Resolving stunting (length-for-age (LAZ) <−2 z scores below the reference median( 1 )) continues to elude public health practitioners, as its risk factors are tightly aligned with poverty but its prevention and remedy are apparently far more complex( Reference Headey 2 ). Stunting is associated with an increased risk of early childhood morbidity and mortality( Reference Black, Allen and Bhutta 3 ) and often persists into adulthood with lifelong and intergenerational consequences for health, human capital and economic development( Reference Victora, Adair and Fall 4 , Reference Dewey and Begum 5 ). Patterns differ somewhat by region, but stunting tends to emerge in the first few months of life and worsen into the 2nd year before stabilising but typically persisting in the absence of intervention( Reference Victora, de Onis and Hallal 6 ). In Bangladesh, the prevalence of stunting has fallen over the past decade, but national estimates continue to place it at over 40 %, and the prevalence is even higher among rural populations and those of lowest socio-economic status( 7 , 8 ).
A pervasive subclinical disease of the small intestine, termed ‘environmental enteric dysfunction’ or EED, may help explain the burden and persistence of stunting in low- and middle-income countries. EED is thought to result from chronic exposure to environmental pathogens and toxins( Reference Humphrey 9 – Reference Mapesa, Maxwell and Ryan 11 ), and may lead to impaired growth through malabsorption of nutrients and chronic systemic inflammation (SI)( Reference Crane, Jones and Berkley 12 , Reference Guerrant, Oria and Moore 13 ). Observational studies have reported high prevalence of EED in numerous settings( Reference Goto, Panter-Brick and Northrop-Clewes 14 – Reference Manary, Abrams and Griffin 16 ), but findings are mixed regarding associations between markers of EED and attained length or rate of linear growth( Reference Lunn, Northrop-Clewes and Downes 17 – Reference Guerrant, Leite and Pinkerton 23 ). Further, the extent to which EED contributes to stunting in rural Bangladesh has been explored only minimally in the existing literature.
In a prior study, we reported that lactulose:mannitol ratio (L:M) and other markers of EED and SI were widely elevated in a subset of 18-month-old children completing participation in a community-based randomised controlled trial of complementary food supplements (CFS) in rural northwest Bangladesh( Reference Campbell, Schulze and Shaikh 24 ). Despite not seeing effects of supplementation on markers of EED or SI, cross-tabulations with baseline anthropometric measures revealed some unexpected positive associations between 6-month size and subsequent gut dysfunction, suggesting a potential for associations between EED markers and subsequent growth to be confounded by prior anthropometry. These observations highlight a need to investigate EED in relation to growth trajectories using longitudinal models of length and weight that can address this potential confounding. In the present analysis, we examine, in the same study, length and weight and their trajectories from 15–24 months relative to a panel of EED biomarkers to determine the extent to which markers of EED and SI explain variability in children’s growth in their 2nd year of life.
Methods
Setting
Participants were a subset of children enrolled in a community-based randomised controlled CFS trial in the Gaibandha and Rangpur districts of northwest Bangladesh( Reference Christian, Shaikh and Shamim 25 ). The setting is rural and densely populated with agrarian livelihoods and small household landholdings, typical of rural South Asia. The research site has been host to several randomised nutrition and health trials( Reference West, Christian and Labrique 26 – Reference Klemm, Labrique and Christian 28 ) and numerous observational studies( Reference Shamim, Kabir and Merrill 29 – Reference Rah, Shamim and Arju 32 ) to characterise and improve maternal and child health.
In the CFS trial, child and household socio-demographic characteristics were assessed by interviewer-administered questionnaire at enrolment (age 6 months), and anthropometry including length and weight was assessed by trained and standardised interviewers at ages 6, 9, 12, 15, 18 and 24 months. To ensure the quality of anthropometric measurements, a randomly selected subset of measurements was rechecked by expert anthropometrists and interviewers were retrained and re-standardised as needed.
Environmental enteric dysfunction assessment
In a geographically designated subset of trial participants, biospecimens were collected at age 18 months to measure EED and SI. A target sample size of 500 balanced across the five study arms was set based on a primary aim of detecting effects of supplementation on L:M, within the logistical constraints of the methodology and setting. The sample size allowed for detection of a difference in L:M of 0·46 between supplemented groups and the control, assuming L:M standard deviation equal to 0·64 based on prior pilot data from the same study area (K Schulze, unpublished results), α=0·05, β=0·20 and adjusting for multiple comparisons. Biospecimens were collected between October 2013 and May 2014 to reach the target sample size. Urine following lactulose and mannitol dosing, blood for processing to serum and stool samples were collected. The full details of the sample collection and laboratory analyses have been described previously( Reference Campbell, Schulze and Shaikh 24 ). In brief, urine collected for 2–3 h following lactulose and mannitol solution dosing was weighed, mixed with chlorhexidine and stored in liquid N2 pending shipment to a collaborating laboratory at icddr,b, where it was analysed by high-pressure ion chromatography (Dionex; Thermo Fisher Scientific) for concentrations of lactulose and mannitol (CV 3·6 and 9·4 %, respectively). Blood collected during the same field clinic visit was allowed to clot and then, in the project laboratory, centrifuged and the serum transferred to cryovials and stored in liquid N2 for shipment to the Center for Human Nutrition laboratory at JHSPH. A single stool sample for each participant was collected in the household using sterilised collection materials, stored and transported in Styrofoam cold boxes and then processed in the project laboratory and stored in liquid N2 for shipment to JHSPH.
Stool samples were analysed for myeloperoxidase (MPO) and neopterin (NEO), markers of immune activity in the wall of the small intestine( Reference Dabritz, Musci and Foell 33 , Reference Campbell, McPhail and Lunn 34 ), and α-1 antitrypsin (AAT), a marker of protein-losing enteropathy( Reference Crossley and Elliott 35 ), using commercially available ELISA kits (MPO and NEO; ALPCO Diagnostics; AAT; BioVendor, LLC). Serum was analysed for IgG endotoxin core antibody (EndoCAb IgG; Hycult Biotech), a marker of intestinal permeability( Reference Campbell, Elia and Lunn 20 ), and glucagon-like peptide-2 (GLP-2), a growth factor indicative of enterocyte proliferation and repair( Reference Rowland and Brubaker 36 ), as well as C-reactive protein (CRP) and α-1 acid glycoprotein (AGP), markers of SI. EndoCAb and GLP-2 were assessed with commercial ELISA kits (EndoCAb IgG and GLP-2; EMD Millipore), whereas CRP was assessed by chemiluminescent immunoassay (Siemens Diagnostics) and AGP by commercial radial immunodiffusion kit (Kent Laboratories). Standards and controls provided in the kits, along with participant sample-derived control samples, were run in duplicate on each plate and CV monitored.
Data management
Household characteristics assessed at baseline (child age 6 months) included maternal and paternal education and profession, asset ownership and the physical structure of the house. Assets and house structure variables were captured in a wealth score (living standards index (LSI)) according to a method previously developed for this setting( Reference Gunnsteinsson, Labrique and West 37 ), and dichotomised as low and high around the median value within this study sample.
Lactulose and mannitol concentrations were converted to the recovery ratio (L:M) by dividing the total recovered volume of each by the initial dose and expressed as the ratio of percent lactulose recovery to percent mannitol recovery. L:M >0·07 was considered elevated and indicative of EED( Reference Hossain, Nahar and Hamadani 15 ).
Anthropometry at ages 15, 18 and 24 months was used for this analysis to examine growth in the 3 months before and 6 months after the assessment of EED and SI biomarkers. LAZ, weight-for-age (WAZ) and weight-for-length (WLZ) z scores relative to the WHO growth standards( 1 ) and prevalence of stunting, underweight and wasting (LAZ, WAZ and WLZ, respectively, <−2) at each interview time point were calculated. Extreme outlying values (|z score|>6) were omitted from the analysis. Variables for the change in each anthropometric measure from 15 to 18 months and from 18 to 24 months were generated with adjustment for the exact number of days between the pair of measurements.
Statistical methods
Biomarkers were log-transformed before analysis. Two orthogonal scores of EED – a ‘gut inflammation’ (GI) score and a ‘gut permeability’ (GP) score derived with principal component analysis on log-transformed biomarkers (online Supplementary Table S1) as described previously( Reference Campbell, Schulze and Shaikh 24 ) – were used as indicators of EED along with L:M. The derived EED scores were standardised to units of standard deviation around their mean values and oriented to each have minimum value 0 and higher values indicative of worse intestinal health. Children in the five trial arms were combined for this analysis, as no evidence of supplementation effects on markers of EED or SI was observed( Reference Campbell, Schulze and Shaikh 24 ). Final models were adjusted for supplementation group, as well as for child age and household LSI.
To investigate bivariate associations between biomarkers at 18 months and anthropometry at 18 months and changes in anthropometry over the preceding (15–18 months) and subsequent (18–24 months) age intervals, pairwise Pearson’s correlation coefficients were generated for each biomarker – anthropometric measure pair.
Longitudinal models were then developed to harmonise associations between biomarkers and anthropometric measures in individual intervals identified using correlation analysis. Mixed- effects (ME) models were developed for repeated measures of LAZ, WLZ and WAZ, an approach that accounts for observed correlations among repeated anthropometric measures of the same child. A spline term was included for age with a knot at 18 months, in accordance with hypotheses about distinct effects of biomarkers on growth trajectories before and after the 18-month assessment. Models were built in a step-by-step manner with each addition evaluated with P values for the added term(s), likelihood ratio tests relative to the simpler model and visual inspection of residual variance plots, as outlined in Grajeda et al.( Reference Grajeda, Ivanescu and Saito 38 ). Random slopes, autoregressive correlation structure and correlated random intercepts and slopes were sequentially introduced and evaluated.
Markers of EED (L:M, GI score, GP score) and SI (CRP, AGP) were each added to the longitudinal models as fixed effects with interaction terms between biomarkers and the age and age spline terms to determine the extent to which they explained within- and between-child variation in growth trajectories. Separate models were developed for each biomarker for ease of interpretation given minimal collinearity among biomarkers( Reference Campbell, Schulze and Shaikh 24 ). The models were used to generate coefficients estimating the shift in baseline (15 months) anthropometry and in the rate of growth in anthropometric unit per month from 15 to 18 months and 18 to 24 months, respectively, associated with a 1-sd change in biomarker value. Confidence intervals for the individual coefficients and P values for the likelihood ratio test comparing otherwise identical models with and without the biomarker were used to evaluate the explanatory value of the biomarker.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all protocols were approved by the institutional review boards of the Johns Hopkins Bloomberg School of Public Health and icddr,b. Written parental consents were required for participation in the supplementation trial and in the EED biomarker assessment. Analyses were conducted using Stata, version 14.1 (StataCorp).
Results
Of 566 eligible children, parental consent for 539 was obtained for the EED assessment. Households sampled were typical of the setting: all had access to an improved source of water and most (83 %) had an improved sanitation facility, whereas only about 30 % had electricity (Table 1). Most mothers (78 %) had some education, but only 14 % completed high school. Fathers were predominantly employed as farmers (48 %) or day labourers (35 %). Length and weight data were available for 527 (98 %), 539 (100 %) and 513 (95 %) children at the 15-, 18- and 24-month assessments, respectively. Stunting was common, with 45 % stunted at 18 months, and prevalence of underweight and wasting was 37 and 15 %, respectively. L:M, EED scores (GI and GP) and inflammation markers (CRP and AGP) were measured in 476 (88 %), 436 (81 %) and 505 (94 %) children, respectively. Geometric mean of L:M assessed at age 18 months was 0·06 (95 % CI 0·05, 0·06), and 39 % of children had elevated L:M values (>0·07). SI was also common: CRP and AGP were elevated (>5 mg/l and >1g/l( Reference Thurnham, Northrop-Clewes and Knowles 39 ), respectively) in 20 and 56 % of children, respectively, but only 4 % had elevated CRP only with normal AGP. The mean values of the standardised GI and GP scores were 2·9 (sd 1·0) and 2·8 (sd 1·0), respectively, with higher values of each indicative of poorer intestinal health( Reference Campbell, Schulze and Shaikh 24 ).
Table 1 Household and individual characteristics of environmental enteric dysfunction study participants (n 539) (Numbers and percentages; mean values and standard deviations; geometric means (GM) and 95 % confidence intervals)
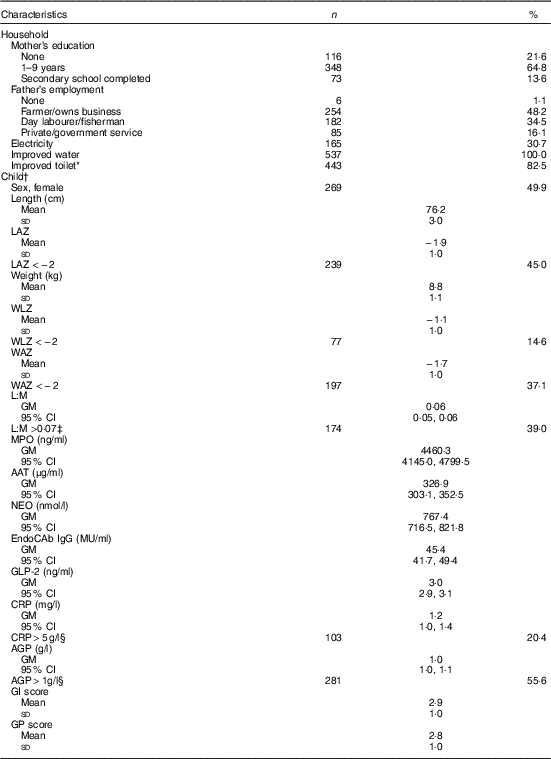
LAZ, length-for-age z score; WLZ, weight-for-length z score; WAZ, weight-for-age z score; L:M, lactulose:mannitol ratio; MPO, myeloperoxidase; AAT, α-1 antitrypsin; NEO, neopterin; EndoCAb IgG, endotoxin core antibody IgG; GLP-2, glucagon-like peptide-2; CRP, C-reactive protein; AGP, α-1 acid glycoprotein; GI, gut inflammation score; GP, gut permeability score.
* Improved toilet defined as household access to a water-sealed or slab latrine.
† Anthropometry and biomarkers measured at age 18 months.
‡ Cutoff for elevated values( Reference Hossain, Nahar and Hamadani 15 ).
§ Cutoff for elevated values( Reference Thurnham, Northrop-Clewes and Knowles 39 ).
In correlation analysis of biomarkers and anthropometry, few associations were observed between the biomarkers and LAZ (Table 2). In contrast to expectations, L:M was positively correlated with change in LAZ between 15 and 18 months (ρ=0·1, P=0·045), such that children with greater L:M at 18 months had grown relatively more in the prior 3 months than those with lower L:M. GP score was positively correlated with LAZ at 18 months (ρ=0·13, P=0·005), but not with change in length in the surrounding periods. GI score and AGP were not correlated with any LAZ measures. Weight trajectories (WLZ and WAZ) were not correlated with L:M, but were with the other scores, in divergent patterns: GI score was associated with declining WLZ and WAZ from 18 to 24 months only (ρ=−0·13, P=0·006 and ρ=−0·14, P=0·005, respectively), whereas GP score and AGP were correlated with WLZ and WAZ in all of the intervals examined. GP score was associated with declining WLZ and WAZ from 15 to 18 (P≤0·01) and 18 to 24 months (P<0·02), but was positively correlated with 18-month WLZ and WAZ (P<0·04). In contrast, higher AGP was associated with lower gains in weight from 15 to 18 months (P<0·001) and somewhat lower WLZ and WAZ (P=0·06) at 18 months, but positively correlated with change in WLZ and WAZ from 18 to 24 months (P<0·001). In all, correlations with length and weight measures suggested that distinct anthropometric trajectories may have preceded the EED assessment. Correlations between the individual biomarkers that comprise L:M and the GI and GP scores and anthropometric measures further elucidate these trends (online Supplementary Table S2).
Table 2 Correlation between biomarkers of environmental enteric dysfunction (EED) and systemic inflammation (SI) measured at age 18 months and anthropometric measures at 18 months and their changes from 15 to 18 months and 18 to 24 months of age
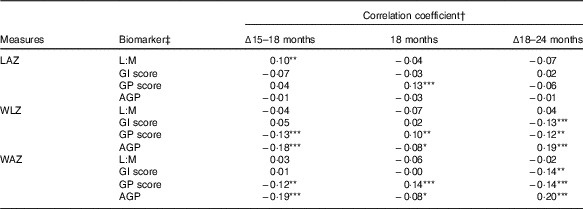
LAZ, length-for-age z score; L:M, lactulose:mannitol ratio; GI, gut inflammation score; GP, gut permeability score; AGP, α-1 acid glycoprotein; WLZ, weight-for-length z score; WAZ, weight-for-age z score.
Statistical significance of the correlation coefficient: * 0·05<P≤0·10; ** 0·01<P≤0·05; *** P≤0·01.
† Values are Pearson’s correlation coefficients.
‡ L:M and AGP were log-transformed before analysis.
Associations of 1-sd increments of EED and SI biomarkers with anthropometric measures at 15 months and growth velocity in z score units per month from 15–18 and 18–24 months, as determined in ME regression models of 15–24 months trajectories, are shown in Table 3, along with the statistical significance of the biomarker’s contribution to each model according to the likelihood ratio test. Longitudinal models included all children with complete data for the relevant variables, and thus sample sizes differed slightly between models to maximise the sample size in each. Overall, only GP score was associated with LAZ trajectory, and its contribution to the model was marginal (likelihood ratio test P=0·08). Children with a 1- sd greater GP score tended to have 0·1 greater LAZ at 15 months, corresponding to 0·28-cm greater length, although their rate of growth from 15 to 24 months was not different from those with lower GP score. Initial WLZ and WAZ at 15 months were greater by 0·16 z (se 0·05) and 0·17 z (se 0·05), respectively, in those with GP score 1 sd greater than the mean, corresponding to 0·16 kg higher weight at 15 months. None of the other biomarkers was associated with differences in 15-month WLZ or WAZ. GP score and AGP were associated with small but highly significant decreases in rate of WLZ and WAZ change from 15 to 18 months (β −0·03 (se 0·01) and β −0·02 (se 0·01) z score units/month, respectively, for GP score and β −0·04 (se 0·01) and β −0·03 (se 0·01) z score units/month, respectively, for AGP). These coefficients correspond to 0·04 kg less weight gained over the 15–18-month period in children with GP score 1- sd greater than the mean and 0·08 kg less weight gained in the same interval in children with 1- sd greater AGP. In the 18–24-month period, GI score and GP score were negatively associated with change in WLZ and WAZ (β −0·01 (se 0·00) for all), whereas AGP was positively associated with WLZ and WAZ (β 0·02 (se 0·00) for both). A 1- sd greater GI or GP score was associated with 0·06 kg less weight gain over the 6 months from 18 to 24 months, whereas a 1- sd greater AGP value was associated with 0·09 kg more weight gained between 18 and 24 months.
Table 3 Associations between environmental enteric dysfunction (EED) and systemic inflammation (SI) biomarkers assessed at age 18 months and repeated anthropometric (anthro.) measures from 15 to 24 months in longitudinal mixed-effects regression modelsFootnote † (β-Coefficients with their standard errors)
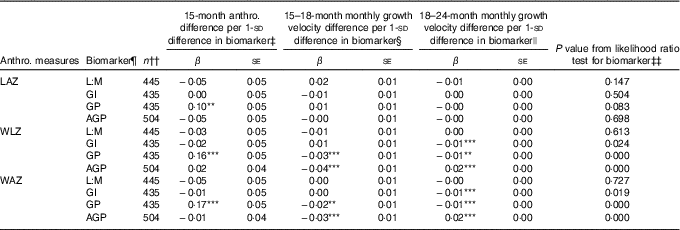
LAZ, length-for-age z score; L:M, lactulose:mannitol ratio; GI, gut inflammation score; GP, gut permeability score; AGP, α-1 acid glycoprotein; WLZ, weight-for-length z score; WAZ, weight-for-age z score; LSI, living standards index.
Significance of the individual coefficient in the mixed-effects model: ** 0·01<P≤0·05; *** P≤0·01. Asterisks are not displayed if the overall likelihood ratio test P value is >0·10.
† Values are coefficients and standard errors from mixed-effects linear regression models with a continuous biomarker term, continuous age term, spline term for age after 18 months, interactions between the continuous biomarker and the age and age spline terms, and random intercepts and random slopes for each child. Models were developed separately for each biomarker. The LAZ models were adjusted for child sex, dichotomous LSI and interactions between LSI and age spline terms, and assigned supplementation group. WAZ and WLZ models were adjusted for sex, dichotomous LSI and supplementation group.
‡ Coefficient for continuous biomarker term in mixed-effects model, which indicates shift in baseline (15 months) anthropometric measure with each standard deviation change in biomarker value.
§ Coefficient for interaction term for continuous biomarker by continuous age interaction in mixed-effects model. This term indicates the shift in the monthly rate of change in the anthropometric measure with age before 18 months for each standard deviation change in biomarker value.
|| Coefficient for interaction term for continuous biomarker by age spline interaction in mixed-effects model. This term indicates the shift in the monthly rate of change in the anthropometric measure with age after 18 months for each standard deviation change in biomarker value.
¶ L:M and AGP were log-transformed, and all biomarkers and scores were centred at their mean values and standardised to standard deviation units.
†† Participants with complete data for the model variables were included in each model.
‡‡ P value for the likelihood ratio test for the addition of the biomarker, biomarker×age interaction and biomarker×age spline interaction terms to the mixed-effects regression model.
In ME models for individual EED biomarkers, of the GI score markers, only AAT was negatively associated with change in WLZ and WAZ between 18 and 24 months (online Supplementary Table S3). Of the GP score markers, GLP-2 was strongly inversely associated with 15-month length and positively associated with 18–24-month change in WLZ and WAZ, whereas EndoCAb, with a small but highly significant inverse association with 15–18-month change in WLZ and WAZ, contributed more to GP score associations with 15–18-month weight gain velocity. EndoCAb was associated with greater 15 months WLZ and WAZ, whereas GLP-2 was associated with lower 15-month WLZ and WAZ, directionality that is concordant given their opposite loadings on the GP score. Associations with CRP were examined but none was observed in simple or ME regression models (results not shown).
Discussion
Among Bangladeshi children participating in a trial of CFS, EED and SI, comprehensively assessed with a panel of biomarkers at age 18 months, were associated with children’s weight-gain trajectories but not their linear growth in the 2nd year of life. The lack of evidence for an EED impact on linear growth occurred despite a high prevalence of stunting in this cohort, an extensive burden of EED and SI assessed with a panel of accepted and novel biomarkers, and the use of longitudinal models to comprehensively characterise growth trajectories from 15 to 24 months of age. Our findings contrast with several studies that have reported associations between EED and linear growth( Reference Lunn, Northrop-Clewes and Downes 17 – Reference Campbell, Elia and Lunn 20 , Reference Arndt, Richardson and Ahmed 22 , Reference Guerrant, Leite and Pinkerton 23 , Reference Panter-Brick, Lunn and Langford 40 , Reference Goto, Mascie-Taylor and Lunn 41 ), but observed links to weight gain suggest that effects may manifest in important health and development outcomes aside from restricted linear growth.
An advantage of our study design is having growth data that precede the EED measurement. This is particularly important for examining EED in the 2nd year of life, as during this period linear growth rates typically slow down compared with greatest growth velocity in infancy, yet smaller children may grow relatively more rapidly than children of a healthy size to recover from prior faltering( Reference Victora, de Onis and Hallal 6 , Reference Richard, Black and Gilman 42 ). These characteristics of linear growth highlight the importance of understanding and accounting for both usual growth dynamics and individual growth history when examining the impact of EED on growth. Our longitudinal models used length measured at multiple time points surrounding the EED assessment to compare growth velocity from 18–24 months among otherwise similar children, including with respect to their initial size at 15 months and growth in the 15- to 18-month interval.
The null association between EED and linear growth differs from findings in several other studies( Reference Lunn, Northrop-Clewes and Downes 17 – Reference Campbell, Elia and Lunn 20 , Reference Arndt, Richardson and Ahmed 22 , Reference Guerrant, Leite and Pinkerton 23 , Reference Panter-Brick, Lunn and Langford 40 , Reference Goto, Mascie-Taylor and Lunn 41 ), although it is not unique to our study( Reference Arndt, Richardson and Ahmed 22 , Reference Denno, VanBuskirk and Nelson 43 , Reference Kosek, Ahmed and Bhutta 44 ). Several factors could contribute to this finding. EED may be relatively transient( Reference Lee, McCormick and Seidman 45 ), such that children with elevated markers at this single time point were not necessarily more burdened with EED over the preceding or subsequent months. A recent publication describing high within-child variability in faecal EED biomarkers over the first 2 years of life corroborates the hypothesis that EED is more transient than chronic in young children( Reference McCormick, Lee and Seidman 46 ). Assuming, then, that EED assessed at 18 months is indicative of present status rather than prior or sustained illness, an effect on growth would most likely be observed in the subsequent months. However, the timing of assessments in the 2nd year of life may have precluded observing prospective associations with growth, as previous reports of associations between EED and subsequent growth have largely been observed in children <15 months old( Reference Lunn, Northrop-Clewes and Downes 17 , Reference Kosek, Haque and Lima 18 , Reference Campbell, Elia and Lunn 20 , Reference Panter-Brick, Lunn and Langford 40 , Reference Goto, Mascie-Taylor and Lunn 41 ). Additionally, the effect of EED at a single time point may be insufficient to measurably alter linear growth in a subsequent 6-month interval, similarly observed by Arndt et al.( Reference Arndt, Richardson and Ahmed 22 ) and Kosek et al.( Reference Kosek, Ahmed and Bhutta 44 ) in a multi-country study. Finally, the association of EED with WLZ that we observed could precede length effects that manifest beyond the available 6-month follow-up, as WLZ changes can portend future LAZ( Reference Richard, Black and Gilman 47 ), with effects on LAZ slow to emerge amidst slowing linear growth rates.
In contrast to length, however, weight gain trajectory was significantly perturbed by EED and SI. Children with elevated AGP gained relatively less weight from 15 to 18 months but seemed to recover from 18 to 24 months, whereas those with elevated GI score gained less weight from 18 to 24 months and those with elevated GP score gained less weight in both intervals. This temporal divergence is consistent with AGP marking the end of an infection followed by catch-up growth rather than indicating sustained subclinical infection, whereas GI and GP scores may indicate current or ongoing conditions that impair subsequent weight gain. Observed WLZ declines could represent impaired muscle or fat deposition, which could precede length growth effects that are slower to emerge. Alternately, short-term WLZ fluctuations could provide some cushion that shields linear growth from mild, transient insults. Previous studies investigating EED and severe acute malnutrition (SAM), defined by WLZ, have reported inverse associations between EED and WLZ at presentation and during recovery with therapeutic feeding( Reference Hossain, Nahar and Hamadani 15 , Reference Behrens, Lunn and Northrop 48 ), although older studies often conflated symptomatic diarrhoeal illness with subclinical EED( Reference Behrens, Lunn and Northrop 48 ). Before this paper, there has been little evidence to show an association of EED with WLZ variation that does not reach the severity of SAM. Still, one new multi-site study reported a similar set of findings to ours, with stronger associations of EED and SI with WLZ than LAZ changes in repeated assessments over the first 2 years of life( Reference Kosek, Ahmed and Bhutta 44 ).
In this study of EED nested within an RCT, an overarching hypothesised pathway from CFS to growth via improved EED was not supported by our findings. In the parent trial, groups assigned to receive CSF had 0·07–0·10 greater mean LAZ and 5–6 percentage point reduction in stunting prevalence at 18 months of age following a year of supplementation relative to an un-supplemented control group( Reference Christian, Shaikh and Shamim 25 ). We found minimal associations, however, between EED and linear growth, which suggests, in combination with previously reported null effects of the CFS on EED( Reference Campbell, Schulze and Shaikh 24 ), that food supplementation affected growth via pathways not mediated by EED. This may be due primarily to a lack of benefit for EED of the particular CFS formulations or the quantities given, or because EED was not a major factor limiting growth in this setting, possibilities that require further investigation to inform future CFS interventions. Supplementation, EED and growth could, alternately, be associated such that supplementation blunts the impact of EED on growth. This could explain the null associations we observe between EED and linear growth, as 4/5 of the participants had received CFS. Analyses stratified by supplementation group did not suggest effect modification of that nature, although the study was not powered to address that possibility.
The strengths of the present study include the very large sample size, comprehensive panel of EED and SI biomarkers including the widely used L:M test, and repeated high-quality anthropometry assessments. Weaknesses include having EED measured only once. The timing of the EED assessment was driven by a primary hypothesis focused on the effects of the supplementation trial on EED markers, which necessitated assessment of EED in the middle of the 2nd year of life, and supported prioritising a larger sample size over repeated EED assessments. The lack of a true gold standard test of EED inhibits the interpretation of the biomarkers where they diverge from L:M, which is a limitation in this field of study more generally.
In this rural Bangladesh cohort where stunting and EED were common, EED exerted little influence over linear growth trajectories during the 2nd year of life, whereas weight gain was relatively more compromised by EED and SI. The high prevalence of EED and SI that we observed in this study along with effects on subsequent weight gain distinct from gains in length suggest potential consequences of EED on body composition, the impact of which may be the subject of future study as we continue to investigate strategies to support optimal growth.
Acknowledgements
The authors thank Margia Arguello, Sarah Baker and Afreen Zaman Khan for assistance with laboratory measures, and John McGready for help with statistical model development.
This work was supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture's Food Aid Nutrition Enhancement Program (2010-38418-21732); National Institutes of Health (NIH) (R21HD081503); and Johns Hopkins Sight and Life Global Nutrition Research Institute. USDA, NIH and Johns Hopkins Sight and Life Global Nutrition Research Institute had no role in the design, analysis or writing of this article.
R. K. C., K. J. S. and P. C. designed research; R. K. C., K. J. S., S. S., R. R., H. A., S. M., K. P. W. and P. C. conducted research; R. K. C. analysed data and wrote the paper; K. J. S. and P. C. made substantial contributions to data analysis and manuscript revisions; and all authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003683





