Phytochemicals are synthesised by plants and are well-known to exhibit health protective properties. A major group of phytochemicals is polyphenols, largely comprised of flavonoids. The basic structure of flavonoids is a benzo-γ-pyrone ring, a functional hydroxyl group that performs antioxidant activities by scavenging free radicals and chelating mineral ions( Reference Kumar and Pandey 1 – Reference Kumar, Mishra and Pandey 3 ). Dietary flavonoids are an integral part of the human diet and have received much attention in recent years as non-essential nutrients. Based on their chemical structure, dietary flavonoids are commonly categorised into six principal subclasses: flavonols, flavones, flavanones, flavan-3-ols and their oligomers and polymers, anthocyanidins and isoflavones. The oligomers and polymers of flavan-3-ols are also labelled as proanthocyanidins as a distinct subclass( Reference Beecher 4 ). The antioxidant properties and related health effects differ among these subclasses( Reference Manach, Williamson and Morand 5 , Reference Williamson and Manach 6 ).
Many epidemiological studies have shown that dietary flavonoids are associated with lower incidences of degenerative diseases such as CVD( Reference McCullough, Peterson and Patel 7 – Reference Wang, Ouyang and Liu 9 ), type 2 diabetes( Reference Zamora-Ros, Forouhi and Sharp 10 , Reference Wedick, Pan and Cassidy 11 ), dementia( Reference Beking and Vieira 12 , Reference Gao, Cassidy and Schwarzschild 13 ) and cancer( Reference Romagnolo and Selmin 14 – Reference Cutler, Nettleton and Ross 16 ) – for example, flavonols showed a protective effect against type 2 diabetes in a Framingham Offspring cohort study( Reference Jacques, Cassidy and Rogers 17 ), and anthocyanidins, flavan-3-ols, flavones and flavonols were individually associated with lower mortality caused by CVD in the Cancer Prevention Study II Nutrition Cohort( Reference McCullough, Peterson and Patel 7 ). However, many knowledge gaps still exist in this field. Some studies have reported inconsistent associations( Reference Nettleton, Harnack and Scrafford 18 – Reference Wang, Lee and Zhang 22 ), and the underlying mechanisms have not been fully clarified( Reference Scholz and Williamson 23 ). Thus, further studies, especially large and long-term trials, are needed to develop dietary recommendations( Reference Williamson and Holst 24 , Reference Gaine, Balentine and Erdman 25 ). One of the critical difficulties in such research lies in the ability to accurately estimate flavonoid intake.
Assessing dietary flavonoid intakes and their major food sources is the first step in documenting associations between flavonoids and diseases. Nonetheless, the lack of data about the flavonoid content of foods has limited our ability to estimate the flavonoid intakes among populations. The major challenges in estimating dietary flavonoid intakes are associated with the high variability in the flavonoid content of foods across different locations and types of cultivation, even within the same crop variety, especially given the large number of individual flavonoid components( Reference Erdman, Balentine and Arab 26 , Reference Haytowitz, Bhagwat and Holden 27 ). To date, several countries have established national databases on flavonoids, including the USA( Reference Bhagwat, Haytowitz and Holden 28 – 30 ) and France( Reference Neveu, Perez-Jimenez and Vos 31 – Reference Rothwell, Perez-Jimenez and Neveu 33 ). In addition, a small number of studies on the estimation of flavonoid intakes and their major dietary sources has been published, but most of these studies have targeted populations other than Korean( Reference Dragsted, Strube and Leth 34 – Reference Bai, Wang and Ren 36 ). A few studies that have focused on the flavonoid intake of Koreans have reported intakes only from fruits and vegetables( Reference Yang, Kim and Yang 37 , Reference Lee, Cho and Park 38 ) or are based on foreign databases( Reference Yeon, Bae and Kim 39 ).
The Korea National Academy of Agricultural Science recently released the Korea Functional Food Composition Table, incorporating the concentrations of flavonoids and other compounds( 40 ). Although the table includes the flavonoid contents of selected foods cultivated in Korea, it still requires further development to assess common Korean diet. We tried to expand the table to construct a new flavonoid database of common Korean foods (KFDB) to cover the food items typically consumed by the Korean population. To the best of our knowledge, this is the first study to assess the flavonoid intake of the Korean population using a flavonoid database including seven flavonoid subclasses (the above-mentioned six subclasses and proanthocyanidins) and covering not only plant foods but also other food groups such as legumes and beverages and alcohols. We aimed to estimate the total and individual flavonoid intakes among Korean adults and determine the major dietary sources of these flavonoids.
Methods
Study population
The Korea National Health and Nutrition Examination Survey (KNHANES), conducted by the Korea Centers for Disease Control and Prevention (KCDC), is a national surveillance system that assesses the health and nutritional status of Koreans. To date, the survey has been executed in five rounds (1998, 2001, 2005, 2007–2009 and 2010–2012). The fourth and fifth rounds were conducted annually, throughout the year to avoid seasonal bias in diet. The subjects of each round were selected to represent the Korean population, aged 1 year and older, using a multistage clustered probability design. Written informed consent was obtained from all the subjects.
Among 45 044 subjects who had completed a 24-h recall in the fourth and fifth KNHANES, 33 581 subjects, aged 19 years and older, were selected for this study. The KNHANES data collection was approved by the KCDC Institutional Review Board, and this study was approved by the Institutional Review Board of the Seoul National University.
Collection of diet and lifestyle information
Trained dietitians conducted a nutrition survey at each participant’s home to obtain information on dietary intake and dietary behaviour. Dietary intake data collected through a 1-d 24-h recall were transformed into individual food intakes using the KNHANES recipe database. Dietary behaviour data collected through questionnaires comprised the following variables. With respect to supplement use, the participants were asked whether they had taken supplements or not in the fourth KNHANES and whether they had taken supplements more than once a week during the month before the interview in the fifth KNHANES. Breakfast consumption was divided into two categories: ‘yes’ (ate breakfast on both the days before the interview) or ‘no’. Food security was divided into four categories: full food security (able to eat an adequate amount and variety of food), marginal food security (able to eat an adequate amount but not variety of food), low food security (sometimes unable to afford enough food) and very low food security (often unable to afford enough food).
A structured questionnaire including information on socio-demographic and lifestyle factors was administered by trained interviewers at mobile examination centres. Household income was divided into four categories: low income (first quartile), middle-low income (second quartile), middle-high income (third quartile) and high income (fourth quartile). Educational level was divided into four categories: elementary school and lower, middle school, high school and college and higher. Regular alcohol consumption was divided into two categories: ‘yes’ (drank more than once a month during the year before the interview) or ‘no’. Current smoking was divided into two categories: ‘yes’ (smoked >100 cigarettes in their lifetime and are still smoking) or ‘no’. Physical activity was divided into two categories: ‘active’ (performed vigorous physical activity that requires a large amount of effort and causes rapid breathing – e.g. running, fast cycling and playing football for more than 20 min – on more than 3 d during the week before the interview) or ‘inactive’.
Construction of the flavonoid database and estimation of flavonoid intake
We constructed a KFDB. The food list in the database is comprised of 3193 foods consumed by the subjects of the fourth and fifth KNHANES. The KFDB was constructed based on the Korea Functional Food Composition Table( 40 ), US Department of Agriculture (USDA) flavonoid database for selected foods( Reference Bhagwat, Haytowitz and Holden 28 – 30 ) and the Phenol-Explorer database( Reference Neveu, Perez-Jimenez and Vos 31 – Reference Rothwell, Perez-Jimenez and Neveu 33 ). For the food items without flavonoid content in these databases, values from published articles were used after quality assessment according to the modified USDA data quality evaluation system( Reference Holden, Bhagwat and Haytowitz 41 ). The values for remaining food items were replaced with estimated values from similar food items, using the moisture conversion factors and logical zeros. Moisture conversion factors developed by the KCDC were applied for food items that have a similar nutrient composition but different processing or preparation methods, based on the criteria developed by Chun et al. ( Reference Chun, Chung and Song 35 ). Logical zeros were applied when the food groups were expected to contain no flavonoids, such as meats and poultry and fish and shellfish. The database included seven major flavonoid subclasses and their thirty-one main flavonoid aglycones: flavonols (quercetin, kaempferol, myricetin and isorhamnetin), flavones (luteolin and apigenin), flavanones (eriodictyol, hesperetin and naringenin), flavan-3-ols (catechin, epicatechin, epigallocatechin, theaflavin, theaflavin-3-gallate, theaflavin 3′-gallate, theaflavin 3,3′-digallate and thearubigin), anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin, peonidin and petunidin), isoflavones (daidzein, genistein and glycetin) and proanthocyanidins (dimers, trimers, 4–6 monomers, 7–10 monomers and >10 monomers).
We linked food consumption data from the KNHANES with the developed KFDB to estimate the subjects’ flavonoid intake. Flavonoid content was expressed as aglycones (mg/d). The individual flavonoid intake from a food item was calculated by multiplying the flavonoid content by the total grams of food intake. The daily individual flavonoid intake was the sum of each individual flavonoid intake from all the food sources reported in the 1-d 24-h recall data. The daily total flavonoid intake was determined by the summation of the daily individual flavonoid intakes.
Dietary sources of flavonoid intake
We determined the major dietary sources of flavonoid intake. To identify the major food group sources of the total and subclasses of flavonoid intake, eighteen food groups were categorised based on the food group criteria of the KNHANES: grains, potatoes and starches, sugars and sweets, legumes and legume products, nuts and seeds, vegetables, mushrooms, fruits, meats and poultry, eggs, fish and shellfish, seaweeds, milk and dairy products, oils and fats, beverages and alcohols, seasonings, prepared foods and others. Furthermore, to estimate the predictive effect of the specific food groups including legumes and legume products, vegetables, fruits and beverage and alcohols, serving size (g) for each food item was assigned based on the Korean Food Guidance System as described by Jung et al.( Reference Jung, Han and Song 42 ). The daily servings of a food item consumed by each subject were calculated by dividing the intake of the food by one serving size of the food item.
Statistical analysis
All the statistical analyses were carried out using SAS software (version 9.3; SAS Institute). Clusters, strata and survey weights were applied to all the analyses to adjust for the complex survey design of the KNHANES, which enabled the results to represent the Korean population. The daily intakes of flavonoids of the subpopulations grouped by socio-demographic and lifestyle factors were described using the means with their standard errors. The differences among flavonoid intakes of the subgroups after adjusting for the total energy intake were determined by Student’s t test and ANOVA using the SURVEYREG procedure. The contribution of each individual flavonoid intake to the intake of total flavonoids and subclasses was calculated as a percentage. The contribution of each food and food group to the intake of total flavonoids and subclasses was also calculated as a percentage. We also carried out multiple regression analysis to determine the extent to which total flavonoid and subclass intakes were explained by the intake of specific food groups. The model included the daily consumption of servings of legumes and legume products, vegetables, fruits and beverages and alcohols as independent variables. Factors that showed a significant difference in the t test and ANOVA were included in the model for statistical control: survey phase, sex, household income, education level, regular alcohol drinking, current smoking, supplement use, breakfast consumption, food security and total energy intake. These variables showed no substantial collinearity or skewness. Statistical significance was accepted at P<0·05, and all the P values were two-sided.
Results
Evaluation of the developed flavonoid database for common Korean foods
The developed KFDB covered 49 % (1579 items of 3193 items) of foods reported in the KNHANES 2007–2012 24-h recall data. The coverage was high in fruits (90 %), seaweeds (85 %), legumes and legume products (79 %), potatoes and starches (73 %), nuts and seeds (72 %), prepared foods (62 %) and vegetables (54 %), but low in oils and fats (33 %), mushrooms (32 %), milk and dairy products (30 %), beverages and alcohols (23 %), seasonings (23 %), grains (22 %), sugars and sweets (19 %) and others (14 %). The coverage for meats and poultry and fish and shellfish was 100 % due to the application of logical zero. As a result, 76 % of the total amount of food consumed by all the participants was covered by the KFDB.
Estimated daily flavonoid intake
The daily flavonoid intakes of subjects are shown in Table 1. The mean total flavonoid intake among the subjects was 318·0 (se 4·1) mg/d. When the total energy intake was adjusted, total flavonoid intake was higher in women (P<0·001), non-regular alcohol consumers (P<0·001), non-current smokers (P<0·001), supplement users (P<0·001) and breakfast eaters (P<0·001) compared with their counterparts. Energy-adjusted total flavonoid intake increased with household income (P<0·001), education level (P<0·001), eating out frequency (P<0·001) and food security (P<0·001). The 50–64 years age group had the highest intake of total flavonoids after energy adjustment, followed by the 30–49, 65–74, ≧75 and 19–29 years age groups. With respect to the flavonoid subclasses, proanthocyanidins were the major contributors (22·3 %) to the total flavonoid intake, followed by flavonols (20·3 %), isoflavones (18·1 %), flavan-3-ols (16·2 %), anthocyanidins (11·6 %) and flavanones (11·3 %); flavones (0·4 %) accounted for the lowest proportion. The contributions of individual flavonoids to the intake of total flavonoid and subclasses are shown in Table 2.
Table 1 Daily dietary flavonoid intakes of Korean adults by socio-demographic and lifestyle factors (Mean values with their standard errors)

P values for the differences by the t tests and ANOVA among the subgroups after adjusting for total energy intake * P<0·05, ** P<0·01, *** P<0·001.
† Household income: low (first quartile), middle-low (second quartile), middle-high (third quartile), high (fourth quartile).
‡ Regular alcohol drinking: ‘yes’ meant that the person drank more than once a month over the past year.
§ Current smoking: ‘yes’ meant that the person smoked >100 cigarettes over his or her lifetime and still smokes.
|| Physical activity: ‘active’ meant that the person performed vigorous-intensity physical activity, which requires a large amount of effort and causes rapid breathing for >20 min once for >3 d/week.
¶ Supplement use: ‘yes’ meant taking dietary supplements.
†† Breakfast consumption: ‘yes’ meant that the person had breakfasts on both the days before the interview.
‡‡ Food security: ‘full’ meant able to eat an adequate amount and variety of food, ‘marginal’ meant able to eat an adequate amount but not variety of food, ‘low’ meant sometimes unable to afford enough food, ‘very low’ meant often unable to afford enough food.
Table 2 Composition of the individual flavonoid intakes among Korean adults

Major dietary sources of flavonoid intake
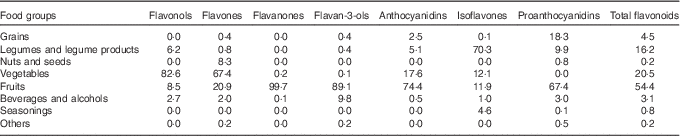
The main food group contributors to flavonoid intake are shown in Table 3. The major dietary food group sources of total flavonoids were fruits (54·4 %), followed by vegetables (20·5 %), legumes and legume products (16·2 %), grains (4·5 %) and beverages and alcohols (3·1 %). The main contributors to the intake of flavonols, flavones and anthocyanidins were vegetables and fruits. Fruits were the most important contributors to the intakes of flavanones and flavonols, and legumes and legume products were the most important contributors to the intake of isoflavones. Proanthocyanidins were obtained mainly from fruits, grains and legumes and legume products.
Table 3 Contribution (%) of food groups to flavonoid intakes among Korean adults
The main individual food contributors to flavonoid intake were also analysed (Table 4). We identified apples (21·9 %), mandarins (12·5 %), tofu (11·5 %), onions (9·6 %) and grapes (9·0 %) as the major sources of the total flavonoids. For flavonols, onions (49·2 %), radish leaves (14·4 %), radishes (9·7 %), soyabeans (6·1 %) and apples (5·5 %) were the main contributors. The most abundant sources of flavones were chili peppers (33·8 %), sweet peppers (12·1 %), watermelons (9·0 %), parsley (8·5 %) and perilla seeds (8·3 %). Mandarins (90·3 %) and oranges (6·5 %) were the exclusive sources of flavanones, and apples (84·9 %) and green tea (7·6 %) were the major sources of flavan-3-ols. Grapes (37·6 %), persimmons (24·3 %) and radishes (16·4 %) were the prime sources of anthocyanidins, and apples (27·8 %), grapes (17·7 %) and sorghum (12·7 %) were the major sources of proanthocyanidins. We identified tofu (66·7 %) as the richest contributor of proanthocyanidins, followed by mandarins (11·7 %), soyabean sprouts (11·6 %), soyabean paste (4·0 %) and soyabeans (2·1 %).
Table 4 Top five food sources contributing to the flavonoid intake among Korean adults

* Cumulative percentage of the food item contributing to flavonoid subclass and total flavonoid intakes.
† Includes radish, raw; radish, dried; radish, pickled; red beet; turnip.
‡ Includes parsley, raw; parsley, dried.
§ Includes fruit juice; fruitade; fruit nectar; fruit-flavoured drink.
|| Includes green tea, powder; green tea, leaves; green tea, infusion.
¶ Includes black tea, tea bag; black tea, infusion; black tea, flavoured.
In the multiple regression analysis, intakes of legumes and legume products, vegetables and fruits were major predictors of total flavonoid intake. Regression estimates indicated that one serving of legumes and legume products, vegetables and fruits per d could increase the total flavonoid intake by 82·31 mg/d (P<0·0001), 3·94 mg/d (P=0·0086) and 78·30 mg/d (P<0·0001), respectively. The consumptions of legumes and legume products, vegetables, fruits and beverage and alcohols were significant predictors of flavonol intake; those of legumes and legume products, vegetables and fruits were strong predictors of isoflavone intake; and those of legumes and legume products and fruits were strong predictors of proanthocyanidin intake. In the model for anthocyanidins intake, fruits consumption was the only significant predictor (Table 5).
Table 5 Regression estimates of food groups for flavonoid intakes among Korean adults (β Coefficients and coefficient of determination)

* Adjusted by survey phase, sex, household income, education level, regular alcohol drinking, current smoking, supplement use, breakfast consumption, food security and total energy intake.
† Flavones, flavanones and flavonols in which model R 2 was <0·150 are not shown.
‡ P values for all the models were <0·0001.
§ Servings of each food group was calculated according to the Korean Food Guidance System.
Discussion
As only a few studies on flavonoid intakes have focused on the Korean population, we investigated the epidemiological characteristics of flavonoid intakes in Korean adults. We constructed a new KFDB and found that the mean daily total flavonoid intake of Korean adults was 318·0 mg/d, and the major food sources were fruits, vegetables and legumes and legume products.
A comprehensive database is an essential part in epidemiological studies for estimating flavonoid intake( Reference Erdman, Balentine and Arab 26 ). The Korea Functional Food Composition Table, published by the National Academy of Agricultural Science, is the only currently available phytochemical database for Korean common foods, but it covers a limited number of food items( 40 ). To fill the gap, we constructed a new flavonoid database for common Korean foods that appeared in the KNHANES (2007–2012) food consumption data. The new flavonoid database covered 49 % of the food items and 76 % of the food intake for thirty-one individual flavonoids in seven flavonoid subclasses, which could enhance the accuracy of flavonoid intake estimation.
The mean daily intake of total flavonoids by Korean adults was 318·0 mg, which was slightly lower compared with other countries. The mean intake of total flavonoids by US adults was estimated to range from 189·8 to 207·30 mg/d based on six of the seven subclasses of flavonoids, excluding proanthocyanidins( Reference Chun, Chung and Song 35 , Reference Chun, Floegel and Chung 43 ), and 344·83 mg/d based on all seven subclasses( Reference Bai, Wang and Ren 36 ). European adults in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort had a mean intake of 370·2–437·2 mg/d when seven subclasses except for thearubigins were included( Reference Zamora-Ros, Sacerdote and Ricceri 44 , Reference Zamora-Ros, Knaze and Lujan-Barroso 45 ); the Spanish cohort had an intake of 376·69 mg/d when all seven subclasses and their thirty-five individual flavonoids were included( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 46 ). In a sample of Chinese adults, the average daily intake of total flavonoids was estimated to be 165·6 mg/d from fruit, vegetable and nut intakes for anthocyanidins, flavonols, flavones and isoflavones( Reference Li, Zhu and Zhang 47 ). The differences in these amounts could be due to the differences in diet among the populations, dietary data collection methods, the extent of flavonoid components included in the databases, cultivars and geographical features of the food sources and other factors.
In the present study, total flavonoid intake was significantly higher among females, especially in the 30–64-year-old group, subjects with higher household incomes and higher education level, non-regular alcohol drinkers, non-current smokers, those who take supplements, those who eat breakfast regularly and those with higher food security, after adjusting for total energy intake. Distinct distributions by sex and age can be explained by the findings of a previous study, which reported that the percentages of Korean adults consuming the recommended daily fruit and vegetable intakes were higher among females than in males and were higher in the ≧40-year-old group than in the other groups( Reference Lee, Cho and Park 38 ). The older adults might adhere more to a traditional Korean diet, which is mainly composed of plant food( Reference Lee, Popkin and Kim 48 ) and pay more attention to health care. People with a lower socio-economic status, as characterised by a low income level, low education level and low food security, were consistently reported to consume less amounts of flavonoids( Reference Zamora-Ros, Forouhi and Sharp 10 , Reference Yang, Kim and Yang 37 , Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 46 ), which seem to be attributable to lower amounts of fruits and vegetables( Reference Irala-Estevez, Groth and Johansson 49 – Reference Hanson and Connor 51 ). Moreover, unhealthy lifestyles that included regular alcohol drinking, current smoking, irregular physical activity and infrequent breakfast consumption also resulted in an inadequate intake of flavonoid-high food items such as fruits and vegetables( Reference Hur, Jang and Oh 50 – Reference Friel, Newell and Kelleher 53 ). The significant difference in flavonoid intake between supplement users and non-users in our study was also shown by other researchers( Reference Chun, Chung and Song 35 , Reference Chun, Floegel and Chung 43 ). The prevalence of chronic diseases may be influenced by these socio-demographic and lifestyle factors through the mediation of different flavonoid intakes.
The top five food items contributing to the total flavonoid intake in Korean adults were apples, mandarins, tofu, onions and grapes. A US study reported that the major food sources of daily flavonoid intakes were tea, wines, beers, citrus fruits and apples( Reference Bai, Wang and Ren 36 ), and the EPIC study also reported that fruits, tea, wines and fruit juices were the main food sources of total flavonoid intake( Reference Zamora-Ros, Sacerdote and Ricceri 44 ). As soyabean products such as soyabean paste and tofu are part of the traditional Korean diet, legumes and legume products contributed far more to the total flavonoid intake in the Korean population compared with that of the US and European populations, whereas tea and wines contributed far less in Koreans.
In addition to the differences in total flavonoid intake, the differences in flavonoid compositions have been observed across studies. In this study, proanthocyanidins (22·3 %, 70·8 mg/d) contributed the most to the total daily flavonoid intake, followed by flavonols (20·3 %, 64·6 mg/d), isoflavones (18·1 %, 57·5 mg/d) and flavan-3-ols (20·5 %, 51·4 mg/d). Zamora-Ros et al.( Reference Zamora-Ros, Forouhi and Sharp 10 ) reported similarly that proanthocyanidins (44·0 %, 182·7 mg/d) were the primary contributors to the total flavonoid intake in EPIC-InterAct study participants, largely from fruits, wines, chocolate and juices. Among European countries, different amounts of proanthocyanidin intake were observed. The southern region, including France, Italy and Spain, consumed more proanthocyanidins than the Central and Northern regions( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 46 , Reference Vogiatzoglou, Mulligan and Luben 54 , Reference Knaze, Zamora-Ros and Lujan-Barroso 55 ). On the other hand, Bai et al.( Reference Bai, Wang and Ren 36 ) reported proanthocyanidins as secondary contributors (28·5, 98·29 mg/d) to the total flavonoid intake in US adults, largely from wines, legumes, tea, strawberries and plums. However, Korean adults consumed a large amount of proanthocyanidins from fruits, grains and legumes and legume products.
Several studies on flavonoid intake have reported that flavan-3-ols are the greatest contributors to the total flavonoid intake, 156·5 mg/d in the USA study with three flavan-3-ol monomers, theaflavins and thearubigins in their database( Reference Chun, Chung and Song 35 , Reference Wang, Chung and Song 56 ), and 182·4 mg/d in the EPIC study with seven monomers and theaflavins in their database( Reference Zamora-Ros, Sacerdote and Ricceri 44 ). In a study by Otaki et al.( Reference Otaki, Kimira and Katsumata 57 ) that did not include proanthocyanidins and anthocyanidins in their analysis, Japanese women obtained flavonoids mainly from flavan-3-ols, comprised of only three flavan-3-ol monomers (80·0 %, 1277 μmol/d; 386·30 mg/d). The flavan-3-ol intake level in the present study (50·6 mg/d) was very low compared with those in the studies of other populations. Our finding was supported by a study that estimated the daily flavan-3-ol intake for its twelve components from only plant food sources among Korean adults at 30·0 mg/d for men and 22·5 mg/d for women( Reference Yang, Kim and Yang 37 ), suggesting that Koreans consume a relatively small amount of flavan-3-ols. Considering that the major sources of flavan-3-ols in other countries are tea, fruits and wine, the low consumption of tea and red wine by Korean adults may be the reason for this. Koreans obtained flavan-3-ols mostly from apples (84·9 %), followed by green tea, grapes, black tea and strawberries.
The largest difference has been found in the amount of isoflavone intake. Koreans consumed a lot more isoflavones than Western people. US and European people consume very small amounts of isoflavones, ranging from 0·10 to 2·35 mg/d( Reference Zamora-Ros, Forouhi and Sharp 10 , Reference Chun, Chung and Song 35 , Reference Bai, Wang and Ren 36 , Reference Zamora-Ros, Sacerdote and Ricceri 44 , Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 46 , Reference Ovaskainen, Torronen and Koponen 58 , Reference Dilis and Trichopoulou 59 ), whereas Asians consume 6·2–54·3 mg/d( Reference Arai, Watanabe and Kimira 60 , Reference Messina, Nagata and Wu 61 ). Dietary patterns influenced by cultural habits might be attributable to this disparity. For example, Asians consume more soyabeans and soyabean products, which are abundant in isoflavones than Western people. In addition to soya foods, fruits, grains and coffee are good dietary sources of isoflavones( Reference Zamora-Ros, Forouhi and Sharp 10 , Reference Lee, Cho and Park 38 ). We found that the major individual food sources of isoflavones in Korean adults were tofu (66·7 %) and mandarins (11·7 %), followed by soyabean sprouts, soyabean paste and soyabeans.
The estimated flavonol intake of 64·6 mg/d was higher than that in the USA (12·8–18·1 mg/d)( Reference Chun, Chung and Song 35 , Reference Bai, Wang and Ren 36 ), Europe (18·7–51·0 mg/d)( Reference Zamora-Ros, Forouhi and Sharp 10 , Reference Zamora-Ros, Sacerdote and Ricceri 44 , Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 46 , Reference Perez-Jimenez, Fezeu and Touvier 62 ), Japan (58·4 μmol/d, 17·5 mg/d)( Reference Otaki, Kimira and Katsumata 57 ) and China (19·13 mg/d)( Reference Zhang, Li and Cao 63 ). In accordance with these studies, which showed that flavonols are abundant in tea, fruits and vegetables, a significant amount of flavonol intake in our study was from vegetables and fruit. Meanwhile, flavones were the smallest contributors to total flavonoids, and the intake amount (1·0 mg/d) of Korean adults was smaller than that of the above-mentioned populations. This can be explained by the relatively lower flavone content and lower intake of its top five food sources. Yeon et al.( Reference Yeon, Bae and Kim 39 ) found a lower estimated flavanone intake in Korean middle-aged men and women (0·78 mg/d) than ours; this difference appears to be due to the more comprehensive flavonoid databases used in the present study.
Korean adults in the present study had a flavanone intake level of 35·9 mg/d. Flavanone intake was predominantly from fruits (99·7 %) such as mandarins, oranges, fruit drink, grapefruits and kumquats. The flavanone intakes of Finnish adults were estimated to be 31 mg/d( Reference Ovaskainen, Torronen and Koponen 58 ), of French adults 26 mg/d( Reference Perez-Jimenez, Fezeu and Touvier 62 ), of Spanish adults 50·44 mg/d( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 46 ) and of US adults 22·38 mg/d( Reference Bai, Wang and Ren 36 ). The anthocyanidin intake also varied among different studies. Finnish adults consumed the highest amount of anthocyanidins (47 mg/d), followed by Korean adults (37·0 mg/d) and Mediterranean people (9–37·42 mg/d)( Reference Zamora-Ros, Knaze and Lujan-Barroso 45 , Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 46 , Reference Dilis and Trichopoulou 59 , Reference Zamora-Ros, Knaze and Lujan-Barroso 64 ), whereas US adults consumed only 9·20 mg/d( Reference Bai, Wang and Ren 36 , Reference Kita, Tada and Ito 65 ). The dietary sources of anthocyanidins in Korean adults were fruits, vegetables, especially grapes, persimmons, radishes and strawberries, as reported in other studies( Reference Bai, Wang and Ren 36 , Reference Lee, Cho and Park 38 ), whereas legumes and legume products, such as soyabeans, were the unique contributors of anthocyanidin intake in Korean adults.
In our study, legumes and legume products, vegetables and fruits were strong predictors in the regression model of flavonoid intake by specific food group intake in Korean adults. If an individual consumes one more serving of legumes and legume products, vegetables and fruits per d, he or she can expect to have 82·31, 3·94 and 78·03 mg increase in the amount of total flavonoids per d, respectively. The higher regression coefficient of legumes and legume products and fruits than that of vegetables may be partly due to the higher weight of one serving. For anthocyanidins, only fruits were significant independent predictors, and one serving of fruits may lead to an increase of 21·98 mg/d of anthocyanidin intake. These findings can provide basic information for developing dietary recommendations for flavonoid intakes.
In this study, the expanded database that focused on the foods commonly consumed by Koreans, with greater coverage, allowed us to estimate the flavonoid intakes and their dietary sources among Koreans. This database can also be used in future studies to elucidate the health benefits of dietary flavonoids. However, there are several limitations to this study. First, our results could be underestimated because of the limited coverage of food items in the KFDB and the exclusion of dietary supplements in our analysis. Second, the accuracy of some flavonoid contents in the KFDB might be lower than expected. For example, thearubigin contents that we included in our database is a crude estimation quantified by an indirect method( Reference Zamora-Ros, Knaze and Romieu 66 ). Nevertheless, the inclusion of thearubigins may not have much impact on the estimation of total flavonoids as tea, the only food contributor of thearubigins, is not highly consumed in our population. Finally, the use of a single 24-h recall might not be sufficient to reflect an individual’s usual diet due to large intra-individual variability. Further research using more a comprehensive database and more accurate dietary assessment methods could improve the quality of our study.
Considering that the antioxidant capacity of flavonoids is reported to be related with health benefits( Reference Pietta 67 ), construction of a database including a wider scope of antioxidant materials, such as antioxidant vitamins and carotenoids, for foods and dietary supplements may enhance our understanding of the associations between the total antioxidant intake and human health, as suggested by a few studies( Reference Chun, Floegel and Chung 43 , Reference Yang, Chung and Chung 68 ). Furthermore, the estimated intake of dietary antioxidants does not always reflect the bioavailability of these antioxidants. Thus, the use of biochemical indicators, for instance, the plasma total antioxidant capacity that considers the antioxidant capacity in the human body, could enhance the efficacy of flavonoid research( Reference Schlesier, Harwat and Bohm 69 , Reference Wang, Chun and Song 70 ).
In conclusion, dietary flavonoid intakes of Korean adults were relatively low compared with other countries and their major food sources were fruits, vegetables and legumes and legume products. The findings of this study could facilitate further investigation on the health benefits of flavonoids among Koreans and provide the basic information for establishing recommended flavonoid intakes for Koreans.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2014R1A2A2A01003138). NRF had no role in the design, analysis or writing of this article.
The authors’ responsibilities were as follows: H. J. formulated the research questions and critically reviewed the manuscript; S. S. contributed to the research design; S. J. carried out database construction, data analysis and wrote the draft of the manuscript.
There are no conflicts of interest.








