The relevance of fetal growth for a child’s health and development has been emphasised by pooled longitudinal analysis gathering over 400 000 repeated measurements until 2 years of age in low- and middle-income countries. A narrow postnatal timeframe for the onset of growth faltering was characterised, as incident stunting peaked from birth to 3 months without reversal at a sufficient rate to overturn its magnitude in early life(Reference Benjamin-Chung, Mertens and Colford1). Suboptimal growth patterns may configure a whole-population condition in these countries, as data from the Demographic and Health Surveys from 1993 to 2015 support that children experience slower growth in comparison to international standards across the entire distribution of anthropometric indicators during the first years of life(Reference Roth, Krishna and Leung2).
Therefore, preventive strategies with downstream effects are critical to improving growth trajectories since the antenatal period. From a life course perspective, maternal diet is recognised for its influence on fetal outcomes(Reference Stang and Huffman3) and has important implications in a global scenario of nutritional inadequacies and increasing risk for chronic diseases among women in the preconception period and during pregnancy(Reference Victora, Christian and Vidaletti4–Reference Wang, Wang and Darling6).
Ultra-processed foods (UPF) are convenient, ready-to-consume products manufactured through intense industrial processes resulting in fractioning and extensive modification of whole foods, with frequent use of flavouring agents, cosmetic additives and other substances not usually available in domestic kitchens(Reference Monteiro, Cannon and Levy7). Larger UPF participation in the diet has been associated with poorer nutritional quality in nationally representative samples(Reference Martini, Godos and Bonaccio8) and, more specifically, among pregnant women(Reference Ben-Avraham, Kohn and Tepper9). With the substantial expansion in sales noted worldwide(Reference Baker, Machado and Santos10) and emerging evidence on the potential mechanisms driving their detrimental influence on human health(Reference Srour, Kordahi and Bonazzi11), a focus on the relationship between maternal UPF consumption and fetal growth is warranted.
Recently, maternal UPF consumption during the periconceptional period was found to impair embryonic growth from 7 to 11 weeks of gestation in a cohort study conducted in the Netherlands. At the 11th week, fetal crown-rump length and embryonic volume were significantly smaller by –1·34 mm and –0·45 cm3, respectively, among participants whose percentage of energy intake from UPF was in the highest quartile in comparison with those in the lowest quartile(Reference Smit, Hojeij and Rousian12). These novel findings elicit further investigation into the impact of UPF consumption on fetal growth in late pregnancy. Particular interest should be paid to whether associations from the embryonic period are sustained, and if differential implications arise for the components of growth according to distinct fetal planes in ultrasound assessments. A need for studies in lower resource settings should also be underlined, as prospective investigations with available ultrasound fetal growth parameters are scarce in these areas that vastly concentrate the burden of malnutrition.
Using data from a birth cohort study with follow-up since the antenatal period in the Brazilian Amazon, the present study explored associations between the frequency of UPF consumption during pregnancy and fetal head circumference (HC), abdominal circumference (AC) and femur length (FL) assessed at > 24 gestational weeks. The potential role of maternal UPF consumption was examined at different levels of the distribution of each ultrasound fetal growth parameter, considering international standards. Findings should fill an existing knowledge gap and contribute to the timely promotion of healthier growth trajectories.
Subjects and methods
Study design and population
The MINA-Brazil Study (Maternal and Child Health and Nutrition in Acre, Brazil) is a population-based birth cohort conducted in Cruzeiro do Sul, a municipality in the Brazilian Amazon with an estimated population of 81 519 inhabitants as of 2015. Located in an endemic malaria region, the setting combines disparities in socio-economic and health access indicators, thereby impacting the provision of antenatal care. Details regarding the MINA-Brazil Study are provided elsewhere(Reference Cardoso, Matijasevich and Malta13).
We retrieved data from participants whose recruitment took place during pregnancy. From February 2015 to January 2016, pregnant women were screened weekly at thirteen primary health care units of the Family Health Strategy in the urban area of Cruzeiro do Sul while booking an antenatal care appointment. Inclusion criteria were the following: (a) up to 20 gestational weeks according to the last menstrual period; (b) with fixed residence in the city and (c) intending to give birth at the only local maternity hospital(Reference Cardoso, Matijasevich and Malta13). A total of 587 participants were enrolled and invited to a baseline interview, followed by prospective clinical evaluations including ultrasound examinations, with confirmation of gestational age(Reference Lourenço, Lima and Vivanco14).
For the present analysis, we considered all live-born singletons with an available ultrasound examination in late pregnancy (> 24 weeks), depicting fetal growth parameters. We excluded twin pregnancies, miscarriages or abortions, stillbirths and those with an unknown pregnancy outcome, as regularly monitored until delivery by the research team at the maternity hospital. The analytical sample comprised 417 participants or 78 % of eligible live-born singleton pregnancies (Fig. 1).
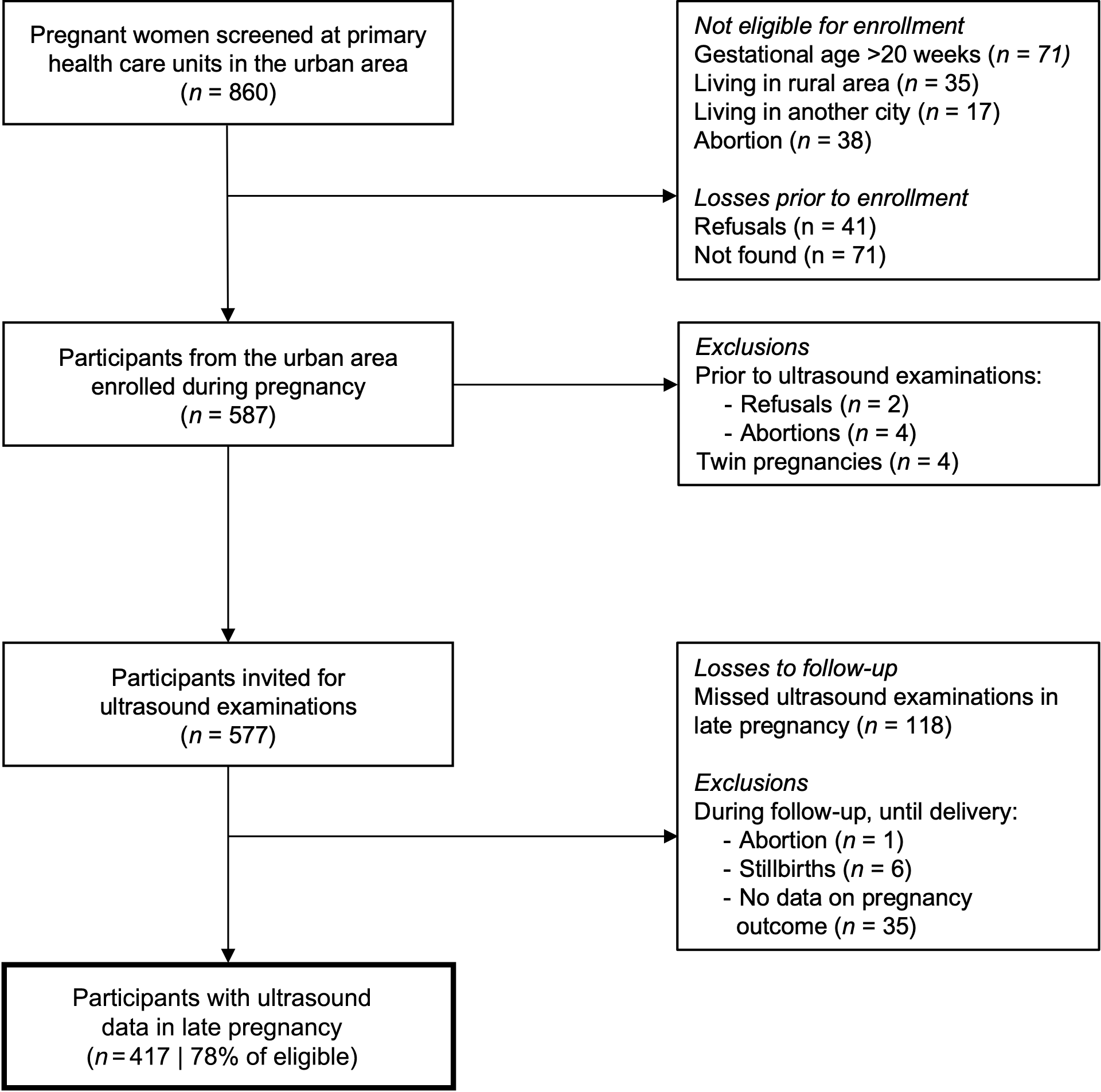
Fig. 1. Flow diagram for the analytical sample retrieved from the MINA-Brazil Study.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board of the School of Public Health, University of São Paulo (protocol number 872.613/2014). Written informed consent was obtained from all subjects or their legal guardians in cases of teenage pregnancies.
Field procedures and data collection
Trained research assistants conducted structured interviews during home visits with each participant at baseline (median (interquartile range) gestational age: 17 (14, 19) weeks). Socio-demographic factors were assessed, including age, self-reported skin colour (as defined by the Brazilian Institute of Geography and Statistics: white, mulatto, black, yellow or indigenous), schooling (≤ 9 or > 9 years, i.e. up to middle school or high school and above), ownership of household assets to generate a wealth index (divided into tertiles)(Reference Filmer and Pritchett15) and assistance from the Bolsa Família conditional cash transfer program (yes or no). Obstetric and pre-pregnancy health characteristics were reported regarding primiparity, planned pregnancy and history of morbidities, including elevated blood pressure, diabetes mellitus and malaria (yes or no).
During follow-up in the antenatal period, participants were invited for up to two clinical evaluations at primary health care units when the research team collected anthropometric, health and dietary information and performed ultrasound examinations. For this analysis, data referring to late pregnancy (> 24 weeks) were considered at a median gestational age of 27·5 (interquartile range: 26·9, 28·6) weeks.
Participants reported their weight before conception and had their current weight ascertained in duplicate using a portable scale with a precision of 100 g (Tanita Corporation, model UM061). Height was measured in duplicate using a portable stadiometer to the nearest millimetre (Alturexata®). Calibrated equipment and standard procedures were used(16); weight and height were allowed to vary up to 100 g and 0·2 cm between measurements, respectively, and mean values were used. Pre-pregnancy nutritional status was ascertained according to BMI, calculated by dividing the pre-pregnancy weight (kg) by height (m) squared. Among participants aged ≥ 19 years, pre-pregnancy BMI was categorised using criteria from the WHO as underweight (< 18·5 kg/m2), normal weight (≥ 18·5 to < 25·0 kg/m2), overweight (≥ 25·0 to < 30·0 kg/m2) and obesity (≥ 30 kg/m2)(16). For those aged < 19 years, categories were defined according to BMI-for-age z-scores as underweight (< –2 z), normal weight (≥ –2 to ≤ +1 z), overweight (> +1 to ≤ +2 z) and obesity (> +2 z)(Reference de Onis, Onyango and Borghi17).
Following recommendations from the Institute of Medicine, gestational weight gain during follow-up was calculated and divided by the corresponding number of gestational weeks until evaluation in late pregnancy. Considering the pre-pregnancy BMI, participants were categorised into insufficient, adequate or excessive gestational weight gain(Reference Rasmussen18,19) . Smoking status during pregnancy (yes or no) was assessed during interviews conducted with participants.
We evaluated UPF consumption in the past month using an adapted frequency questionnaire for food groups(Reference Augusto, Cobayashi and Cardoso20,Reference Cardoso, Tomita and Laguna21) with the following response options: never, 1–3 times per month, 1–3 times per week, 4–6 times per week, 1 time per day, 2–3 times per day and ≥ 4 times per day. UPF were defined within the NOVA classification system, reflecting the extent and purpose of industrial food processing(Reference Monteiro, Cannon and Levy7). The adapted questionnaire considered the most relevant UPF consumed in the study setting, including soft drinks and powdered juices, industrialised cookies and crackers, chips and instant noodles, as described in previous qualitative and quantitative analyses(Reference Sato, Couto and Wells22,Reference Malta, Neves and Lourenço23) . The frequency of UPF consumption was categorised as no/monthly, weekly or daily.
Trained field physicians performed ultrasound examinations using a portable SonoSite TITAN machine (SonoSite Inc.). Standardised self-scoring quality criteria for the placement of callipers and ellipses with proper visualisation of landmarks in all fetal planes(Reference Salomon, Bernard and Duyme24) were employed to capture images in the cephalic, abdominal and femoral planes, following protocols from the INTERGROWTH-21st Project(Reference Papageorghiou, Sarris and Ioannou25). For external quality control, an independent expert obstetrician conducted a blinded re-evaluation of images; for all planes, > 94 % of the images were deemed satisfactorily acquired(Reference Lourenço, Lima and Vivanco14). Ultrasound fetal growth parameters included HC, AC and FL. Z-scores for gestational age were calculated for HC, AC and FL, according to the INTERGROWTH-21st Project standards(Reference Papageorghiou, Ohuma and Altman26).
Statistical analysis
The fetal HC, AC and FL z-scores in late pregnancy were the main outcomes of interest. The main exposure was the frequency of UPF consumption, as assessed in the month before the ultrasound examination. Socio-demographic and pregnancy characteristics were initially described in the study population and compared by UPF consumption categories using chi-square or Fisher’s exact tests. We also described the distribution of fetal growth parameters at the 10th, 50th, and 90th percentiles, with equivalent information in millimetres.
Associations between exposure to UPF consumption and fetal growth parameters were investigated by fitting simultaneous-quantile regression models with bootstrapped standard errors at the 10th, 50th and 90th percentiles in the distribution of each outcome. Using a quantile regression approach, we examined whether categories of frequency of UPF consumption had distinct effects on the median and extremes of the distribution of HC, AC and FL. The command sqreg in Stata was used, and the estimation of coefficients at a given percentile was based on the reduction of the median absolute deviation, including check functions with asymmetric weights for the selected percentiles. All equations were estimated simultaneously, with assessment of correlations between parameters for different percentiles and an estimate of the entire variance-covariance matrix of the estimators by bootstrapping(27).
In the unadjusted analysis, crude differences in fetal growth parameters at each selected percentile in the distribution were estimated according to an increasing frequency of UPF consumption in late pregnancy, with respective P values for trends across categories. For final adjusted models, adjustment for covariates was based on their conceptual relevance for fetal growth conditions and potential associations with the exposure and included household wealth index and maternal schooling, age, height, pre-pregnancy weight, pre-pregnancy elevated blood pressure, primiparity, smoking during pregnancy and adequacy of gestational weight gain up to the ultrasound examination. Considering no/monthly UPF consumption as the reference category, adjusted differences in fetal HC, AC and FL and 95 % confidence intervals (95 % CI) according to weekly and daily UPF consumption were estimated at the 10th, 50th and 90th percentiles. Differences in coefficients between percentiles were tested with interquantile regressions using the command iqreg (90th v. 10th, 90th v. 50th and 50th v. 10th percentiles). The analytical sample of this study (n 417) was deemed sufficient for detecting outcome differences of effect size 0·25 in comparing categories of UPF consumption (no/monthly v. weekly and daily), with 80 % power and 5 % significance level.
All analyses were performed using Stata version 15 (StataCorp).
Results
Among the 417 participants included in this study, the mean age was 24·7 (sd: 6·5) years and 29·5 % had ≤ 9 years of schooling. As shown in Table 1, 44 % of the women were primiparous and planned their pregnancies. While 61·3 % had an adequate weight status before conception, gestational weight gain until the assessment of ultrasound fetal growth parameters was classified as insufficient for 20·0 % and excessive for 57·2 % of the study population. Successfully followed-up participants were less frequently from households in the poorest tertile of wealth and reported smoking to a lesser extent during pregnancy than eligible pregnant women who missed ultrasound examinations in late pregnancy (n 118, 43·2 % and 10·2 %, respectively, P < 0·05). No differences were noted between the groups regarding age, skin colour, schooling, pre-pregnancy nutritional status, elevated blood pressure, primiparity, planned pregnancy and gestational weight gain.
Table 1. Characteristics of participants according to ultra-processed food consumption (UPF) in the MINA-Brazil study (n 417)
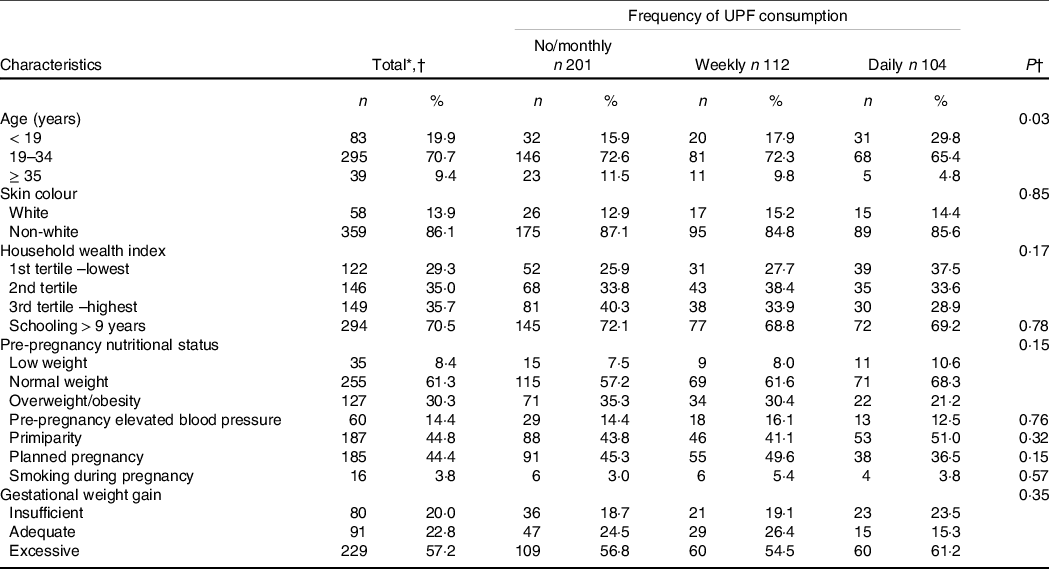
* Totals may vary due to missing observations.
† Characteristics were compared by categories of frequency of UPF consumption using chi-square or Fisher’s exact tests.
UPF were consumed on a weekly or daily basis by 26·9 % and 24·9 % of pregnant women, respectively. There was an inverse association between age and the frequency of UPF consumption (P 0·03). Other socio-demographic and obstetric factors were not significantly associated with UPF consumption in the study population.
According to ultrasound scans performed at 27·5 (interquartile range: 26·9, 28·6) weeks, median HC, AC and FL z-scores were close to the expected average fetal size by gestational age according to international standards. At the 10th percentile in the distribution of each fetal growth parameter, values were close to –1 z-score, ranging from –1·32 to –1·19 z-score. On the other hand, values at the 90th percentile were 0·97 z-score for HC, 1·65 z-score for AC and 1·35 z-score for FL (Table 2).
Table 2. Percentiles in the distribution of fetal growth parameters in late pregnancy among participants of the MINA-Brazil study (n 417)
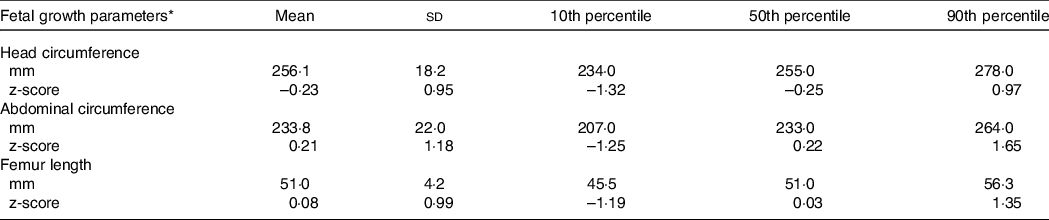
* Head circumference, abdominal circumference and femur length z-scores for gestational age were calculated according to the INTERGROWTH 21st Project standards.
In the unadjusted analysis, difference estimates for fetal growth parameters according to an increasing frequency of UPF consumption were generally negative across percentiles in each distribution, as illustrated in Fig. 2. For HC, values were significantly lower at the 90th percentile following exposure to more frequent UPF consumption (–0·16 z-score, P < 0·05 for linear trend).
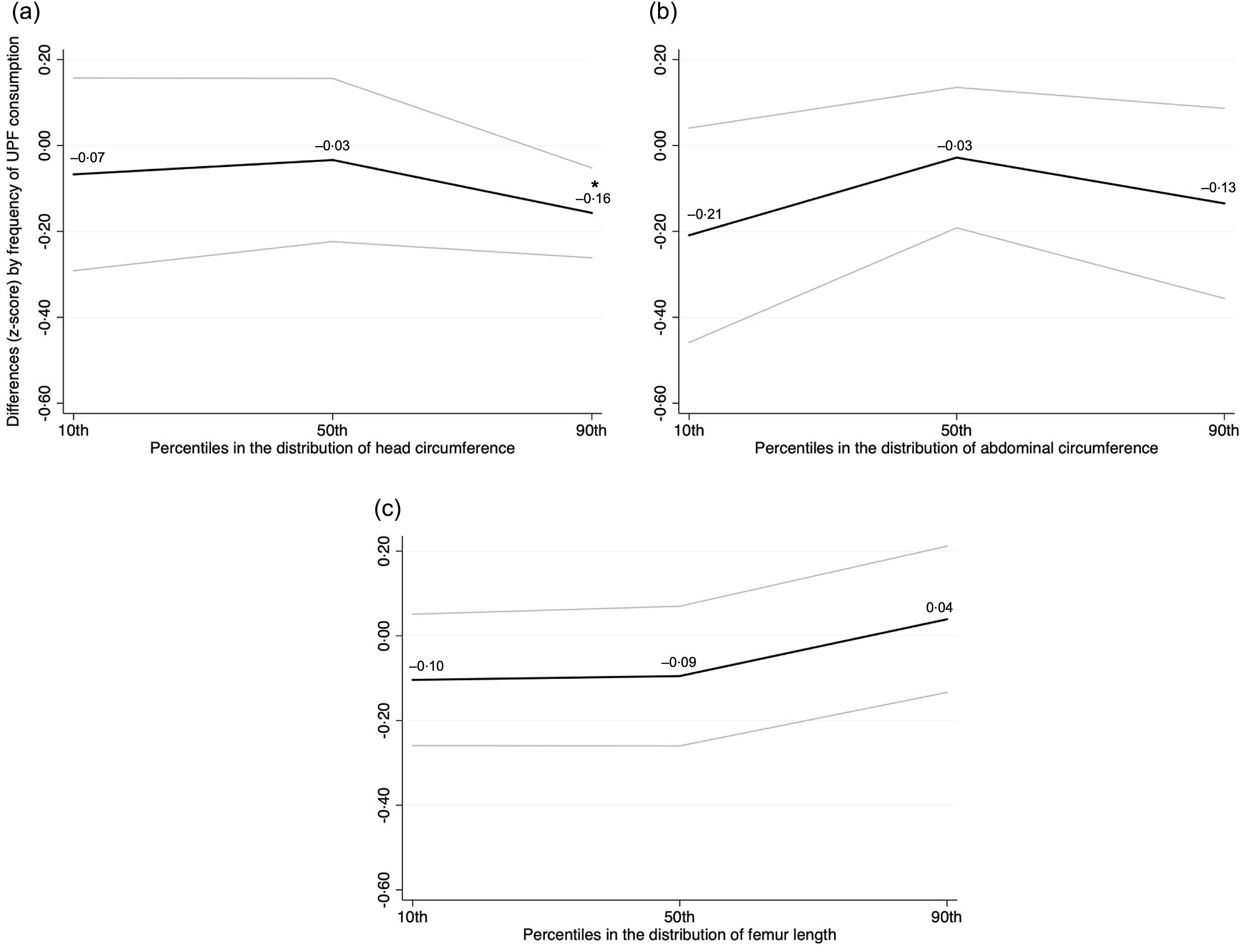
Fig. 2. Crude associations of fetal growth parameters in late pregnancy according to an increasing frequency of ultra-processed food consumption among participants of the MINA-Brazil Study (n 417). Head circumference (panel A), abdominal circumference (panel B), and femur length (panel C) z-scores for gestational age were calculated according to the INTERGROWTH 21st Project standards. Coefficients (black lines) and 95 % confidence intervals (95 % CI; grey bands) for each fetal growth parameter were estimated using simultaneous-quantile regression models with bootstrapped standard errors for the 10th, 50th and 90th percentiles in the distribution. Estimates are differences (z-scores) according to increasing categories of ultra-processed (UPF) food consumption (no/monthly consumption as the reference category). *P < 0·05 for linear trend.
Adjusted associations from simultaneous-quantile regression models of fetal growth parameters in late pregnancy according to categories of frequency of UPF consumption are presented in Table 3. In comparison with pregnant women who reported no/monthly consumption, we observed that weekly UPF consumption was negatively associated with HC and FL at different portions of their distributions. HC values at the 90th percentile were affected by –0·39 z-score (95 % CI: –0·78, –0·01), while no association was noted at the 10th or 50th percentiles. Median FL values decreased by –0·32 z-score (95 % CI: –0·60, –0·04).
Table 3. Adjusted associations between fetal growth parameters in late pregnancy and frequency of ultra-processed food consumption among participants of the MINA-Brazil study (n 400)
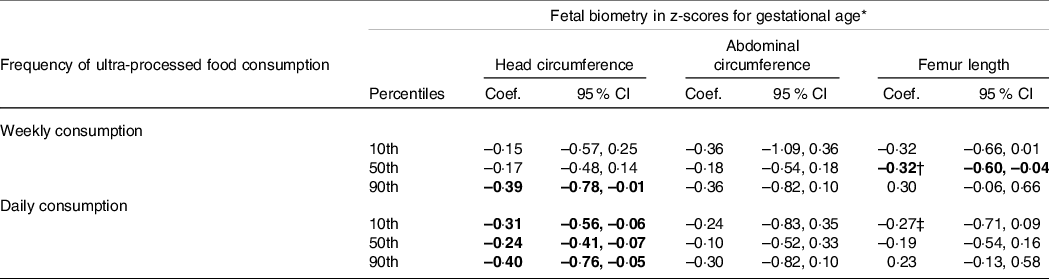
* Head circumference, abdominal circumference and femur length z-scores for gestational age were calculated according to the INTERGROWTH 21st Project standards. Coefficients and 95 % confidence intervals (95 % CI) for each fetal growth parameter were estimated using simultaneous-quantile regression models with bootstrapped standard errors for the 10th, 50th and 90th percentiles in the distribution. Estimates are differences (z-scores) according to each category of ultra-processed (UPF) food consumption (no/monthly consumption as the reference category), with adjustment for household wealth index and maternal schooling, age, height, pre-pregnancy weight, pre-pregnancy elevated blood pressure, primiparity, smoking during pregnancy and adequacy of gestational weight gain up to the ultrasound examination. Values in bold are significantly different from null.
† Coefficients estimated at the 50th and 90th percentiles were significantly different according to interquantile regression.
‡ Coefficients estimated at the 10th and 90th percentiles were significantly different according to interquantile regression.
Daily exposure to UPF, in turn, was negatively associated with fetal growth particularly in the cephalic plane, as smaller z-scores were noted throughout the HC distribution (Table 3). In contrast to those who were less exposed to UPF, foetuses from pregnant women with daily UPF consumption had a –0·31 z-score (95 % CI: –0·56, –0·06) smaller HC at the 10th percentile. The difference reached –0·40 z-score (95 % CI: –0·76, –0·05) at the 90th percentile, which corresponds to approximately –4 mm. The frequency of UPF consumption was not associated with AC.
These associations were not attenuated by socio-economic indicators, including household wealth index and maternal schooling. In fact, there was an independent and beneficial association between maternal schooling and all three fetal growth parameters at the lower bound of the distributions (data not shown). At the 10th percentile, a positive difference was observed for HC (0·65 z-score; 95 % CI: 0·15, 1·16), AC (0·68 z-score; 95 % CI: 0·05, 1·31) and FL (0·42 z-score; 95 % CI: 0·03, 0·82) among foetuses of pregnant women with > 9 years of schooling in comparison to ≤ 9 years.
Discussion
This study showed that an increased frequency of UPF consumption during the antenatal period was negatively associated with fetal HC and FL in late pregnancy. While such relationship was apparent for these skeletal components of fetal growth, it was not pivotal for AC. Daily maternal exposure to UPF was particularly unfavourable regarding the cephalic plane, with significant negative differences detected at all levels of HC distribution compared with the lowest frequency of UPF consumption.
Previous studies on the relationship between maternal diet and ultrasound fetal growth parameters in the second and third trimesters of pregnancy have explored specific food items and have been mainly conducted in high-income countries. A higher frequency of seafood consumption was related to a + 4 mm larger AC at 20–24 gestational weeks, but not to HC or FL, in overweight participants in a French hospital-based mother–child cohort(Reference Drouillet, Kaminski and De Lauzon-Guillain28). Increasing maternal consumption of cow’s milk in the first trimester was positively associated with birth weight according to the Generation R Study; when compared with < 1 glass of milk per day, consumption of > 2–3 daily glasses was associated with +1 mm (95 % CI: 0·1, 1·9) higher HC in the third trimester, while no effect was detected on FL(Reference Heppe, van Dam and Willemsen29). Among Korean pregnant women(Reference Jang, Kim and Lee30), a slightly higher fetal biparietal diameter at late pregnancy (mean 36·4, sd: 2·5 weeks) was observed among participants who reported a daily fruit and vegetable consumption ≥ 519 g (8·99 cm v. 8·92 cm among those consuming < 519 g).
Findings from the Rotterdam Periconceptional Cohort were the first to identify impairment of embryonic growth following exposure to higher UPF consumption, indicating an effect size comparable to maternal smoking of > 10 cigarettes per day(Reference Smit, Hojeij and Rousian12). In the present study, the analysis relied on a measure of frequency of UPF consumption and a detailed dietary assessment was not obtained from pregnant women, preventing the estimation of associations based on the relative contribution of UPF for total energy intake. Taking these differences into account, our results suggest that the effect of maternal UPF consumption is sustained after the first trimester of pregnancy. Especially in a lower resource setting and considering previous data relating UPF consumption to socio-economic status in pregnant women(Reference Gomes, Malta and Papini31,Reference Naspolini, Machado and Fróes-Asmus32) , it is important to notice that the present associations were independent of household wealth index and maternal schooling.
The focus on UPF consumption seems critical not only because of a profile featuring higher energy density, added sugar and saturated fat, as well as reduced fibre, protein and micronutrient intake(Reference Martini, Godos and Bonaccio8). There is also evidence that ultra-processing may constitute a distinct yet complementary dimension of diet healthiness, adding to the effects attributed to nutritional quality(Reference Julia, Baudry and Fialon33). Thus, mechanistic considerations for the role of UPF span from poor nutritional characteristics to the production of potentially toxic compounds during food processing and packaging, in addition to the presence of several food additives and disruption of the physical structure of the food matrix during the industrial manufacturing of these products. Negative repercussions to human health could encompass altered nutrient bioavailability in conjunction with damage to gut microbiota composition, metabolism and function(Reference Srour, Kordahi and Bonazzi11), with pregnancy and intergenerational implications(Reference Sonnenburg, Smits and Tikhonov34,Reference Grech, Collins and Holmes35) .
Here, we highlight that the associations of frequency of UPF consumption during the antenatal period were specific to skeletal structures in fetal growth. Conducted in a 6-week-long period representing growth before sexual maturation and closure of growth plates in humans, an in vivo trial described stunted linear growth not attributable to energetic deficiency among young female rats fed a UPF diet when compared with the control group(Reference Zaretsky, Griess-Fishheimer and Carmi36). This finding was explained by extensively compromised bone quality, including reduced bone mineral density and higher porosity, as depicted by micro-computed tomography and histological analysis. Transcriptome analysis revealed that gene expression in the UPF group was consistent with disruption of the endochondral ossification process through a compromised balance between bone matrix extracellular formation and degradation, as well as disorganisation of the growth plate structure due to imbalances in proliferation and differentiation, and altered mineralisation and vascularisation(Reference Zaretsky, Griess-Fishheimer and Carmi36). These results imply that the growing skeleton is a particular target for the harmful effects of UPF and are consistent with the associations observed in the present investigation.
Among the fetal bone structures affected by increased exposure to UPF, fetal HC is considered a consistent proxy of brain volume, with a peak intrauterine growth velocity at 16–17 gestational weeks(Reference Ohuma, Villar and Feng37). In our study population, HC values were evaluated at a median 27·5 gestational weeks. In the Generation R Study, brain volume assessed by MRI at the age of 10 years was associated with summary measures of fetal growth, notably from late pregnancy to birth(Reference Silva, El Marroun and Sammallahti38). A Mendelian randomisation study reported that a 1 sd increase in infant HC according to ten SNP as instrumental variables resulted in 0·14-fold significantly higher intelligence test scores; no evidence for causal relationships was found for birth weight or length(Reference Qian, Gao and Yan39). Taken together, these findings may raise concerns regarding the medium- to long-term implications of daily maternal UPF consumption in negatively affecting HC at all levels of its distribution during pregnancy.
Fetal FL, in turn, has been associated with linear growth, with little contribution to explaining neonatal body fat(Reference Lee, Balasubramaniam and Deter40), similarly to HC. Meta-analysis showed that isolated short FL at 18–28 gestational weeks was associated with higher odds of several poor perinatal outcomes(Reference D’Ambrosio, Vena and Marchetti41), including preterm birth (OR: 3·09, 95 % CI: 1·57, 6·08), low birth weight (OR: 3·24, 95 % CI: 2·34, 4·48) and Apgar score < 7 at 5 min (OR: 3·56, 95 % CI: 1·87, 6·77). In our analysis, the results for FL were limited to a negative difference in median values among foetuses exposed to weekly UPF consumption. As we were not able to detect a more consistent dose–response association, further investigation would be important to confirm such findings.
Our study could not ascertain a significant relationship between the frequency of UPF consumption and AC. This ultrasound parameter is considered an indicator of subcutaneous fat and abdominal organ development, and its growth velocity is relatively steady across gestational ages(Reference Ohuma, Villar and Feng37). Consequently, we may not completely rule out associations in later gestational ages, since there should be room for variability until birth. In this sense, one previous investigation with a convenience sample of pregnant women in the USA reported that each 1 % increase in energy intake from UPF in the second trimester was associated with a 0·62 % higher neonatal total body adiposity(Reference Rohatgi, Tinius and Cade42).
The importance of this study should be considered in light of experimental evidence both in vivo and at the community level. Among young rats, impaired bone quality and growth due to UPF exposure could be managed through a transition to a balanced diet. Correction of the femora and vertebral length, growth plate structure and upturn of overall body weight and length were observed after a prolonged rescue protocol, although recovery of cortical parameters was only partial(Reference Griess-Fishheimer, Zaretsky and Travinsky-Shmul43). Furthermore, a training program for advising on healthy eating, with a focus on restricting UPF consumption, was designed for primary healthcare professionals involved in antenatal care. This intervention resulted in a significant reduction in the percentage of energy intake from UPF reported by pregnant women, mostly between the first and second trimesters of pregnancy(Reference Gomes, Malta and Louzada44). This period seems fairly favourable for the promotion of diets based on in natura and minimally processed, culturally appropriate foods. Such food choices are more nutritive and have the potential to strengthen sustainable food systems with regard to the environment (promoting biodiversity and short food supply chains), economy (stimulating local production) and society (supporting family farmers and traditional eating habits)(45).
Some limitations of this study should be considered when interpreting our results. First, pregnant women who missed ultrasound examinations were from poorer households and more frequently smoked. Attrition is a common issue in cohort studies, with a more successful follow-up among better-off and healthier participants(Reference Catherine, Lever and Marcellus46). Even though 78 % of those eligible were included in this study and no significant association was observed between UPF consumption and wealth index or smoking, a role for these factors in affecting fetal size is recognised(Reference Pereira, Da Mata and Figueiredo47). Thus, selection bias cannot be disregarded, with potential overestimation of associations in our analytic sample. Second, exposure to UPF was assessed according to the frequency of food group consumption. We could not investigate diet quality in more detail or ascertain the percentage of total energy intake from UPF, disentangling components of food preparations and meals. This could incur in some degree of exposure misclassification, with possible implications to the magnitude of estimates toward the null. However, the questionnaire used in this study has held consistent results in previous investigations(Reference Augusto, Cobayashi and Cardoso20,Reference Malta, Neves and Lourenço23) , and our models considered the adequacy of gestational weight gain(Reference Minami, J-P and Noguchi48). Moreover, as associations were evident with the frequency of UPF consumption in the present study, its usefulness as a screener could be further explored, especially in large epidemiological investigations. Third, the outcomes of interest were depicted according to a single ultrasound scan, as multiple measurements in late pregnancy were not available. Attained growth can be considered a ‘distance’ measure(Reference Ohuma, Villar and Feng37), in a sensitive period in pregnancy that enabled the first investigation, to our knowledge, of the implications of exposure to UPF for different fetal growth parameters.
Several strengths ought to be outlined as well. With a prospective design, this study was conducted in a low-resource setting and followed a strict protocol for quality control of ultrasound examinations(Reference Lourenço, Lima and Vivanco14). Fetal growth parameters were expressed as z-scores according to gestational age based on international standards(Reference Papageorghiou, Ohuma and Altman26). Interpretation of estimates abides, therefore, a prescriptive approach – the negative associations of the exposure to UPF are deemed not in relation to absolute measures of HC and FL but refer to how fetal growth should have occurred. Finally, we did not focus on associations with average growth only, but could rather explore distinct portions in the distribution of outcomes, which can be a valuable approach for tracking percentiles that raise concern for growth faltering since before birth.
In conclusion, this study showed that an increased frequency of maternal UPF consumption in the antenatal period was negatively associated with fetal growth, as assessed by ultrasound examinations in late pregnancy. Negative associations between maternal UPF consumption and skeletal components of HC and FL were observed but were not apparent for AC. These findings reinforce the relevance of maternal diet in a life-course approach for promoting healthier infant growth trajectories, with potential repercussions on body composition. Interventions aimed at restricting UPF consumption before and during pregnancy would be beneficial for perinatal outcomes.
Acknowledgements
The authors are thankful to all participants, professional health workers and research team members of the MINA-Brazil Study Group involved in fieldwork for this study, as well as the Municipal Health Secretariat, all primary health care units, and the Juruá Women’s and Children’s Hospital of Cruzeiro do Sul. Members of MINA-Brazil Study Group are Marly Augusto Cardoso (PI), Alicia Matijasevich, Bárbara Hatzlhoffer Lourenço, Maíra Barreto Malta, Marcelo Urbano Ferreira, Paulo Augusto Ribeiro Neves (University of São Paulo, São Paulo, Brazil); Ana Alice Damasceno, Bruno Pereira da Silva, Rodrigo Medeiros de Souza (Federal University of Acre, Cruzeiro do Sul, Brazil); Simone Ladeia-Andrade (Oswaldo Cruz Institute, Fiocruz, Rio de Janeiro, Brazil) and Marcia C. Castro (Harvard T.H. Chan School of Public Health, Boston, USA).
The MINA-Brazil Study received funding from the Brazilian National Council for Scientific and Technological Development (CNPq, grant number 407255/2013-3); the Maria Cecília Souto Vidigal Foundation and the São Paulo Research Foundation (FAPESP, grant number 2016/00270–6). BHL was a recipient of the Fulbright Junior Faculty Member Award, Cycle 2021–2022; and of a Junior Visiting Professor scholarship from the Coordination for the Improvement of Higher Education Personnel (CAPES-PRINT, grant number 88887·569688/2020–00). MAC is a recipient of a senior research scholarship from CNPq (grant number 303794/2021-6). The funding agencies had no role in the design, data collection, analysis and interpretation or the writing of this article.
B. H. L., M. C. C. and M. A. C. conceptualised the study, developed data collection materials and oversaw quality assurance protocols. P. M. S., P. A. R. N., E. V. and D. L. L. were involved in fieldwork. M. C. C. and M. A. C. supported project administration and funding. B. H. L. planned and conducted data analysis, interpreted study results and wrote the original manuscript draft. All authors read, critically reviewed and approved the final manuscript as submitted.
There are no conflicts of interest.








