Exogenous enzymes used in poultry diet have usually resulted in improved growth performance, enhanced flock uniformity as well as reduction of nutrient waste being released to the environment. The improvement in performance is closely associated with improvement in nutrients and energy utilization which is primarily related to availability of more nutrients and energy from the feed ingredients. Energy utilization in poultry is usually expressed in terms of metabolizable energy (ME) which accounts for energy loss in the excreta. Though improvements in animal performance and bone mineralization have been reported, the influence of phytase on ME has not been consistentReference Namkung and Leeson1–Reference Onyango, Bedford and Adeola4. Similarly, a large number of studies reported an improvement both in performance and ME when carbohydrases were used in diets based on wheat, rye or barleyReference Bedford and Schulze5–Reference Bedford and Morgan7. In some studies in which carbohydrases were used however, improvement in growth performance was reported without improvement in MEReference Hong, Burrows and Adeola8, Reference Wu, Ravindran, Thomas, Birtles and Hendriks9.
Net energy (NE) is another measure of energy utilization, this response criterion considers the efficiency of ME utilization, and hence it has been argued that it might be more sensitive than ME for determining efficiency of energy use in poultryReference De Groote, Morris and Freeman10–Reference Pirgozliev, Rose, Kettlewell and Bedford12. NE is the amount of energy that is available to the animal after ME has been used to support the heat increment of feeding. This NE is available both for maintenance and production (NEp). There is the possibility that NEp can be used as a more sensitive measure of energy utilization by the chickens receiving enzyme because it takes into account the efficiency of utilization of ME for growth.
NE can be determined using the carbon–nitrogen method or by the comparative slaughter technique. The carbon–nitrogen method for NE determination was used in evaluating the efficacy of endo-β-d-mannanase in a recent studyReference Daskiran, Teeter, Fodge and Hsiao13 using chickens. The comparative slaughter approach has been used recently for determination of NE for ruminantsReference Ouellet, Seoane, Lapierre, Flipot and Bernier14, Reference Early, Mahgoub and Lu15, pigsReference Nieto, Miranda, García and Aguiler16, fishReference Lupatch, Kissil and Sklan17 and chickensReference Pirgozliev, Rose, Kettlewell and Bedford12, Reference Sakomura, Silva, Couto, Coon and Pacheco18.
The objective of the present experiment therefore was to evaluate the use of NEp, determined using the comparative slaughter method, in studying the effectiveness of enzyme supplementation of broiler feed. In addition, the study related the effect of enzymes on energy utilization to the age of the broilers.
Materials and methods
Enzymes
The enzymes used had phytase, xylanase, amylase and protease activities. An Escherichia coli-derived phytase (Phyzyme XP; Danisco Animal Nutrition, Marlborough, UK) was used and was supplemented in the diet to provide 1000 FTU/kg diet (as-fed basis). The enzyme cocktail used had xylanase, amylase and protease (XAP) activities (Avizyme 1505; Danisco Animal Nutrition). It was added to provide per kg diet (as-fed basis) 650, 1650 and 4000 U of xylanase, amylase and protease, respectively. One phytase unit (FTU) was defined as the quantity of enzyme required to liberate 1 μmol inorganic P/min, at pH 5·5, from an excess of 15 μm-sodium phytate at 37°C. One unit (U) of xylanase was defined as the quantity of the enzyme that liberates 1 μmol xylose equivalent/min. One unit of amylase was defined as the amount of the enzyme catalysing the hydrolysis of 1 μmol glucosidic linkage per minute and one protease unit was defined as the quantity of the enzyme that solubilized 1 μg azo-casein/min.
Animals, diets and experiment design
Day-old male broiler chicks (480) were used for the present study. At 1 d old, the chicks were allocated into four slaughter groups of similar average body weight (48·1 (sd 0·02) g) consisting of 30, 150, 150 and 150 chicks. The similar initial body weight for all slaughter groups ensured that each group was representative of the other groups. One of the slaughter groups comprising thirty chicks constituted the initial slaughter group killed at day 0. The thirty birds in the initial slaughter group were further allocated into six replicate cages of equal body weight, with five birds per replicate cage.
The remaining three slaughter groups of 150 chicks each made up the final slaughter groups that were killed by CO2 asphyxiation at days 7, 14 and 21. On day 0, 150 broilers in each of the remaining slaughter groups were allocated to five dietary treatments in a randomized complete block design, the chicks were blocked by body weight. Each treatment had six replicate cages with five chicks per replicate cage. The dietary treatments were: (1) a positive control (PC) diet that was formulated to meet National Research Council19 nutrient requirement for broilers, (2) a negative control (NC) diet formulated to meet 94 % of ME and 53 % of non-phytate P requirement, (3) NC diet plus an E. coli phytase, (4) NC diet plus XAP, and (5) NC plus phytase and XAP. The diets were maize–wheat–soyabean based and were fed as a mash, the wheat served as an additional source of NSP. Chromic oxide was added to the diets as an indigestible marker to enable determination of digestibility. The compositions of the PC and NC diets are presented in Table 1.
Table 1 Ingredient composition of the experimental control diets
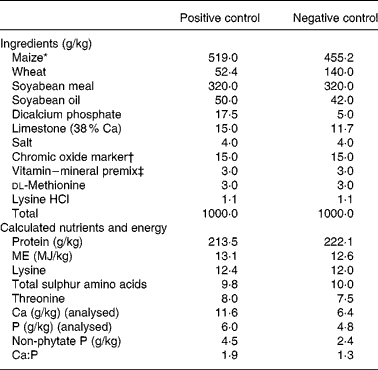
FTU, phytase unit.
* Danisco phytase (Phyzyme XP) premix formulated to contain 100 FTU/g and Danisco XAP (Avizyme 1505) premix formulated to contain 65, 165 and 400 U/g xylanase, amylase and protease, respectively, replaced ground maize in the NC diet at the rate of 10 g/kg providing per kg diet 1000 FTU, 650, 1650 and 4000 U phytase, xylanase, amylase and protease, respectively.
† Prepared as 1 g chromic oxide added to 4 g ground maize.
‡ Vitamin–mineral premix contained per g premix: retinol, 548 μg; cholecalciferol, 22 μg; dl-α-tocopherol, 3·34 mg; menadione sodium bisulphite, 1·46 mg; cyanocobalamin, 13·2 μg; biotin, 18·4 μg; choline chloride, 257 mg; folic acid, 330 μg; niacin, 14·69 mg; d-pantothenic acid, 3·67 mg; pyridoxine hydrochloride, 1·1 mg; riboflavin, 1·83 mg; thiamine mononitrate, 735 μg; Cu (as copper sulphate), 1·48 mg; I (as calcium iodate), 370 μg; Fe (as ferrous sulphate), 14·69 mg; Mn (as manganese oxide), 22·02 mg; Se (as sodium selenite), 100 μg; Zn (as zinc oxide), 14·69 mg.
Body weight and feed intake data of the birds were recorded weekly. Grab excreta samples were collected from each cage in the last 3 d of each week to enable determination of ME. The excreta were immediately frozen before being dried in a forced air oven to a constant weight. Excreta were pooled within each pen and ground prior to analyses. Chickens were killed in four phases. Thirty broiler chicks that were used as the initial slaughter group were not fed but were killed at day 0 by CO2 asphyxiation. Every 7 d thereafter 150 chicks were killed by CO2 asphyxiation to serve as the final slaughter group for days 7, 14 and 21. On slaughter days, after weighing the birds, feed was withdrawn for about 4 h before asphyxiation by CO2. The birds were subsequently frozen after slaughter and prior to processing. All animal handling procedures were approved by the Purdue University Animal Care and Use Committee.
Chemical analysis
The diets were analysed for enzyme activity and nutrient composition. Excreta and diets samples were analysed for gross energy in order to determine the ME. Samples were dried at 105°C in a drying oven (Precision Scientific Co., Chicago, IL, USA) for 24 h for DM determination. Gross energy was determined in bomb calorimeter (Parr 1261; Parr Instruments Co., Moline, IL, USA) using benzoic acid as a calibration standard. Chromium concentration in the diets and excreta samples was determined using the method of Fenton and FentonReference Fenton and Fenton20.
The whole intact chicken (feathers, head, feet and all organs) was frozen immediately after being killed and later processed. All the chicks in the same cage were processed together, after chopping and coarse-grinding individual chickens, they were thoroughly mixed and two subsamples (approximately 200 g each, wet weight) were taken, finely ground and freeze-dried. The two subsamples were mixed together after drying and ground again. Hence chemical analysis was on one sample from each cage and not from individual chickens. The ground carcase samples were analysed for gross energy, diethyl ether extractable fat and nitrogen. Nitrogen was determined using a combustion method (Leco FP analyser model 602600; Leco Corp., St Joseph, MI, USA) with EDTA as a calibration standard.
Calculations
ME (MJ/kg) was calculated as follows:
where GEi is gross energy (MJ/kg) in feed; GEo is the gross energy (MJ/kg) in excreta, C i is the concentration of chromium in the diets; and C o is the concentration of chromium in the excreta.
Net energy for production (NEp) was calculated as follows:
Heat production (HP), which consists of the heat increment of feeding and fasting HP is calculated as the difference between NEp and ME intake:
where ME intake (MEI) was calculated using the following formula:
Energy retained as fat (REf) and as protein (REp) were calculated as follows:
The values 38·2 and 23·6 kJ/g are energy values per gram of fat and protein, respectively, and were according to Larbier and LeclercqReference Larbier, Leclercq and Wiseman21.
Because excreta were collected the last 3 d of each week, ME intake was determined for each week (days 7, 14 and 21). The ME intake for chickens killed at day 7 was calculated as shown earlier using days 0–7 feed intake. The ME intake for chickens killed at day 14 was calculated by adding the ME intakes from the 0–7 and 8–14 d periods. The ME intake for chickens killed at day 21 was calculated by adding the ME intakes from the 0–7, 8–14 and 15–21 d periods.
Statistical analysis
Data on growth performance of broilers were analysed as a randomized complete block design using the General Linear Model procedures of SAS22. The last four treatments in the experiment were analysed as a 2 × 2 factorial arrangement of treatments to elucidate the main effects of phytase, XAP and possible interactions. Because of the possibility of influence of ME intake on other energy utilization response criteria, ME intake was used as a covariate in the analysis of energy utilization response data. The data on efficiency of ME intake use for energy retention, energy retained as fat and as protein were first arcsine transformed before analysis by the General Linear Model procedures of SAS. The relationships among energy utilization responses were determined by correlation using the CORR procedure of SAS. Data were obtained on energy utilization responses at three different periods (days 7, 14 and 21) and hence it is possible to compare the response of chickens to the dietary treatments at these periods. Therefore, means are presented for main effects of five diets and three periods and possible diet × period interactions. Where there are no significant diet × period interactions, only the main effects means are presented, whereas simple effects means are presented when there are significant interactions. Means of PC and NC diets were compared using orthogonal contrasts to elucidate the effect of the dietary treatments.
Results
Analysed phytase activity was 894 and 1208 FTU/kg feed, respectively for treatments 3 and 5. For treatments 4 and 5, respectively, the analysed enzyme activities (U/kg) were 575 and 634 for xylanase; 1862 and 1987 for amylase; and 3166 and 3279 for protease. The analysed activities of xylanase, amylase, protease and phytase were ≤ 100 U/kg in treatments 1 and 2.
Table 2 shows the data on growth performance of the broiler chickens used. Feeding the nutritionally marginal NC diet to the broilers depressed (P < 0·05) both weight gain and gain:food at all periods. There were significant effects of diets and periods (P < 0·001) on both weight gain and gain:food. Weight gain increased with age in all treatments whereas gain:food decreased with age. There was diet × period interaction (P < 0·001) only for weight gain. Phytase improved (P < 0·05) weight gain at all periods, there were no effects of XAP at any period, but there was XAP × phytase interaction (P < 0·05) only in the 0–14 d period. The interaction at this period was explained by phytase improving weight gain more in the absence than in the presence of XAP. There were no effects of XAP nor phytase × XAP interaction on gain:food at any period but phytase improved (P < 0·05) gain:food at the 0–14 and 0–21 d periods.
Table 2 Growth performance of broilers receiving phytase or cocktail of xylanase, amylase and protease (XAP) individually or in combination
(Mean values for six replicate cages with five broilers per replicate cage)
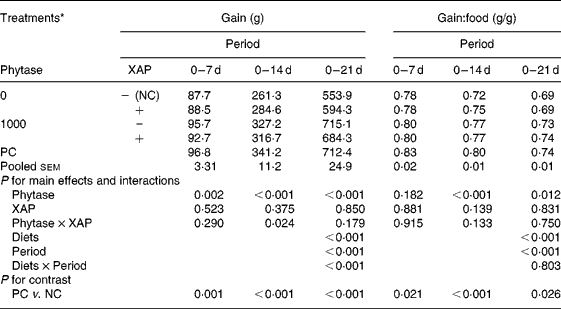
NC, negative control; PC, positive control.
* See Table 1 for details of control diets.
Table 3 shows the result of MEI and NEp of the experimental broilers. There were effects (P < 0·001) of diet and period on MEI and NEp as well as diet × period interaction (P < 0·01). The interactions observed were due to the differences in the effects of the enzymes at different periods as described later. For example, effects of XAP on MEI and NEp were only observed in the 0–7 d period. There were no effects of phytase supplementation alone or in combination with XAP on MEI from 0 to 7 d. In the 0–14 and 0–21 d periods, however, MEI was higher (P < 0·01) in phytase-supplemented diet and there was a trend (P < 0·10) towards phytase × XAP interaction for MEI and NEp only in the 0–21 d period. Phytase supplementation improved (P < 0·01) NEp in the 0–14 and 0–21 d periods.
Table 3 Metabolizable energy and net energy for production of broilers receiving phytase or cocktail of xylanase, amylase and protease (XAP) individually or in combination
(Mean values for six replicate cages with five broilers per replicate cage)

NC, negative control; PC, positive control.
* See Table 1 for details of control diets.
Table 4 shows the result of partitioning of the energy deposited into that deposited as fat or protein in the carcase. Carcase energy deposited as fat increased as the chicken matured (P < 0·01) and there was a diet × period interaction (P < 0·01) for this response criterion as the following explanation of the simple effects shows. Energy deposited as fat was lower (P < 0·05) in the broilers receiving XAP only in the 0–7 d period. In the 0–14 and 0–21 d periods, phytase supplementation increased REf (P < 0·01) and had a trend (P < 0·10) to decrease REf in the 0–7 d period; there were no phytase × XAP interactions in any period. Also REf was lower (P ≤ 0·001) in NC compared to PC treatment in the 0–14 and 0–21 d periods. There were no effects of any dietary treatment on energy retained as protein in 0–7 d. Carcase energy deposited as protein increased as the chickens matured (P < 0·01) and there was diet × period interaction (P < 0·05) for this response criterion. The source of diet × period interaction was the observation that phytase supplementation improved (P < 0·01) REp only in the 0–14 and 0–21 d periods and there was a trend for phytase × XAP interaction (P < 0·10) for REp in 0–21 d only. In addition, REp was greater (P ≤ 0·01) in PC compared to NC treatment.
Table 4 Carcass energy deposition as fat and protein in broilers receiving phytase or cocktail of xylanase, amylase and protease (XAP) individually or in combination
(Mean values for six replicate cages with five broilers per replicate cage)

NC, negative control; PC, positive control.
* See Table 1 for details of control diets.
HP of the broilers receiving the experimental diets is shown in Table 5. There were effects (P < 0·05) of diet and period as well as diet × period interaction on HP. HP of the broilers increased as broilers matured (P < 0·01), the diet × period interactions are explained as follows. Supplementation of XAP decreased (P < 0·01) HP in both the 0–7 and 0–14 d periods only, phytase tended to increase HP (P < 0·10) only in 0–14 d period and there were phytase × XAP interactions (P < 0·05) only in the 0–7 and 0–14 d periods. Phytase increased HP in the 0–14 and 0–21 d periods (P < 0·01). Phytase × XAP interaction in the 0–7 d period is explained by the observation that phytase alone increased HP but the combination of phytase and XAP decreased HP; whereas in the 0–21 d period phytase increased HP more in the absence of XAP than in the presence of XAP. HP was greater (P < 0·05) in PC compared to NC treatment in the 0–14 and 0–21 d periods, but there were no differences in HP between the two treatments in the 0–7 d period.
Table 5 Heat production (kJ/d) in broilers receiving phytase or cocktail of xylanase, amylase and protease (XAP) individually or in combination
(Mean values for six replicate cages with five broilers per replicate cage)
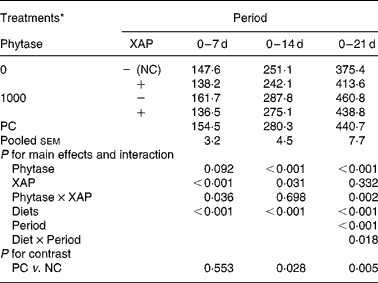
NC, negative control; PC, positive control.
* See Table 1 for details of control diets.
The data on efficiency of use of MEI for NEp, REp or REf are shown in Table 6. In general, efficiency of MEI use for NEp, REp and REf increased (P < 0·01) as broilers matured. There was significant (P < 0·05) diet effect on K REp in the 0–14 d period, this is due to the improvement in K REp observed in the treatment with XAP supplementation during the same period. No other dietary treatment effects were observed for the efficiency of MEI use data. In Table 7 are the ME values of the diets for the chicks at different periods. The overall trend was that ME increased (P < 0·01) with age in all the treatments. There was also significant (P < 0·01) diet effect as well as diet × period interaction (P < 0·01). Although phytase supplementation improved (P < 0·05) ME at all ages, ME was lower (P < 0·01) in diets supplemented with XAP in the 0–7 and 7–14 d periods, whereas in the 14–21 d period, XAP supplementation tended to increase ME (P < 0·10) thus accounting for the interactions observed for diet and period. There were no phytase × XAP interactions on ME at any age of the broiler chicks. There were no differences between the ME in PC and NC treatments at any period.
Table 6 Efficiency of metabolizable energy (ME) use for tissue energy deposition in broilers as influenced by supplementation of phytase or cocktail of xylanase, amylase and protease (XAP) individually or in combination
(Mean values for six replicate cages with five broilers per replicate cage)
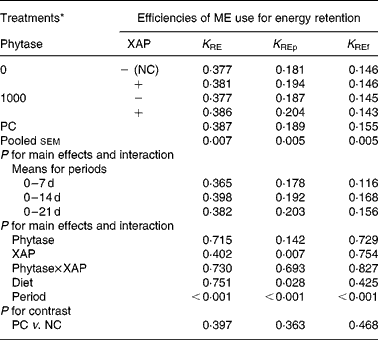
K RE, efficiency of ME use for carcass energy retention; K REf, efficiency of ME use for energy retained as fat; K REp, efficiency of ME use for energy retained as protein; NC, negative control; PC, positive control.
* See Table 1 for details of control diets.
Table 7 Metabolizable energy (MJ/kg DM) concentration of diets fed to broilers receiving phytase or cocktail of xylanase, amylase and protease (XAP) individually or in combination
(Mean values for six replicate cages with five broilers per replicate cage)
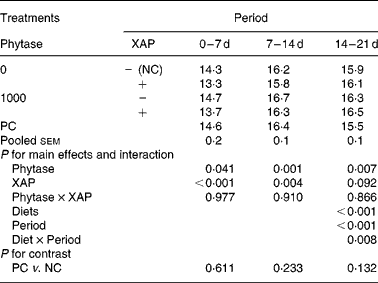
NC, negative control; PC, positive control.
* See Table 1 for details of control diets.
Table 8 shows the correlation coefficients among the various energy utilization response criteria. Body weight was more highly correlated with NEp (r 0·999) compared with ME (r 0·666). HP was less highly correlated with NEp (r 0·980) compared with ME intake (r 0·997). Energy retained as protein was more highly correlated with NEp (r 0·988) than energy retained as fat (r 0·950). All correlations were significant (P < 0·01).
Table 8 Correlation matrix for the energy utilization response criteria of broilers to supplementation of carbohydrases or phytase individually or in combination*
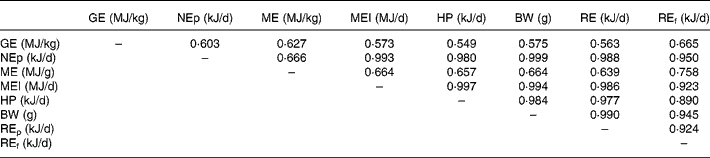
BW, body weight; GE, carcass gross energy; HP, heat production; ME, metabolizable energy; MEI, metabolizable energy intake; NEp, net energy for production; REf, energy retained as fat; REp, energy retained as protein.
* Correlation was run on ninety observations.
Discussion
The experimental diets (except for the PC diet) were formulated to be marginally deficient in ME, P and Ca and because these would be limiting for growth, the effects of enzymes that are capable of enhancing energy and P utilization would be expected to be more pronounced. The improvement in performance observed when phytase alone or combined with XAP were used has been reported in the literatureReference Cowieson and Adeola2, Reference Cowieson, Singh and Adeola23, Reference Olukosi, Cowieson and Adeola24. It is noteworthy that the enzymes were effective early in age in improving growth performance especially because at this age the birds might be limited in their capacity to produce the digestive enzymesReference Mahagna and Nir25. Exogenous enzyme supplementation can reduce the energy needs for producing some of the digestive enzymes as has been observed for chicks receiving supplemental amylase and proteaseReference Mahagna, Nir, Larbier and Nitsan26. Enzyme supplementation did not improve gain:food from day 0 to 7 but phytase improved the response criterion in the 0–14 and 0–21 d periods, however, weight gain was improved by phytase alone at all ages.
The use of NEp as a measure of energy utilization response to enzyme supplementation is predicated on the premise that the quantity of feed consumed and body weight gain can be measured in energy terms (ME intake and NEp, respectively). NEp can be determined as the difference between the gross energy content of the body at the end of a specified period and the gross energy content of the body at the beginning of the period. Also, because energy can not be lost but be converted into other forms, differences in energy intake and energy deposited can be accounted for in energy used for maintenance of the animal. There is a dearth of information in poultry on the response to phytase using the NE approach. Daskiran et al. Reference Daskiran, Teeter, Fodge and Hsiao13 using the carbon–nitrogen method showed that a carbohydrase improved NE in a maize–soyabean meal without any change in ME. However, addition of the enzyme to the same diet with added guar gum did not lead to any improvement in NE.
The results from various experiments have not been consistent with regards to the influence of phytase or carbohydrases on ME, whereas some have reported improvement in ME in response to phytaseReference Namkung and Leeson1, Reference Cowieson and Adeola2, Reference Shirley and Edwards27 or carbohydrasesReference Zanella, Sakomura, Silverside, Fiqueirdo and Pack28, Reference Palander, Näsi and Järvinen29 others did not see an improvement in ME when the enzymes were usedReference Shirley and Edwards3, Reference Tejedor, Albino and Rostagno30. Interestingly, in most of these studies, there were improvements in weight gain and nutrient utilization. In the current study, phytase supplementation alone or in combination with XAP improved NEp and REp. The improvement in energy utilization may be due to improvement in nutrient and energy availability because additional energy and nutrients made available would be deposited in the carcase and hence improvement in weight gain would represent an improvement in energy deposited in the tissues gained, this energy would be deposited either as fat or protein. Boekholt et al. Reference Boekholt, Van der Grinten, Scheurs, Los and Leffering31 observed that when protein is not limiting in the diets of broilers, extra energy available in the diet is used for both fat and protein retention.
NE for production has accounted for ME used for HP and maintenance, this may be the reason for the differences in the effect of the enzymes on ME and NEp as observed in the current study. For example, phytase supplementation improved ME at the 0–14 and 0–21 d periods and tended to improve ME at the 0–7 d period in the present study, phytase did not improve NEp in the first week of the study. Furthermore, although ME was lower in XAP treatment in the 0–7 and 8–14 d periods the same effect was not observed for NEp. Similarly, Daskiran et al. Reference Daskiran, Teeter, Fodge and Hsiao13 reported that carbohydrase improved NE in a maize–soyabean meal diet without improving ME.
An indication of the possibility of a higher sensitivity of NEp as a measure of energy utilization compared to ME was the higher correlation between NEp and body weight in comparison to correlation between ME and body weight. MacleodReference Macleod32 similarly reported a high correlation between HP, MEI and energy retention in their study. Intuitively, the relationship between NEp and body weight seems obvious and a strong relationship between the two is expected. However, it should be noted that NEp is a product of both body weight and the gross energy of the carcase. In the current study, gross energy content of the carcase explained approximately 60 % of the variation in NEp, showing a strong relationship between the two. Hence, NEp is not only dependent on body weight but also on the amount of energy deposited in the carcase which is an indication of how effectively the enzyme used facilitated energy utilization.
HP was higher in broilers receiving phytase or a combination of phytase and XAP in comparison to those receiving NC diet. Energy costs involved are those for tissue respiration which include those tissues like muscles as well as those for energy-dependent nutrient transportation like Na-K ATPase. Spratt et al. Reference Spratt, McBride, Bayley and Leeson33 noted that vital organs like the liver, respiratory tissues as well as the gastro-intestinal tract may consume up to 30 % of the fasting HP and that the total cost of maintenance may take up to 75 % of total fasting HP. Experimental evidence point to the fact that the use of enzymes usually leads to reduction in the weight and relative proportion of energetically active organs like the gastro-intestinal tract and pancreas as shown in data from pigsReference Shelton, Southern, Bidner, Persica, Braun, Cousins and McKnight34, Reference Kies, Gerrits, Schrama, Heetkamp, Van der Linden, Zandstra and Verstegen35 and poultryReference Wu, Ravindran, Thomas, Birtles and Hendriks9, Reference Esteve-Garcia, Brufau, Perez-Vendrell, Miquel and Duven36. Hence it would seem that the cause of higher HP in enzyme treatments in the current study would be due to maintenance of skeletal muscles and fat as well as energy associated with energy-dependent nutrient and mineral absorption processesReference Kies, Gerrits, Schrama, Heetkamp, Van der Linden, Zandstra and Verstegen35.
The use of phytase leads to the release of nutrients and minerals which need to be transported and used by the animals and this may increase expenditure of energy. JohnsonReference Johnson37 pointed out the fact that higher HP usually accompanies a higher plane of nutrition. Hence, both in the PC diet as well as those with added phytase, which both had higher planes of nutrition in comparison with the NC diet, it would be expected that HP would be higher in broilers receiving these diets. Interestingly, Johnson et al. Reference Johnson, Johnson and Baldwin38 demonstrated that the weights of liver and gastro-intestinal tract expand or contract in response to metabolic demands. Also, part of HP is the thermic effect of feeding which is the quantity of energy yield as a result of various processes that accompany feeding. Perhaps the most significant for broilers will be the conversion of various energy sources in the diet into the primary energy store in the animal, namely fat. Van Milgen et al. Reference Van Milgen, Noblet and Dubois39 reported that the efficiency of using nutrients for lipid deposition in the body was in this order: lipid > starch > protein. Hence, the phytase-induced increase in digestibility of nutrients would increase the nutrients available for energy storage in the body; this may increase the energy required for these physiological processes.
Energy deposited as protein was higher than energy deposited as fat in the current study and this may be another reason for the high HP observed in the enzyme treatments. It requires more ATP to deposit 1 kcal protein than it does to deposit the same quantity of energy as fat therefore it is likely that the high proportion of energy deposited as protein is responsible, at least in part, for the high HP in the current study. The energetic efficiency of protein deposition is generally between 0·40 and 0·60 whereas that for fat deposition is between 0·44 and 0·80Reference Larbier, Leclercq and Wiseman21, Reference Lawrence, Fowler and Lawrence40. The efficiency of ME intake utilization for protein and fat deposition is lower in the present study than reported earlier because whereas the energetic efficiencies reported by Larbier and LerclecqReference Larbier, Leclercq and Wiseman21 and Lawrence and FowlerReference Lawrence, Fowler and Lawrence40 are for ME intake above maintenance requirement, in the current study the efficiencies of total MEI for fat and protein deposition were determined. MacleodReference Macleod32 similarly found that HP was higher in broilers receiving high-protein diets. Because fat tissues contribute less to HP than muscleReference Close, Wiseman and Cole41, high protein accretion by the broilers in the current study engendered by enzyme supplementation may have contributed to high HP observed in enzyme-supplemented diets. Macleod et al. Reference Macleod, Whitehead, Griffin and Jewitt42 reported that fasting HP was higher in broiler lines selected for leanness compared with those selected for fatness which is indicative of higher maintenance energy requirement in the chickens selected for leanness.
However, the high HP observed is not a disadvantage because the broilers retained considerable portion of energy intake in muscle deposition. The efficiency of protein retention should not be confused with efficiency of lean tissue gain. Because deposition of lean tissue necessitates accretion of water, this makes it more efficient to use feed energy to deposit lean rather than fat tissuesReference Van Milgen and Noblet43. It is significant that energy deposited as protein in the current study was numerically higher than energy deposited as fat. On one hand, 1 g fat contains more joules than 1 g protein, thus a higher quantity of energy deposited as protein indicates a higher proportion of protein being deposited in the carcase in comparison to fat being deposited. On the other hand, because it is more efficient to utilize feed energy to deposit lean tissue as opposed to fat, the higher energy being deposited as protein indicates that the phytase used promoted efficient utilization of feed energy.
Lopez and LeesonReference Lopez and Leeson44 reported that broilers deposited more N in their body compared with other birds of intermediate growth potential. Leeson and SummersReference Leeson, Summers, Leeson and Summers45 showed that generally, fat deposition increases with age whereas protein deposition decreases with age in broiler carcase. Sanz et al. Reference Sanz, Flores and Lopez-Bote46 and Bregendhl et al. Reference Bregendahl, Sell and Zimmerman47 reported higher content and retention of protein than fat in broilers up to 21 d of age similar to what was observed in the current study. The reason for the higher proportion and retention of protein than fat in those studies and the current one is likely because the broiler chicks at that age (0–21 d) were still actively growing and have not reached the stage at which fat deposition can overtake protein deposition.
It is also noteworthy that the carcase energy deposited as fat was lower in broilers receiving enzyme-supplemented diets in comparison with those receiving the PC diet whereas energy deposited as protein in phytase-supplemented diets was similar to that observed in the PC diet. In fact the ratio of REp:REf was increased with phytase supplementation; this may indicate a preference for protein retention and it also may indicate that the enzyme preferentially promoted lean tissue gain in contrast to fat gain. Energy deposited as protein explained approximately 99 % of the variation in NEp whereas energy deposited as fat explained 95 % of the variation. Hence, whereas the two response variables were very strongly correlated with NEp, the higher correlation between NEp and REP is an indication that deposition of protein was favoured over deposition of fat in the current study. All these point to the possibility that phytase supplementation may actually enhance the efficient utilization of dietary energy by broilers. Hellwing et al. Reference Hellwing, Tauson and Skrede48 similarly reported a higher energy retained in protein compared to energy retained in fat in broilers receiving bacteria protein meal. Obviously, modern broilers have the genetic potential to deposit more lean tissue compared to fat and hence the phytase used in the current study seems to enhance the ability of the broilers to meet that potential.
In conclusion, although ME and NEp are both measures of energy utilization, data in the current study suggest that NEp may be more sensitive than ME when assessing energy utilization response to phytase in broilers. Phytase alone or combined with XAP improved NEp in the current study. Furthermore, determination of NEp by comparative slaughter technique allows the partitioning of energy deposition and hence allows the assessment of the effect of enzyme use on efficiency of energy utilization. In view of the laboriousness of the comparative slaughter technique, however, the use of less invasive methods for quantifying body composition may make it appealing to use NEp as a measure of energy utilization in response to dietary interventions.
Acknowledgements
The authors gratefully acknowledge the assistance of Jason Fields in maintaining the broiler chicks and the animal holding facility. The technical assistance of Pat Jaynes with the chemical analyses is also appreciated. The current study was funded by Danisco Animal Nutrition, Marlborough, Wiltshire, UK. This is journal paper no. 2007-18 098 of the Purdue University Agricultural Research Programs.










