Clinical evidence of the efficacy of some probiotics in the improvement of symptoms in irritable bowel syndrome (IBS) has recently emerged(Reference Quigley and Flourie1–Reference Camilleri3). The variability of the effects reported has highlighted the product and strain specificity, which is supported by the high variability in their properties (anti-inflammatory activity, effect on motility or visceral sensation)(Reference Quigley and Flourie1, Reference Spiller2). However, due to the complexity of the pathophysiology of IBS which remains poorly understood to date, specific research is required to demonstrate these benefits in a more general population with minor digestive complaints. Only a few studies have been focused on the effects of probiotics on gastrointestinal (GI) well-being in a general population. Most of these studies have investigated the effect of probiotics on bowel habits and were conducted in small sample sizes (from thirty to eighty subjects). Improvement of stool frequency and/or volume or weight, and stool consistency has been shown(Reference Johansson, Nobaek and Berggren4–Reference Matsumoto, Takada and Shimizu8). Unfortunately, only one study(Reference Johansson, Nobaek and Berggren4) has investigated the impact on a specific digestive symptom (i.e. flatulence), showing a positive effect on this parameter; therefore, the real end-benefits of such functional changes for the consumers are open to question.
Studies in human subjects have been designed to investigate the effect of a fermented milk (FM) product containing Bifidobacterium lactis DN-173 010 and yoghurt starters Lactobacillus bulgaricus and Streptococcus thermophilus. The ability of the strain B. lactis DN-173 010 to survive through the entire digestive tract has been shown in human subjects(Reference Duez, Pelletier and Cools9–Reference Rochet, Rigottier-Gois and Ledaire12), demonstrating the ability of this strain to exert its health effect all along the GI tract. This probiotic food has been shown to improve digestive comfort and symptoms in IBS with predominant constipation (IBS-C)(Reference Agrawal, Houghton and Morris13, Reference Guyonnet, Chassany and Ducrotté14) as well as transit time in both healthy(Reference Marteau, Cuillerier and Méance15–Reference Méance, Cayuela and Turchet17) and IBS-C populations(Reference Agrawal, Houghton and Morris13). These data support the ability of this specific FM to improve some GI functions in both healthy and IBS populations.
While the effect of probiotics on GI well-being and digestive symptoms has been widely investigated in IBS, only the impact on bowel function has been studied in healthy populations. The present randomised, double-blind, controlled clinical trial was designed to assess the effects of a FM on GI well-being, digestive symptoms and health-related quality of life (HRQoL) among adult women without GI disorders.
Experimental methods
Study population
Subjects (n 240) were recruited in Germany from one clinical centre (Harrison clinical centre, Munich). They were all women, aged 18–60 years, with normal weight or overweight (BMI 18–30 kg/m2), without a diagnosis of any digestive disease including functional bowel disorders such as IBS. Only individuals with a bowel movement frequency within the normal range (3–21 per week) were recruited. At study entry, a screening questionnaire was used to determine the frequency of four different digestive symptoms (i.e. discomfort or abdominal pain, bloating, flatulence/passage of gas, borborygmi/rumbling stomach) in the past month. Frequency of each symptom was assessed using a six-point Likert scale (never = 0; 1 d per month = 1; 2–3 d per month = 2; 1 d per week = 3; >1 d per week = 4; every day = 5) leading to an overall symptom score ranging from 0 (no symptoms) to 20 (all symptoms every day). To be considered for inclusion, all subjects had to have a digestive symptoms score between 8 and 16 or at least one digestive symptom with a score >4. Subjects were usual consumers of dairy products.
Subjects were excluded from the study if they had any significant systemic disease or if they were under prescription for medication for digestive symptoms. Antibiotic ingestion within the month before the entry in the study was also an exclusion criterion.
Individuals with known lactose intolerance or with dietary habits which might interfere with the assessment of the study product (for example, slimming or vegetarian diets) or known allergy to the study product components were also excluded.
Throughout the study, the subjects were not allowed to consume any probiotic (including food supplements) or fermented dairy product other than those provided. They were encouraged to continue with all the other aspects of their dietary and physical exercise habits.
Study protocol
The study was a single-centre, randomised, double blind, controlled, parallel-group study assessing the effect of daily consumption of a FM containing B. lactis DN-173 010 (FM group) v. a non-fermented dairy product (control group).
During a 2-week run-in period, baseline values were obtained for the outcome parameters with a weekly assessment of frequency of digestive symptoms and bowel function (bowel movement and stool consistency). The questionnaire used for the assessment of the frequency of digestive symptoms (abdominal pain/discomfort, bloating, flatulence/passage of gas and borborygmi/rumbling stomach) during this run-in period was different to the screening questionnaire used at study entry. The frequency of each individual digestive symptom was evaluated weekly with a five-point Likert scale that ranges from 0 (never) to 4 (every day of the week) leading to a composite score ranging from 0 (no symptoms) to 16 (all symptoms every day). Only subjects having a mean score between 2 and 12 during this run-in period and also meeting the other randomisation criteria (normal bowel movement frequency, no consumption of antibiotics) were randomised to consume during 4 weeks two products per d (one at breakfast and one at the evening meal) in a double-blind trial. After this first 4-week period, subjects entered a 4-week period of wash-out without consumption of any specific product (for study flow design, see Fig. 1). Thereafter, subjects started another 4-week period in which all subjects consumed the test product (two servings per d). During this open period, all subjects were informed that they consumed the test product without knowing the commercial name of the product. The purpose of this open period was to specifically investigate the potential placebo effect. Results of this period will be published elsewhere.

Fig. 1 Study flow design. ITT, intention-to-treat population (defined as all randomised subjects); FM, fermented milk; FAS, full analysis set population (defined as all randomised subjects having any efficacy data available under product consumption).
The study protocol was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee Bayerische Landesaerztekammer (Munich, Germany). All volunteers gave written informed consent before inclusion in the study.
Study products
The test product was a FM (Activia®; Danone) containing B. lactis DN-173 010 (strain number I-2494 in the French National Collection of Cultures of Micro-organisms (CNCM), Paris, France) together with the two classical yoghurt starters, S. thermophilus (CNCM strain number I-1630) and L. bulgaricus (CNCM strain numbers I-1632 and I-1519), and Lactococcus cremoris (CNCM strain number I-1631). The test product contains 1·25 × 1010 colony-forming units (cfu) B. lactis DN-173 010 per serving and 1·2 × 109 cfu S. thermophilus and L. bulgaricus per serving.
The control product was a milk-based non-fermented dairy product without probiotics and with a low content of lactose ( < 4 g/serving), which is similar to the content of lactose in the test product. The control product was acidified using an enzymic process which mimics the acidification process that occurs during the fermentation of milk by B. lactis and yoghurt symbiosis. Both the test and control products were without flavour and had a similar appearance, texture and taste. Each serving contained 125 g. Both products were specifically prepared for the study and provided by Danone Research (Palaiseau, France).
Compliance was calculated on the basis of the data reported by subjects in their diaries and the number of non-used servings returned.
Assessments and study endpoints
Gastointestinal well-being
The overall assessment of GI well-being was self-evaluated by subjects weekly from the first week of product consumption (week 1) to the end of the study with the following question: ‘How do you consider in the past seven days, your GI well-being (intestinal transit, stool frequency and consistency, abdominal pain/discomfort, bloating, flatulence/passage of gas, borborygmi/rumbling stomach) compared to the period before beginning the consumption of the study product?’. This global rating is a combined scale leading to a fifteen-point Likert scale ( − 7, 0,+7). In a first step, subjects indicate if their GI well-being has remained the same, improved, or worsened (three-point Likert scale) and in a second step, subjects with improving or worsening symptoms rate the degree of change on a seven-point scale. This scale was adapted from the scale developed by Guyatt et al. (Reference Guyatt, Deyo and Charlson18) and used in IBS studies(Reference Irvine, Whitehead and Chey19).
As recommended in guidelines for the design of treatment trials for functional GI disorders(Reference Irvine, Whitehead and Chey19), each subject was classified as a responder or a non-responder. The definition of a responder was based on the recommendations for global assessment of symptom relief in IBS trials(Reference Irvine, Whitehead and Chey19), which is the only available recommendation for such kinds of assessment. A responder was defined as a subject having an improvement in their GI well-being, i.e. answering ‘improved’ on the three-point Likert scale, on at least 2 weeks over the 4-week double-blind period of product consumption. The same criterion was applied to define the responders for the 4-week wash-out.
Digestive symptoms
The frequency of four individual digestive symptoms (abdominal pain/discomfort, bloating, flatulence/passage of gas and borborygmi/rumbling stomach) was evaluated weekly with a five-point Likert scale that ranges from 0 (never) to 4 (every day of the week) throughout the study. A composite score was calculated ranging from 0 to 16.
Bowel function
Bowel movements were reported daily throughout the study as well as stool consistency for each stool passed according to the Bristol stool form scale(Reference Heaton, O'Donnell and Braddon20). In order to assess the normalisation of stool consistency, scores of stool consistency were recoded as follow: 0 = type 4 (like a sausage or snake, smooth and soft); 1 = types 3 (like a sausage but with cracks on surface) and 5 (soft blobs with clear-cut edges); 2 = types 2 (sausage shaped but lumpy) and 6 (fluffy pieces with ragged edges, a mushy stool); 3 = types 1 (separate hard lumps like nuts, difficult to pass) and 7 (watery, no solid pieces, entirely liquid).
Health-related quality of life
HRQoL of subjects was assessed by self-administration of two questionnaires, the Food and Benefits Assessment (FBA)(Reference Guyonnet, Chassany and Picard21) and the Psychological General Well-Being Index (PGWBI)(Reference Dupuy, Wenger, Mattson, Furberg and Elinson22). The questionnaires were completed at baseline and after 4 and 8 weeks corresponding to the end of the periods of product consumption and of the wash-out period, respectively.
The FBA questionnaire has been developed and validated according to international recognised guidelines used for patient-reported outcomes and aims at assessing specifically the benefits of a food or a diet on HRQoL. This questionnaire comprises forty-one items, making it possible to calculate scores for seven dimensions (snacking, vitality, well-being, physical appearance, aesthetics, digestive comfort and disease prevention). The scores range from 0 to 100 (best).
The PGWBI (a generic questionnaire) measures psychological well-being and distress and is composed of twenty-two items which constitute six dimensions (anxiety, depression, self-control, positive well-being, general health and vitality). The scores of all dimensions can be summarised to provide a global score(Reference Dupuy, Wenger, Mattson, Furberg and Elinson22). The scores range from 0 to 100 (best).
The FBA digestive comfort dimension score and the PGWBI global score were defined as the main scores for HRQoL analysis. Other dimensions of both questionnaires were considered as secondary HRQoL criteria.
The magnitude of the changes in the digestive comfort dimension of the FBA was assessed in two ways: (i) clinical relevance of the difference in the score changes between groups; (ii) comparison of rate of responders. This was done using the minimal important difference (MID) method, which is one of the methods allowing the magnitude of the effects in HRQoL(Reference Walters and Brazier23) to be ascertained(Reference Juniper, Guyatt and Willan24). The MID corresponds to the minimal difference in the digestive comfort score for which the subjects perceived a benefit. The populations used to calculate the MID values were: (i) subjects reporting an improvement of their GI well-being at week 4 of +2 or +3; (ii) subjects reporting an improvement of their GI well-being at week 4 of +4 or +5. Finally, the difference in the score changes between groups was considered as clinically significant if this difference was superior to the MID value (i) and a responder will be defined as a subject having an improvement in their baseline score of digestive comfort dimension of at least the value of the MID (ii).
Subjects recorded daily in their diary the consumption of study products, medications started during the study and forbidden products (fibres, other fermented dairy products), as well as any adverse events.
Statistical methods
The sample-size calculation was based on the main outcome, the overall assessment of GI well-being over the first 4 weeks of the study (evaluated with a three-point Likert scale) for the main criteria (ordered categorical data). The trial sample size required to give a power of 80 % for detecting a significant difference between the control and test products of 20 % more subjects improved in the test-product group at 4 weeks was calculated to be at least eighty-four subjects per group. Taking into account that all subjects who withdrew prematurely were not replaced, 100 subjects per product group were randomised.
The intention-to-treat population corresponds to all the randomised subjects. All the analyses of efficacy were performed on the full analysis set (FAS) population which corresponds to all randomised subjects having any efficacy data available under product consumption. This definition of the FAS population is in agreement with the International Conference of Harmonisation guidances(25).
Baseline demographic data were compared between groups using the Wilcoxon test. Overall assessment of GI well-being (fifteen-point scale), frequency of digestive symptoms, and stool frequency and consistency were analysed using a repeated-measures ANOVA (on raw data or on change from baseline or on the ranks according to the normality assessment) with time, treatment group, interaction time × product and baseline score as fixed factors for each period.
One-factor non-parametric ANOVA based on the ranks was carried out by time, i.e. by week, when the normality of the residual of the repeated-measures parametric or non-parametric model analysis over the 4-week period was not assessed.
The measures on the three-point scale of overall assessment of GI well-being are multinomial and repeated, so in this case the methodology used is the generalised estimation equations analysed with the GENMOD procedure.
The OR was used to report GI well-being results and corresponds to the odds to be in a better condition for the overall assessment of GI well-being and to the odds to be responder for GI well-being in the FM group v. the control group.
The analysis of the score differences for all the dimensions of the FBA and PGWBI questionnaires, at weeks 4 and 8 v. baseline, between the FM and control groups was done using a parametric or non-parametric covariance analysis according to the normality of the assessment and the model residuals (treatment group and baseline score as fixed factors and primary care centre as a random factor).
The responder rates for overall assessment of GI well-being, the digestive comfort dimension of the FBA, were analysed at weeks 4 and 8 by a logistic regression to compare the products.
Analyses during the wash-out period were performed only on parameters for which significant differences were shown during the double-blind period.
Results
Subjects
Figure 1 describes the flow of subjects through the protocol. From the 253 contacted subjects, 217 were included in the present study and 202 were randomised (102 subjects assigned to the FM group and 100 to the control group), corresponding to the intention-to-treat population. Of the subjects, five did not complete the entire 4-week double-blind period and were lost to follow-up (no data available under product consumption), giving an FAS population of 197. Of these, five additional subjects prematurely stopped the study during the wash-out period.
The compliance during the study was 99·2 and 99·3 % for the control and FM groups, respectively.
Baseline characteristics of subjects
The subjects showed no significant differences between the FM product and control groups (Table 1).
Table 1 Baseline characteristics of subjects: comparison between groups
(Mean values and standard deviations)
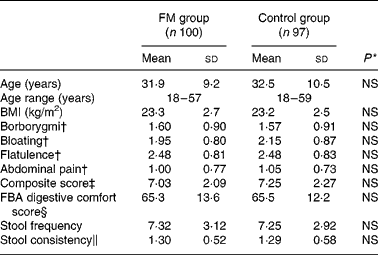
FM, fermented milk; FBA, Food and Benefits Assessment.
* No significant differences were found between the groups for all the variables tested.
† The frequency of individual digestive symptoms was assessed with a five-point Likert scale from 0 (never) to 4 (every day of the week).
‡ The composite score of the frequency of digestive symptoms ranged from 0 to 16.
§ The FBA questionnaire digestive comfort score ranged from 0 to 100 (best).
∥ Stool consistency ranged from 0 to 3.
Double-blind period
Gastrointestinal well-being
The main outcome (i.e. overall assessment of GI well-being over the 4-week period) was analysed on the three-point scale level of this assessment (Table 2). The percentage of women reporting an improvement in their GI well-being was significantly (P = 0·006) higher in the FM group v. the control group (OR 1·7; 95 % CI 1·17, 2·45). The weekly analysis of the fifteen-point scale score showed a significant higher score at weeks 1 (P = 0·001) and 3 (P = 0·047) in the FM group compared with the control group.
Table 2 Overall assessment of gastrointestinal (GI) well-being in the full analysis set population (n 197) during the double-blind and the wash-out periods

FM, fermented milk; NS, OR not statistically different (P>0·05), with OR < 1.
* P < 0·05, ** P < 0·01.
† Analysis of the percentage of subjects in a higher class was done using the GENMOD procedure.
‡ Raw scores ranged from − 7 to +7. Analysis of the raw scores was done by week using rank analysis for each time.
§ A responder was defined as a subject having an improvement of their GI well-being for at least 2 weeks among the 4 weeks. Analysis of the rate of responders was done using a logistic model.
The percentage of responders for GI well-being was significantly (P = 0·025) higher in the FM group v. the control group (52·0 v. 36·1 %, respectively; OR 1·92; 95 % CI 1·09, 3·40) (Table 2).
Frequency of digestive symptoms
Weekly changes during the period of product consumption, the mean change over this 4-week period for the frequency of each individual digestive symptoms, as well as composite scores within the FM and control groups are shown in Table 3. Changes in borborygmi frequency showed a significantly (P = 0·016) higher decrease in the FM group compared with the control group over the 4-week period (least squares mean − 0·22; 95 % CI − 0·40, − 0·04). The decrease in flatulence frequency was significantly higher in the FM group than in the control group at weeks 1 (P = 0·041), 2 (P = 0·028) and 4 (P = 0·008), whereas no significant differences were observed in bloating score as well as in abdominal pain or discomfort score. Comparisons of the effect of the test product on a composite score showed an overall significant (P = 0·044) decrease over the 4-week period in the FM group when comparing with the control group (least squares mean − 0·57; 95 % CI − 1·12, − 0·02).
Table 3 Changes in frequency of digestive symptoms in the full analysis set population (n 197) during the 4 weeks of the double-blind period†
(Mean values and standard deviations)

FM, fermented milk; NV, not valid.
* P < 0·05.
† FM group, n 100; control group, n 97. Changes from baselines in the frequency of digestive symptoms and composite score were compared between groups over the 4 weeks (least squares mean) with the mixed linear model. When the statistical model was not valid for the overall comparison (NV), changes from baselines were analysed by week using a non-parametric model.
‡ The frequency of individual digestive symptoms was assessed with a five-point Likert scale from 0 (never) to 4 (every day of the week).
§ The composite score of frequency of digestive symptoms ranged from 0 to 16.
Bowel function
Stool frequency and stool consistency data are shown in Table 4. Stool frequency did not differ between the FM and control groups. A significant (P = 0·02) decrease of the stool consistency was observed in the FM group v. the control group.
Table 4 Changes in bowel frequency and stool consistency in the full analysis set population (n 197) during the 4 weeks of the double-blind period*
(Mean values and standard deviations)
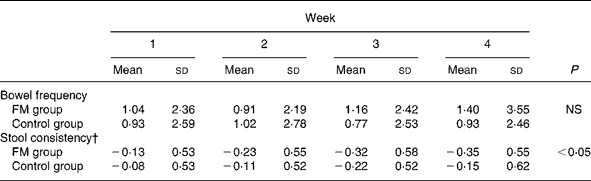
FM, fermented milk.
* FM group, n 100; control group, n 97. Changes from baselines were compared between groups over the 4 weeks with mixed linear models on the ranks.
† Stool consistency ranged from 0 to 3.
Health-related quality of life
Results of the FBA digestive comfort dimension score, and of the PGWBI global score are shown in Table 5. The digestive comfort dimension score, the primary HRQoL endpoint, significantly increased (P = 0·027) after 4 weeks of product consumption in the FM group as compared with the control group. The observed difference between group (3·56) is inferior to the MID (10·9). When the rate of responders for the digestive comfort dimension was analysed, a trend in favour of a higher percentage of responders was shown at week 4 in the FM group as compared with the control group (41·0 v. 27·8 %; P = 0·053). No difference was observed between groups for other HRQoL dimensions. PGWBI scores did not differ between groups over time.
Table 5 Health-related quality of life: digestive comfort dimension score of the Food and Benefits Assessment (FBA) questionnaire and the global score of the Psychological General Well-Being Index (PGWBI) questionnaire in the full analysis set population (n 197) at baseline and end of the double-blind period
(Mean values and standard deviations)

FM, fermented milk.
* P < 0·05.
† Changes from baselines in the scores were compared between groups with the mixed linear model.
‡ The digestive comfort score ranged from 0 to 100 (best).
§ The global score ranged from 0 to 100 (best).
Wash-out period
The percentage of women reporting an improvement in their GI well-being was not significantly different in the FM group v. the control group as well as for the difference in the weekly analysis of the raw score (Table 2). At the end of the wash-out period, the percentage of responders strongly decreased without difference between the groups (8·0 v. 11·3 % for the FM and control groups, respectively; OR 0·68; 95 % CI 0·26, 1·77).
No significant difference in the changes of frequency for each individual symptom was observed between groups. Comparisons of the composite score showed an overall significant (P = 0·044) decrease over the 4-week period in the control group when comparing with the FM group.
Stool frequency as well as score of stool consistency did not differ between the FM product and control groups (data not shown). No difference was observed between the groups for all HRQoL dimensions at the end of the wash-out period compared with baseline values (data not shown).
Discussion
The present randomised, double-blind, controlled clinical study shows the ability of the tested FM to improve GI well-being in women reporting minor digestive disorders. This beneficial effect is supported by both the OR (1·7) for the improvement of GI well-being and the 15 % difference in the rate of responders for GI well-being over the control. Furthermore, this probiotic product decreased the composite score of the frequency of GI symptoms. This overall symptom improvement was mainly due to improvements in several individual gas-related symptoms (for example, borborygmi and flatulence).
When studying the literature, it can be concluded that it is indeed difficult to perform clinical studies assessing the impact of food and probiotics on GI well-being in the general population. Several studies have reported effects of probiotics on bowel function in healthy populations(Reference Johansson, Nobaek and Berggren4–Reference Yamano, Iino and Takada7, Reference Marteau, Cuillerier and Méance15) but none of them has assessed the effect on digestive comfort or GI symptoms. Indeed, research has focused on the assessment of potential negative sensations from the gut associated with the consumption of both fibres(Reference Goetze, Fruehauf and Pohl26) and probiotics(Reference Hernando-Harder, von Bunau and Nadarajah27). Subject-reported assessment is endorsed to be the more meaningful and accurate measure for GI symptoms as compared with physician-reported assessments(Reference Irvine, Whitehead and Chey19). Thus, in the present study, an overall assessment of GI well-being was chosen to capture the improvement in different GI symptoms as well as in bowel function, which is in line with recent recommendations for the assessment of probiotics in IBS(Reference McFarland and Dublin28). Assessment of the frequency of digestive symptoms, HRQoL and bowel function were considered as secondary endpoints.
Gut-derived symptoms are part of the normal physiological digestive process and are experienced by the general population(Reference Cummings, Antoine and Azpiroz29, Reference van Kerkhoven, Eikendal and Laheij30). However, the efficacy of probiotics on digestive symptoms was only reported in the IBS population(Reference Moayyedi, Ford and Talley31). The present study shows that a specific probiotic food that has been shown to improve digestive symptoms in IBS(Reference Agrawal, Houghton and Morris13, Reference Guyonnet, Chassany and Ducrotté14) is also able to improve GI well-being and digestive symptoms in individuals who do not suffer from GI diseases. The comparison of the clinical benefit between these studies can be done using the number needed to treat (NNT)(Reference Nuovo, Melnikow and Chang32, Reference Altman, Schulz and Moher33), which is based on the difference of responders between groups. The NNT of the present study (6·25) is similar to the one observed (5·7) in a previous IBS study(Reference Guyonnet, Chassany and Ducrotté14) and within the same range as NNT calculated (4 and 7·3) in recent reviews of IBS probiotics trials(Reference McFarland and Dublin28, Reference Moayyedi, Ford and Talley31).
Previous studies investigating the effect of probiotics on the GI tract have mainly shown an increase in bowel frequency(Reference Larsen, Nielsen and Kaestel5–Reference Matsumoto, Takada and Shimizu8). In the present study, GI well-being and symptoms improved without change of bowel frequency. The fermented dairy product used in the present study is also able to decrease transit time in both healthy(Reference Marteau, Cuillerier and Méance15–Reference Méance, Cayuela and Turchet17) and IBS populations(Reference Agrawal, Houghton and Morris13). Several other clinical trials have investigated the effect of either probiotic strain(s) alone, i.e. Propionibacterium (Reference Bouglé, Roland and Lebeurrier34) and VSL#3(Reference Kim, Camilleri and McKinzie35) or FM(Reference Sairanen, Piirainen and Grasten36, Reference Bartram, Scheppach and Gerlach37) and have failed to demonstrate a positive effect on transit time. This suggests that the benefits exerted by one probiotic product could not be extrapolated to other probiotics.
HRQoL measures were used to ascertain the relevance of the observed improvement of both GI well-being and symptoms. We acknowledge that despite the significant and evident effect on GI well-being and symptoms, the improvement of HRQoL remains less pronounced and is not so clear-cut. In order to provide meaningful interpretation of the HRQoL changes, the clinical significance of the changes in the digestive comfort dimension of the FBA questionnaire was assessed using a priori definitions of MID(Reference Juniper, Guyatt and Willan24). Using this very rigorous method of assessment, a trend in favour of the tested product was obtained for the rate of responders whereas the statistically significant difference in the score changes was below the threshold of clinical relevance. Obviously, it is more difficult to improve HRQL in a non-disease population than in a population suffering from a chronic disease such as a digestive pathology(Reference Guyonnet, Chassany and Ducrotté14). A longer trial is necessary to achieve higher improvement in HRQoL, as this parameter is known to change slowly, especially in this population which presents high baseline scores, i.e. good HRQoL.
Another limitation in the interpretation of the results of the present study is the multiple statistical comparisons. Due to the lack of data in this population, the statistical comparisons between groups for all the outcomes are required to detect a positive effect. Although these data of this explorative study show consistent positive effects across the different outcomes, further studies aiming at investigating more specifically some outcomes (for example, GI well-being or HRQoL) are required to confirm these beneficial effect.
The effect of the study product was not maintained after the cessation of its consumption. It was noticeable that a dramatic loss of the effect of the product was observed within the first week of ceasing to use the product whereas a slow but gradual loss of the effect was observed during the following 3 weeks of the follow-up period. Interestingly, it was shown that the level of B. lactis in the faeces of healthy volunteers consuming the study product followed a similar kinetics, with a maximal level reached after 4 weeks of consumption followed by a decrease within a week after discontinuation of use of the product and a non-detectable level after 4–5 weeks(Reference Collado, Moreno and Cobo38). Therefore, the disappearance within the GI tract of the specific B. lactis strain of the study product may support the observed decrease of the effect of this product. Given the fact that alterations of gut microbiota have been identified in IBS(Reference Quigley and Flourie1, Reference Parkes, Brostoff and Whelan39), investigations aiming at assessing the impact of this product on gut microbiota in healthy population as well as in IBS would allow us to determine whether the effects on GI functions and sensations could be associated with gut microbiota modifications.
In conclusion, the present study demonstrated that this probiotic food containing the specific B. lactis DN-173 010 strain is able to improve GI well-being as well as GI symptoms in a population of women reporting minor digestive disorders. These data, taken together with previous data obtained on GI transit and in IBS, suggest that this specific probiotic food may represent a promising nutritional and safe solution for the management of GI symptoms. Future studies are required to explore longer durations of product consumption and the impact on HRQoL. Investigations of gut microbiota modifications will allow the determination of mechanisms of action and could allow the determination of which population could benefit from the consumption of such kinds of probiotics.
Acknowledgements
The authors thank Stéphane Doat for providing products and Dr De La Motte and Dr Hausleiter for the management of the experimental phase.
The present study was supported by a grant from Danone.
D. G. was responsible for the study design and for the analysis of the study results. S. J. and O. C. were involved in the study design definition and in the analysis of the results. L. M. was responsible for the statistical analysis. A. S. was responsible for the overall study management.
D. G., A. S. and S. J. are employees of Danone Group. O. C. is a consultant of Danone Research.








