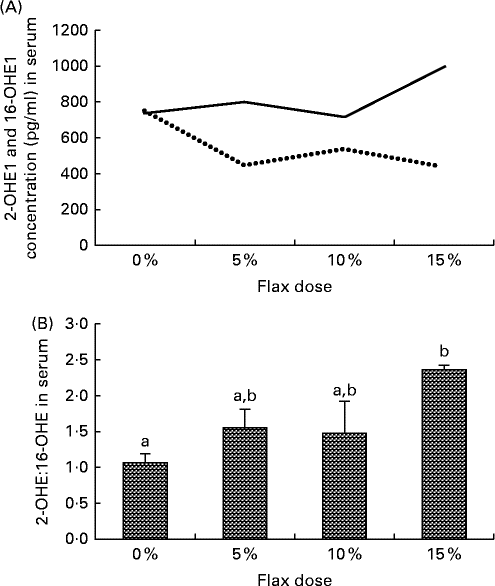
Fig. 6 Serum levels of 2-hydroxyestrone (2-OHE1) and 16-hydroxyestrone (16-OHE1). (A) 2-OHE1 (![]() ) and 16-OHE1 (
) and 16-OHE1 (![]() ) were analysed in the serum samples from control and flaxseed-supplemented diet groups using the 2-hydroxyestrone and 16-hydroxyestrone Estramet Double ELISA Kit (n 3 for the 5, 10 and 15 % groups; n 9 for the control group). (B) 2-OHE1:16-OHE1 ratio in the serum samples of chickens fed varying doses of flaxseed-supplemented diets. Values are means, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA).
) were analysed in the serum samples from control and flaxseed-supplemented diet groups using the 2-hydroxyestrone and 16-hydroxyestrone Estramet Double ELISA Kit (n 3 for the 5, 10 and 15 % groups; n 9 for the control group). (B) 2-OHE1:16-OHE1 ratio in the serum samples of chickens fed varying doses of flaxseed-supplemented diets. Values are means, with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA).
Cytochrome p450 enzymes expression
CYP1B1 (cytochrome p450, family 1, subfamily B, polypeptide 1), CYP1A1 (cytochrome p450, family 1, subfamily A, polypeptide 1) and CYP3A4 (cytochrome p450, family 3, subfamily A, polypeptide 4) enzymes metabolise E2 to 4-hydroxyestradiol (4-OHE2)( Reference Tsuchiya, Nakajima and Kyo 38 ), 2-hydroxyestradiol (2-OHE2) and 16-hydroxyestradiol (16-OHE2; Estriol), respectively( Reference Yager and Leihr 39 ). Ovarian tissue expression of CYP1B1 and CYP3A4 mRNA decreased proportionally with the dose of flaxseed in the diet (Fig. 7(A) and (B)). CYP1A1 mRNA expression in the liver increased with an increase in the amount of flaxseed in the diet (Fig. 8(A)), but its expression was negligible in the ovary. The mRNA expression of CYP3A4 and CYP1B1 in the liver remained unchanged between the groups (Fig. 8(B) and (C), respectively).
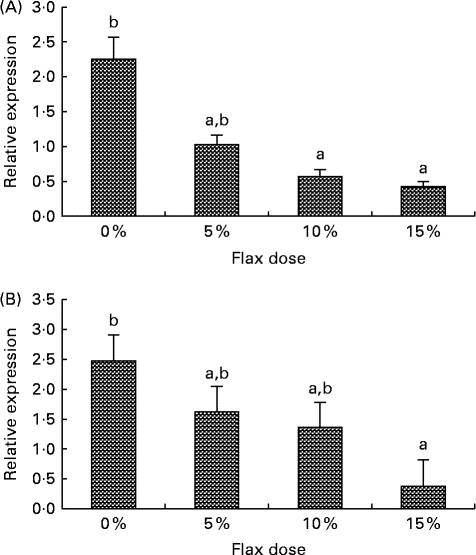
Fig. 7 Cytochrome p450, family 1, subfamily B (CYP1B1) and cytochrome p450, family 3, subfamily A (CYP3A4) enzyme mRNA expression in the ovary tissue. (A) CYP1B1 mRNA expression was measured using real-time quantitative PCR (qPCR). Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA). (B) CYP3A4 mRNA expression was measured using real-time qPCR. Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·01; one-way ANOVA).
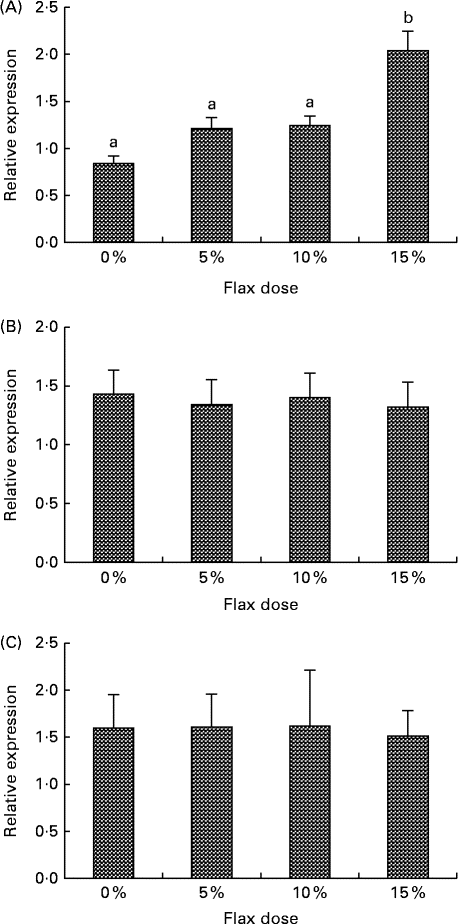
Fig. 8 Cytochrome p450, family 1, subfamily A (CYP1A1) (A), cytochrome p450, family 1, subfamily B (CYP1B1) (B) and cytochrome p450, family 3, subfamily A (CYP3A4) (C) mRNA expression in the liver. mRNA levels were quantified using real-time quantitative PCR. Values are means (n 6), with standard deviations represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA).
Oestrogen receptor expression decreases with flax diet
An increase in the dosage of flax in the diet was observed to parallel a decrease in ERα mRNA expression (Fig. 9(A)). mRNA levels were significantly decreased in the 10 and 15 % flaxseed diet groups as compared to the control group. ERα protein was predominantly expressed in the granulosa cells of the chicken ovarian follicle and appeared to decrease in the 15 % flaxseed diet group (data not shown).
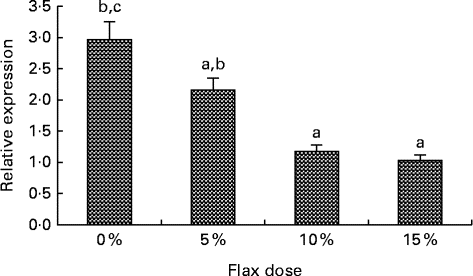
Fig. 9 Oestrogen receptor α (ERα) expression in the ovaries. ERα mRNA levels were analysed using real-time quantitative PCR in the different diet groups. Values are means (n 6), with standard deviations represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA).
Discussion
In our previous studies, we demonstrated that feeding 2·5-year-old chickens a 10 % flaxseed diet for 1 year reduced the severity but not the incidence of ovarian cancer( Reference Ansenberger, Richards and Zhuge 32 ). When younger (22-week-old) chickens in their first lay were fed a flaxseed diet for 4 years, the severity as well as incidence of ovarian cancer was found to be reduced( Reference Eilati, Bahr and Hales 6 ). These findings suggest that flaxseed diet is most effective in combating ovulation-induced inflammation when it is consumed at the start of ovulation and continued throughout the reproductive life. The objective of the present study was to determine the optimum dose of flaxseed in the diet by analysing the levels of surrogate end points that are representative of its effect on ovarian cancer initiation and progression.
We previously established that COX-2 enzyme expression increases with age, and the consumption of flaxseed decreases COX-2 expression( Reference Eilati, Hales and Zhuge 35 ). COX enzymes act on n-6 FA to generate series-2 pro-inflammatory PG such as PGE2, and their action on n-3 FA generates series-3 anti-inflammatory PG( Reference Larsson, Kumlin and Ingelman-Sundberg 40 ). Flaxseed is a rich source of n-3 FA, which competes as a substrate with n-6 FA for COX enzymes. This in turn leads to a possible decrease in the synthesis of pro-inflammatory PG, which are known to stimulate the release of inflammatory cytokines( Reference Sheibanie, Yen and Khayrullina 41 ) that are associated with cancer. In the present study, we observed that an increase in the percentage of flaxseed in the diet led to an increase in the n-3 FA:n-6 FA ratio and a concurrent statistically significant reduction in the ovarian PGE2 levels in hens that were fed a 15 % flaxseed diet (Fig. 1(B)). COX-1 mRNA and protein expression was not altered significantly with a flaxseed diet, which was consistent with our previous finding( Reference Eilati, Bahr and Hales 6 ) that suggested a flax diet did not have any effect on COX-1 expression in normal young chickens (Figs. 2 and ). Also consistent with our previous results, we observed that COX-2 protein expression decreased significantly with a 10 and 15 % flax diet (Fig. 3(D)). With the present study, we confirm our previous results( Reference Eilati, Bahr and Hales 6 , Reference Eilati, Hales and Zhuge 35 ) and take a step further by demonstrating that not only does a flax diet decrease the levels of inflammatory PGE2 ( Reference Eilati, Hales and Zhuge 35 ), but it does so in a dose-dependent fashion. This further suggests that the anti-apoptotic and pro-angiogenic( Reference Arico, Pattingre and Bauvy 42 ) COX-2 enzyme can be targeted by a flaxseed diet.
Phase I of oestrogen metabolism begins with hydroxylation, which is initiated by the CYP (cytochrome p450 enzymes) CYP1B1, CYP1A1 and CYP3A4 acting at different positions on E2 to form 4-OHE2, 2-OHE2 and 16-OHE2, respectively. Phase II of oestrogen metabolism includes conjugation by glucuronidation, sulfation or O-methylation by catechol-O-methyl transferase( Reference Tsuchiya, Nakajima and Yokoi 43 ). The 4-OHE2, 2-OHE2 and 16-OHE2 metabolites are known to have varying degrees of oestrogenicity themselves( Reference Yager and Leihr 39 ). The corticle follicles and small follicles in the chicken ovary produce E2 ( Reference Lee and Bahr 44 ), which thus provides a substrate for these metabolising enzymes. The 4-OHE2 is further oxidised to oestrogen-3, 4-quinones, which can form depurinating DNA adducts that can cause mutations that result in the initiation of cancer( Reference Zhuge, Lagman and Ansenberger 45 ). The 16-OHE2 is known to be pro-carcinogenic( Reference Telang, Suto and Wong 46 ), and evidence suggests that the 16-OHE2 increases the risk of breast cancer because it increases the rate of cell proliferation by binding covalently to the ER( Reference Davis, Bradlow and Wolff 47 ). The 2-OHE2 has a relatively low binding affinity for ER and has no carcinogenicity, unlike some other E2 catechols. For example, Liehr et al. demonstrated that the 2-OHE2 could not induce kidney tumours in hamsters, whereas the 4-OHE2 could( Reference Liehr, Wan-Fen and Sirbasku 48 ). The 2-OHE2 is also known to protect against hormone-dependent cancers by interfering with the binding of sex hormones to the sex steroid binding protein( Reference Martin, Haourigui and Pelissero 49 ). It is also converted to 2-methoxyestradiol at a much faster rate by catechol-O-methyl transferase, as compared to the other catechol oestrogens. The 2-methoxyestradiol has anti-proliferative and anti-angiogenic properties( Reference LaVallee, Zhan and Herbstritt 50 ).
The serum analysis in the present study suggested that the 2-OHE1 increased considerably in the 15 % flax diet, but the 16-OHE1 decreased in all of the flax diet samples and significantly in the 15 % group. A decrease in the 2-OHE1:16-OHE1 ratio can be correlated with an increased risk for a number of cancers, including breast, endometrium and cervical( Reference Bradlow, Hershcopf and Martucci 51 – Reference Bell, Crowley-Nowick and Bradlow 54 ). In the present study, we observed that the 2-OHE1:16-OHE1 ratio increased significantly in the 15 % flax diet as compared to the control group. Consistent with the 16-OHE1 levels, we observed that a flaxseed diet led to a down-regulation of CYP3A4 mRNA expression in the ovaries of chickens (Fig. 7(B)). CYP1A1 expression could not be detected in the ovaries of either control or flax-fed birds, but its expression in the liver increased with a flax diet (Fig. 8(A)). The mechanism through which flaxseed regulates the expression of these CYP enzymes is currently being investigated.
E2 induces CYP1B1 expression in the female reproductive tract as well as in ER-positive breast cancer cells and ER-overexpressing endometrial cancer cells( Reference Tsuchiya, Nakajima and Kyo 38 ). We have previously shown that CYP1B1 expression is up-regulated in cancerous chicken ovaries and is highest in the post-ovulatory follicle 3, which is usually buried inside the ovary, where the E2 concentration is high( Reference Zhuge, Lagman and Ansenberger 45 ). E2 levels in the ovary are about 100-fold higher than circulating levels( Reference Ho 55 ). The high oestrogen levels in the ovary could not only facilitate increased CYP1B1 expression, but they could also provide a large substrate pool for CYP1B1, which would result in an increase in genotoxic 4-OHE2 catechol levels. Different doses of flaxseed in the diet did not alter E2 concentrations in the chicken sera (data not shown); however, concentrations in the ovary were not measured. In the present study, CYP1B1 mRNA expression was seen to decrease in the ovary with a flax diet in a dose-dependent manner (Fig. 7(A)). A decrease in CYP1B1 expression in the ovary with a flax diet demonstrates the anti-oestrogenic and possibly anti-oncogenic properties of flaxseed.
Oestrogen stimulates cell proliferation and can promote lymph node metastasis in ER-positive ovarian cancer tumours but not in ER-negative cancers( Reference Spillman, Manning and Dye 56 ), which demonstrates the significance of ER in promoting tumour growth and mobilisation in ovarian cancer. CYP1B1 is primarily regulated by the aryl hydrocarbon receptor and its dimerising partner, the aryl hydrocarbon receptor nuclear translocator( Reference Zhang, Savas and Alexander 57 ). Tsuchiya et al. ( Reference Tsuchiya, Nakajima and Kyo 38 ) have shown that E2 can up-regulate CYP1B1 through ER independent of the aryl hydrocarbon receptor, which further suggests that ER plays a role in inducing oestrogen-mediated toxicity. We observed that feeding hens a flaxseed-supplemented diet led to a dose-dependent decrease in the expression of ERα mRNA (Fig. 9). A decrease in ERα expression did not alter the egg-laying frequency of these birds, which indicates that flaxseed does not impair the normal functioning of the ovary. We have previously published that hens that were fed a diet of 10 % flaxseed had an average egg-laying frequency of about 71 % per week( Reference Eilati, Hales and Zhuge 35 ). From one of our recent studies, we know that hens that were fed a 15 % flaxseed diet produced eggs at a frequency of 69·4 % per week, whereas the control group produced eggs at 72·23 % per week. These data clearly indicate that the different doses of flaxseed diet do not affect the egg-laying frequency of the birds. The effect of a flaxseed diet on ERα could be a result of the flaxseed lignan SDG, which is ultimately converted to the weakly oestrogenic/anti-oestrogenic EL and ED. The partial agonist/antagonist property of these compounds could be responsible for decreasing ER activity and eventually its expression, given that ER transactivates its own expression( Reference Hales, Zhuge and Lagman 10 ).
In our previous long-term study, we showed that a 10 % flaxseed-supplemented diet decreased the levels of inflammatory PGE2, which also correlated with a reduced incidence and severity of ovarian cancer( Reference Eilati, Bahr and Hales 6 ). We observed that PGE2 levels were also higher in older chickens regardless of pathology, which suggests that there are other factors that contribute to ovarian cancer initiation and dissemination. The present findings indicate that a 15 % flaxseed dose is most effective in decreasing the levels of the highly potent inflammatory PG PGE2, carcinogenic oestrogen metabolites and ER in the ovaries of normal young chickens without having any toxic effects. The findings suggest that a 15 % flaxseed diet can beneficially affect a number of clinically relevant surrogate endpoints that are implicated in cancer. This is especially significant because it was recently demonstrated that ER-positive ovarian cancer is more chemo-resistant than ER-negative ovarian cancer, which makes it extremely challenging to treat( Reference Brasseur, Leblanc and Fabi 58 ). The ER-positive status of these tumours possibly plays an important role in the progression of the disease.
Taken together, the chemo-preventative properties of flaxseed alleviate the expression of inflammatory PGE2, ER, toxic oestrogen metabolites and enzymes which might contribute to the development of ovarian cancer. As a result, the present data strongly supports early dietary intervention using flaxseed as a preventative approach for ovarian cancer.
Acknowledgements
We are extremely grateful to the poultry farm management, including Chet Utterback, Pam Utterback, Doug Hilgendorf and Carl Parsons, for helping us with the diet formulations. Dr Richard van Breeman and his MS lab members helped us extensively with the enterolactone and enterodiol analysis at the University of Illinois, Chicago. We also thank Dr Janice Bahr for her continued guidance and Dr Karen Hales for her valuable suggestions.
The present work was funded by the NIH RO1 AT00408 grant.
The study was conceptualised by D. B. H. in order to understand the effect of different doses of flaxseed on inflammation/oxidative stress, PG-associated pathways and oestrogen-associated pathways. It was included as a specific aim in the NIH RO1 AT00408 grant.
The immunohistochemistry (COX-1, COX-2) and quantitative PCR (CYP1A1 (liver), CYP1B1 (liver and ovary), CYP3A4 (liver and ovary) and ERα) experiments were performed by A. D. In addition, A. D. processed the serum samples and wrote and formatted the manuscript. M. A. G. F. performed the quantitative PCR and Western blotting for COX-1 and COX-2. E. E. measured the PGE2 levels in the ovary tissue. S. M. performed the proliferating cell nuclear antigen and TdT-mediated dUTP nick end labelling staining on the ovary tissue sections. C. G. processed the samples for the enterolactone and enterodiol analysis and also measured the SDG levels in the diets. C. S. analysed the n-3 FA and n-6 FA levels in the ovaries. T. K. analysed the 2-OHE1 and 16-OHE1 in the serum samples.
The authors have no known conflicts of interest.
The authors have nothing to disclose.














