Cyclophosphamide (CP) is an alkylating agent from the oxazaphosphorines group that is widely used to treat a variety of malignant diseases including Hodgkin's disease, non-Hodgkin's lymphoma, many types of leukaemia, multiple myeloma, neuroblastomas, breast cancer, adenocarcinomas of the ovary and certain malignant neoplasms of the lung(Reference McCarroll, Keshava and Cimino1). The therapeutic dose of CP in cancer patients is usually 100–200 mg/kg (orally and daily) or 600–1000 mg/m2 (intravenously every 3–4 weeks)(Reference McCarroll, Keshava and Cimino1). Although it is an effective chemotherapeutic agent, CP intake is associated with many serious side effects and toxicity including mutagenicity, carcinogenicity, teratogenicity, myelosuppression, immunosuppression, cardiac toxicity, lung toxicity and urotoxicity, which are mainly mediated by the reactive oxygen species and lipid peroxide formation(Reference Haque, Bin-Hafeez and Parvez2–Reference Arafa4). Also, a significant increase in micronuclei and gene mutations was found in the peripheral blood lymphocytes of nurses, pharmacists and female workers occupationally exposed to CP during its production or distribution(Reference Anderson, Bishop and Garner5, Reference Burgaz, Karahalil and Bayrak6). Immunosuppression, particularly of humoral immunity, is a common consequence of long-term CP-chemotherapy in cancer patients(Reference Bin-Hafeez, Ahmad and Haque7, Reference Zandvoort, Lodewijk and Klok8). CP-induced immunosuppression is reported to prompt various types of infection, which explains why an impressive part of the morbidity and mortality in cancer patients treated with such chemotherapeutic agents is caused by infections(Reference Zandvoort, Lodewijk and Klok8, Reference Angulo, Jimenez-Diaz and Garcia-Bustos9). Based on the type of secondary tumours induced by CP in different animal species as well as in humans, it was concluded that CP is a systemic carcinogen (i.e. an agent that causes tumours at sites that are distal from the portal of entry, regardless of the route of exposure). This conclusion is in accordance with the assessed mutagenicity data showing that CP is a systemic mutagen(Reference McCarroll, Keshava and Cimino1). In addition, there is evidence suggesting that CP-associated cancers may occur in humans up to several years after drug treatment has ceased(Reference McCarroll, Keshava and Cimino1). The cytotoxic side effects of potentially useful chemotherapeutic agents such as CP, particularly in high-dose regimens and long-term use, on the non-target tissues are a major concern precluding their clinical use. Therefore, extensive studies have been initiated in the last decade to identify various detoxifying and protective agents that help in reducing or eliminating the undesirable side effects of CP(Reference Haque, Bin-Hafeez and Parvez2–Reference Arafa4, Reference Bin-Hafeez, Ahmad and Haque7, Reference Sharma, Trikha and Athar10–Reference Ramadan, El-Beih and Abd El-Kareem16).
Sweet marjoram is one of the most popular culinary herbs in the world, which was grown in Egypt over 3000 years ago and Egypt produces 90 % of the world's supply. It has also been prescribed in the form of a herbal tea (infusion) in folk medicine for asthma, cold, coughs, cramps, depression, dizziness, gastrointestinal disorders, hay fever, headache, toothache and sinus congestions, and as a diuretic and to promote menstruation(Reference El-Ashmawy, El-Nahas and Salama17, Reference Ghaly, Said and Abdel-Wahhab18). It contains acids (carnosic, oleanolic and ursolic acids), cis-sabinene hydrate, flavonoids (diosmetin, luteolin and apigenin), hydrocarbons (P-cymene and γ-terpinene), phenolic glycosides (arbutin, methyl arbutin, vitexin, orientin and thymonin), phenolic terpenoids (thymol and carvacrol), tannins, sitosterol and triacontan(Reference El-Ashmawy, El-Nahas and Salama17–Reference Vagi, Rapavi and Hadolin20). According to an analysis by the US Department of Agriculture (http://www.nal.usda.gov/fnic/cgi-bin/nut_search.pl), one teaspoon of dried marjoram leaves has 2 kcal (8·4 KJ), 0·36 g carbohydrates, 0·04 g fat, 0·2 g fibre, 0·08 g protein, 12 mg Ca, 9 mg K, 0·3 mg vitamin C, 14·4 μg retinol and small amounts of other minerals and vitamins. Preliminary trials have suggested possible antioxidant properties of the sweet marjoram plant(Reference Vagi, Rapavi and Hadolin20–Reference Heo, Cho and Hong22). It was also found that the Egyptian sweet marjoram and its extracts possessed better antioxidant activities than the Hungarian ones(Reference Vagi, Rapavi and Hadolin20). The protective effect of sweet marjoram cultivated in Egypt against the genotoxicity induced by hydroquinone and lead acetate in mice was recently reported in two different studies(Reference El-Ashmawy, El-Nahas and Salama17, Reference Ghaly, Said and Abdel-Wahhab18). To our knowledge, there are no reports in the literature describing the protective effects of Egyptian sweet marjoram leaves against CP-induced genotoxicity and immunosuppression. Because marjoram leaves are commonly used as a seasoning and a tea, the present study aimed to evaluate and compare the efficacy of Egyptian sweet marjoram leaf powder (MLP) and marjoram leaf aqueous extract (MLE) in alleviating the genotoxicity, immunosuppression and other complications induced by CP in albino rats. Two different doses of Egyptian sweet marjoram leaves (50 and 100 mg/kg body weight) were used in the present study to test whether the obtained modulatory effects were dose dependent. Furthermore, the present study investigated any deleterious effects caused by consuming the sweet marjoram leaves.
Materials and methods
Materials
Colchicines and CP (200 mg/ampoule) were purchased from Sigma-Aldrich and Baxter Oncology GmbH, respectively. Egyptian sweet marjoram (Origanum majorana L., family: Lamiaceae) leaves were purchased and authenticated from a herbal-specialised company (Isis Company). MLE was prepared as described previously(Reference Ramadan, El-Beih and Abd El-Ghffar23) by dissolving amounts equivalent to 50 and 100 mg MLP/kg body weight in glassware containing 0·5 ml boiling distilled water (equivalent to 1·5 and 3 cups of marjoram tea, respectively), then covered and let to stand for 10 min at room temperature. After that, the extracts were filtered and given fresh to the animals.
Adult male Wistar albino rats (Rattus norvegicus), weighing 125–135 g, were obtained from the National Research Centre in Giza, Egypt. The animals were housed in suitable cages and acclimatised to laboratory conditions for a period of 1 week before the commencement of the experiments. The rats were fed standard rodent food pellets (Agricultural-Industrial Integration Company) and double-distilled water. The standard rodent food pellets contained wheat-bran, dried clover, maize, bean-hay, methionine, molasses, salt, in addition to minerals and vitamins mix. The amount of crude proteins, fats and fibres in the food pellets were 12, 2·4 and 14 %, respectively. The energy content of the standard diet was 920·48 kJ/100 g. All animals were humanely treated in accordance with the WHO guidelines for animal care, and the study design was approved by the Ain Shams University Research Ethics Committee.
Experimental design and treatment schedule
Experimental animals were randomly divided into ten groups of five rats each: five healthy groups; five CP-treated groups. In the healthy groups, the rats were treated by oral administration, daily with 50 or 100 mg/kg body weight (low or high dose, respectively) of either MLP suspended in 0·5 ml distilled water (at room temperature) or MLE for 30 d. In the CP-treated groups, non-tumour-bearing rats were given the low or high dose of either MLP or MLE orally, daily for 30 d and received a single intraperitoneal injection of CP (25 or 40 mg/kg body weight on day 25 or 29, respectively) to induce immunosuppression(Reference Ramadan, El-Beih and Badr13, Reference Ramadan, El-Beih and Abd El-Kareem16) or clastogenicity(Reference Shukla, Arora and Taneja12). The healthy control and CP-only-treated animals received 0·5 ml distilled water orally and daily, as vehicle, instead of marjoram preparations for 30 d.
Blood and tissues sampling
The animals were subjected to light diethyl ether anaesthesia before being killed on day 31. Blood was collected into clean test-tubes with or without EDTA. A portion of blood with EDTA was used for complete blood picture analysis by Coulter (Hemat 8 analyser; SEAC) and to estimate the activities of erythrocyte enzymes. Another blood portion without EDTA was used to separate serum, which was divided into samples and preserved at − 70 °C for further analysis. Immediately after killing the animals, the thymus and spleen were separated out of the body, cleaned and weighed. Bone marrow was also collected from the left femur bone. The cellularity of lymphoid organs was measured by a Neubauer counting chamber (Paul Marienfeld GmbH) after lysing the erythrocytes using an erythrocyte lysis solution (AppliChem GmbH).
Measurements
Quantitative measurement of serum Ig concentration, as an indicator for humoral immune response, was performed using IgM and IgG pre-calibrated radial immunodiffusion plates (Biocientifica S.A.) according to the manufacturer's recommendations. Serum reduced and oxidised glutathione (GSH and GSSG, respectively) and malondialdehyde (MDA) concentrations were measured by HPLC (HP1100 series; Agilent Technologies)(Reference Jayatilleke and Shaw24, Reference Karatepe25). Total glutathione was calculated as follows: total glutathione = GSH+GSSG. Serum catalase (CAT) activity was estimated from the rate of formaldehyde formation in the presence of methanol and optimal concentration of H2O2. The produced formaldehyde was measured spectrophotometrically using 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole as a chromogen(Reference Johansson and Borg26). Serum glutathione S-transferase (GST) activity was determined by measuring the conjugation rate of 1-chloro-2,4-dinitrobenzene with GSH, which is directly proportional to the GST activity(Reference Habig, Pabst and Jakoby27). Erythrocyte glutathione peroxidase (GPx; selenium-dependent) activity was indirectly determined by a coupled reaction with glutathione reductase. GSSG, produced upon reduction of an organic hydroperoxide by GPx, was recycled to its reduced state by glutathione reductase and NADPH. The rate of decrease in NADPH is directly proportional to the GPx activity(Reference Paglia and Valentine28). Erythrocyte superoxide dismutase activity was determined by measuring the ability of the enzyme to inhibit the reduction of nitro-blue tetrazolium by superoxide, which was generated by the autoxidation of pyrogallol(Reference Minami and Yoshikawa29). The percentage of change of any parameter = ((T–C)/C) × 100, where T = the mean value of the parameter in the treated group and C = the mean value of the parameter in the control group.
Chromosomal aberrations and mitotic index
The animals received a single intraperitoneal injection of 2 mm-colchicines 2 h before being killed to disrupt spindle-fibre formation, prevent the migration of chromatids after splitting the centromere and generate diploid cells containing pairs of identical chromosomes that are homozygous at all loci(Reference El-Beih, Ramadan and Badr30). Bone marrow cells were stained for chromosomal aberration analysis as described previously(Reference El-Beih, Ramadan and Badr30). Briefly, bone marrow cells were incubated with 6 ml of hypotonic KCl (0·65 %, Sigma-Aldrich) at 37 °C for 20 min. After centrifugation, the sediment was fixed three times in a mixture of methanol and acetic acid (3:1, Sigma-Aldrich) before slides preparation. Then, the slides were air-dried and stained with Giemsa solution (Sigma-Aldrich). Following this, 100 spread metaphases per animal were randomly examined for structural and numerical chromosomal aberrations using 100 × oil immersion objective lenses. The mitotic index was also determined by calculating the percentage of cells in metaphase per 1000 cells for each animal.
Delayed type of hypersensitivity response
The delayed type of hypersensitivity response, as an indicator for cellular immune response, was determined as described previously(Reference Ramadan, El-Beih and Abd El-Kareem16) with some modifications. Briefly, on day 31, the animals were subcutaneously immunised with 2·25 × 1010 sheep erythrocytes (Institute of the Agricultural Researches, Giza, Egypt). On the fifth day of immunisation, the animals were again challenged with 1 × 1010 cells in the left hind footpad. The increase in footpad thickness was measured 24 h after the second challenge by a vernier calliper (Samir and Ali Bookshop, Cairo, Egypt). The right footpad was injected with saline solution and served as trauma control for non-specific swelling.
Statistical analysis
Data are presented as means with their standard errors. Statistical analysis was performed with one-way ANOVA, and the differences among groups were determined by Bonferroni's multiple comparison test(Reference Turner and Thayer31), using GraphPad Prism version 4.03 for Windows (GraphPad Software, Inc.). P values of < 0·05, < 0·01 and < 0·001 were considered statistically significant, highly significant and very highly significant, respectively.
Results
Marjoram leaves alleviated the genotoxicity induced by cyclophosphamide in bone marrow cells of rats
The present study showed that treatment of rats with CP alone induced a very highly significant increase (P < 0·001, t = 44·99, difference between means = − 232·2, 95 % CI − 248·9, − 215·5) in nine of the structural chromosomal aberrations that were detected in bone marrow cells: chromatid gap (1733 %), chromatid break (1900 %), chromatid deletion (454 %), fragment (1450 %), centromeric attenuation (1500 %), centric fusion (2175 %), end-to-end association (1510 %), ring chromosomes (1525 %) and chromosome stickiness (1485 %), compared with the healthy control animals (Fig. 1). Also, it induced a very highly significant increase (P < 0·001, t = 13·05, difference between means = − 16·40, 95 % CI − 20·48, − 12·32) in three of the numerical chromosomal aberrations (aneuploidy) that were found in bone marrow cells of rats: monosomic (2n − 1, 1500 %), trisomic (2n+1, 2800 %) and tetrasomic (2n+2, 2400 %), compared with the healthy control animals (Fig. 2(A)). On the other hand, CP-only-treated rats showed a very highly significant decrease (P < 0·001, t = 25·96, difference between means = 9·46, 95 % CI 8·28, 10·64) in the mitotic index (44 %) of bone marrow cells, compared with the healthy control animals (Fig. 2(B)).

Fig. 1 (A) Different types (CA, centromeric attenuation; CB, chromatid break; CD, chromatid deletion; CF, centric fusion; CG, chromatid gap; CS, chromosome stickiness; E-E, end-to-end association; F, fragment; NM, normal metaphase; RC, ring chromosome) and (B) total count of structural chromosomal aberrations in bone marrow cells of cyclophosphamide (CP) and/or marjoram leaves-treated rats. Values are means, with their standard errors represented by vertical bars. *** Mean values were significantly different from that of the control group (P < 0·001). ††† Mean values were significantly different from that of the CP-only-treated group (P < 0·001). ‡‡‡ Mean values were significantly different from that of the CP-treated group that received the corresponding dose of marjoram leaf powder (MLP) suspension (P < 0·001). §§§ Mean values were significantly different from that of the CP-treated group that received 50 mg/kg body weight of the same marjoram preparation (P < 0·001). MLE, marjoram leaf aqueous extract.
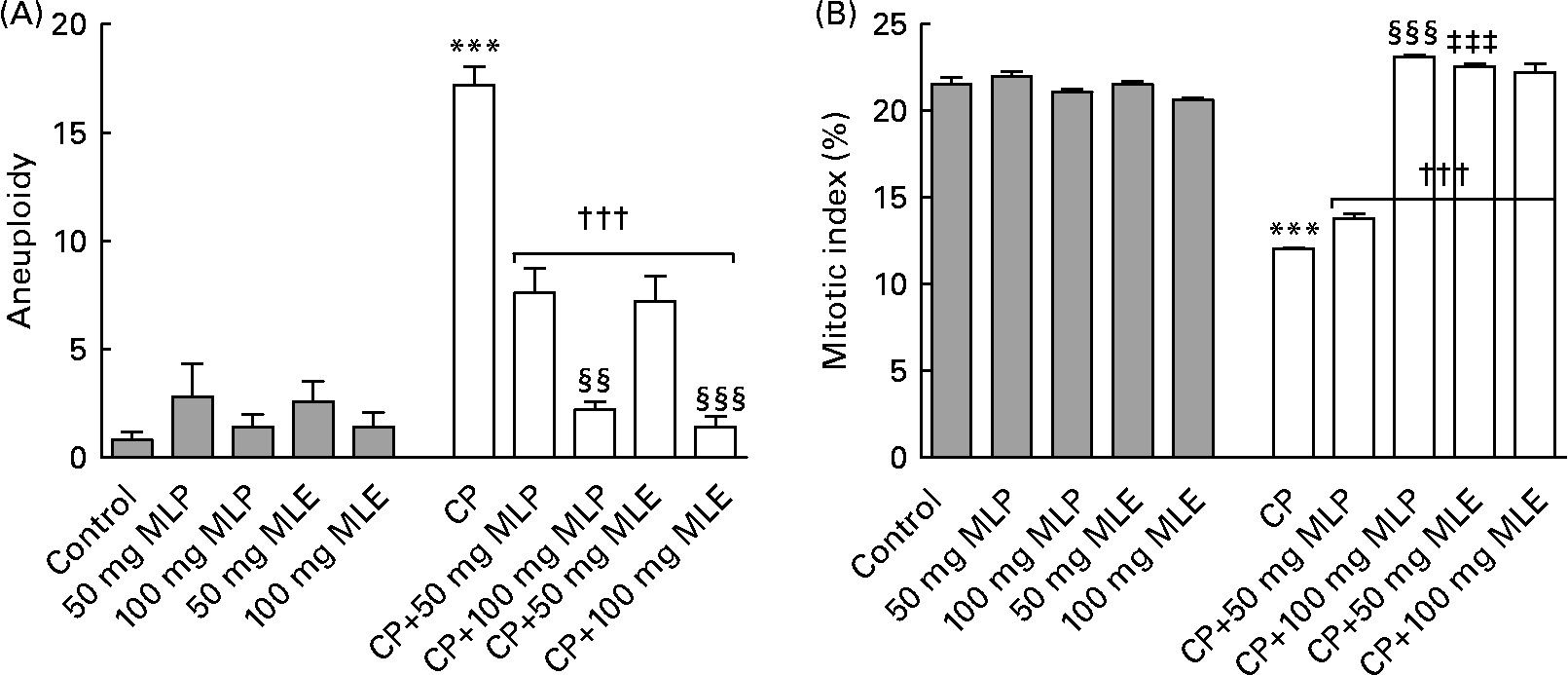
Fig. 2 (A) Aneuploidy numerical chromosomal aberrations and (B) mitotic index in bone marrow cells of cyclophosphamide (CP) and/or marjoram leaves-treated rats. Three types of aneuploidy (monosomic, trisomic and tetrasomic) were detected in the present study. Values are means, with their standard errors represented by vertical bars. *** Mean values were significantly different from that of the control group (P < 0·001). ††† Mean values were significantly different from that of the CP-only-treated group (P < 0·001). ‡‡‡ Mean values were significantly different from that of the CP-treated group that received the corresponding dose of marjoram leaf powder (MLP) suspension (P < 0·001). Mean values were significantly different from that of the CP-treated group that received 50 mg/kg body weight of the same marjoram preparation: §§ P < 0·01, §§§ P < 0·001. MLE, marjoram leaf aqueous extract.
Both MLP and MLE significantly alleviated (14–100 %, P < 0·001, compared with the CP-only-treated group) the increase in structural and numerical chromosomal aberrations and the decrease in mitotic index induced by CP in bone marrow cells of rats (Figs. 1 and 2). These modulatory effects significantly increased by increasing the dose of marjoram in all cases (P < 0·01–0·001), except in the mitotic index of bone marrow cells of CP-treated rats that received MLE where no statistically significant difference in modulation (P>0·05) was found between both doses of MLE (Fig. 2(B)). The modulatory effects of MLE on the increase in the structural chromosomal aberrations (Fig. 1(B)) and the decrease in the mitotic index (Fig. 2(B)) in bone marrow cells of CP-treated rats were significantly higher (10–17 %, P < 0·001) than that of MLP, especially at the low dose.
Marjoram leaves alleviated the immunosuppression induced by cyclophosphamide in rats
The present study showed that treatment of rats with CP alone induced severe blood leucopenia (P < 0·001, t = 8·87, difference between means = 3·99, 95 % CI 2·53, 5·45), which was mainly due to a significant decrease (P < 0·001) in counts of all granulocytes (41–84 %) and agranulocytes (68–76 %), compared with the healthy control animals (Table 1). It also caused a significant decrease (P < 0·05–0·001) in serum IgM (51 %) and IgG (28 %) levels (data not shown) and the delayed type of hypersensitivity response (25 %) as well as weights (64–66 %) and cellularity (54–79 %) of lymphoid organs, compared with the healthy control animals (Figs. 3 and 4).
Table 1 Blood total and differential leucocyte counts (109/l) of cyclophosphamide (CP) with/without marjoram leaves-treated rats
(Mean values with their standard errors)

MLP, marjoram leaf powder; MLE, marjoram leaf aqueous extract.
Mean values were significantly different from that of the control group: *** P < 0·001.
Mean values were significantly different from that of the cyclophosphamide-only-treated group: † P < 0·05, †† P < 0·01, ††† P < 0·001.
Mean values were significantly different from that of the cyclophosphamide-treated group that received 50 mg/kg body weight of the same marjoram preparation: ‡ P < 0·05.
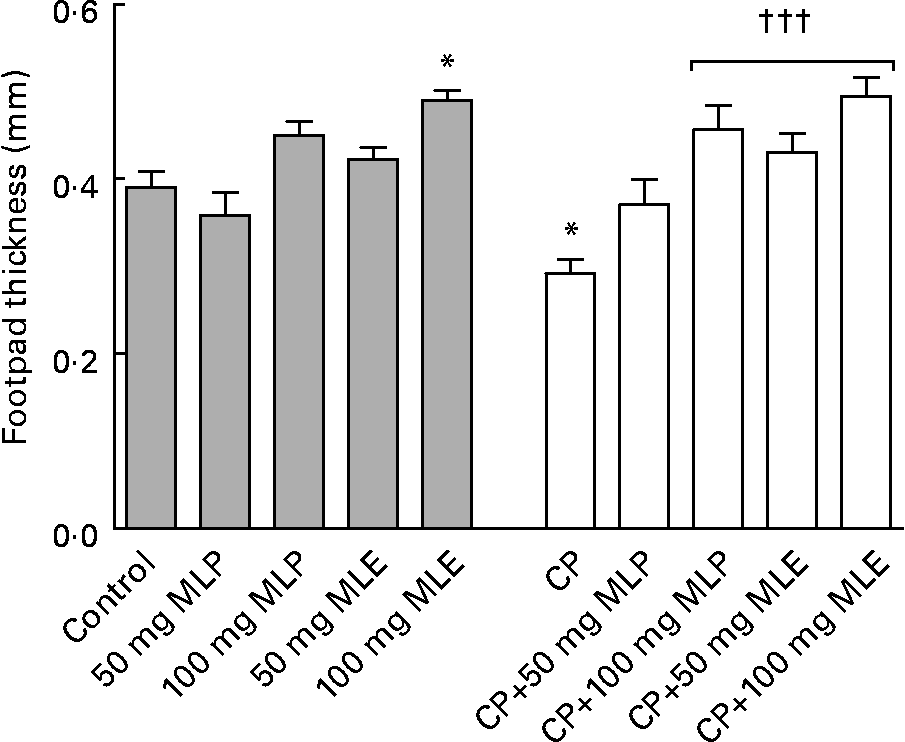
Fig. 3 Delayed type of hypersensitivity response of cyclophosphamide (CP) and/or marjoram leaves-treated rats. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from that of the control group (P < 0·05). ††† Mean values were significantly different from that of the CP-only-treated group (P < 0·001). MLE, marjoram leaf aqueous extract; MLP, marjoram leaf powder.

Fig. 4 (A) Weight (□, thymus; ![]() , spleen) and (B) cellularity (□, bone marrow;
, spleen) and (B) cellularity (□, bone marrow; ![]() , thymus;
, thymus; ![]() , spleen) of lymphoid organs of cyclophosphamide (CP) and/or marjoram leaves-treated rats. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from that of the control group: * P < 0·05, ** P < 0·01, *** P < 0·001. Mean values were significantly different from that of the CP-only-treated group: † P < 0·05, †† P < 0·01, ††† P < 0·001. Mean values were significantly different from that of the CP-treated group that received the corresponding dose of marjoram leaf powder (MLP) suspension: ‡ P < 0·05, ‡‡‡ P < 0·001. Mean values were significantly different from that of the CP-treated group that received 50 mg/kg body weight of the same marjoram preparation: § P < 0·05, §§ P < 0·01, §§§ P < 0·001. MLE, marjoram leaf aqueous extract.
, spleen) of lymphoid organs of cyclophosphamide (CP) and/or marjoram leaves-treated rats. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from that of the control group: * P < 0·05, ** P < 0·01, *** P < 0·001. Mean values were significantly different from that of the CP-only-treated group: † P < 0·05, †† P < 0·01, ††† P < 0·001. Mean values were significantly different from that of the CP-treated group that received the corresponding dose of marjoram leaf powder (MLP) suspension: ‡ P < 0·05, ‡‡‡ P < 0·001. Mean values were significantly different from that of the CP-treated group that received 50 mg/kg body weight of the same marjoram preparation: § P < 0·05, §§ P < 0·01, §§§ P < 0·001. MLE, marjoram leaf aqueous extract.
Both MLP and MLE significantly alleviated (P < 0·05–0·001) all the immunosuppressive activity of CP including leucopenia (94–102 %, Table 1), the decrease in humoral (27–99 %, data not shown) and cellular (47–69 %) immune responses (Fig. 3) and the decrease in weights (76–160 %) and cellularity (74–593 %) of lymphoid organs (Fig. 4), except that the low dose of MLP did not significantly modulate (P>0·05) the decrease in total granulocytes count (Table 1), the cellular immune response (Fig. 3) and bone marrow cellularity (Fig. 4(B)) in CP-plus-MLP-treated rats compared with CP-only-treated rats. Moreover, the delayed type of hypersensitivity response (Fig. 3) as well as the cellularity of thymus and spleen (Fig. 4(B)) in CP-treated animals that received the high dose of MLE exceeded that in the healthy control animals (27–44 %, P < 0·05–0·001). The modulatory effects on the cellularity of spleen and bone marrow significantly increased (P < 0·01 and P < 0·001, respectively) by increasing the dose of MLP (Fig. 4(B)). In addition, the modulatory effects on total agranulocytes count (Table 1) and the cellularity of lymphoid organs (Fig. 4(B)) significantly increased (P < 0·05–0·001) by increasing the dose of MLE. The modulatory effects of MLE on the decrease in the cellularity of bone marrow and spleen in CP-treated rats were significantly higher (132–172 %, P < 0·001) than that of MLP (Fig. 4(B)).
Marjoram leaves alleviated the haematotoxicity induced by cyclophosphamide in rats
The present study showed that treatment of rats with CP alone induced a highly significant decrease (9–35 %, P < 0·01–0·001) in erythrocyte and platelet counts and the Hb content and a highly significant increase (18 %, P < 0·01–0·001) in the mean corpuscular volume and mean corpuscular Hb (Fig. 5), but did not significantly change (1–9 %, P>0·05) the haematocrit value and mean corpuscular Hb concentration (data not shown), compared with the healthy control animals. Both MLP and MLE significantly alleviated (12–60 %, P < 0·05–0·001, compared with CP-only-treated rats) the macrocytic normochromic anaemia and thrombocytopenia caused by CP in rats (Fig. 5). These modulatory effects did not significantly change (P>0·05) by increasing the dose of either MLP or MLE. In addition, there was no significant difference (P>0·05) between the modulatory effects of MLP and MLE on the haematotoxicity induced by CP in rats (Fig. 5).
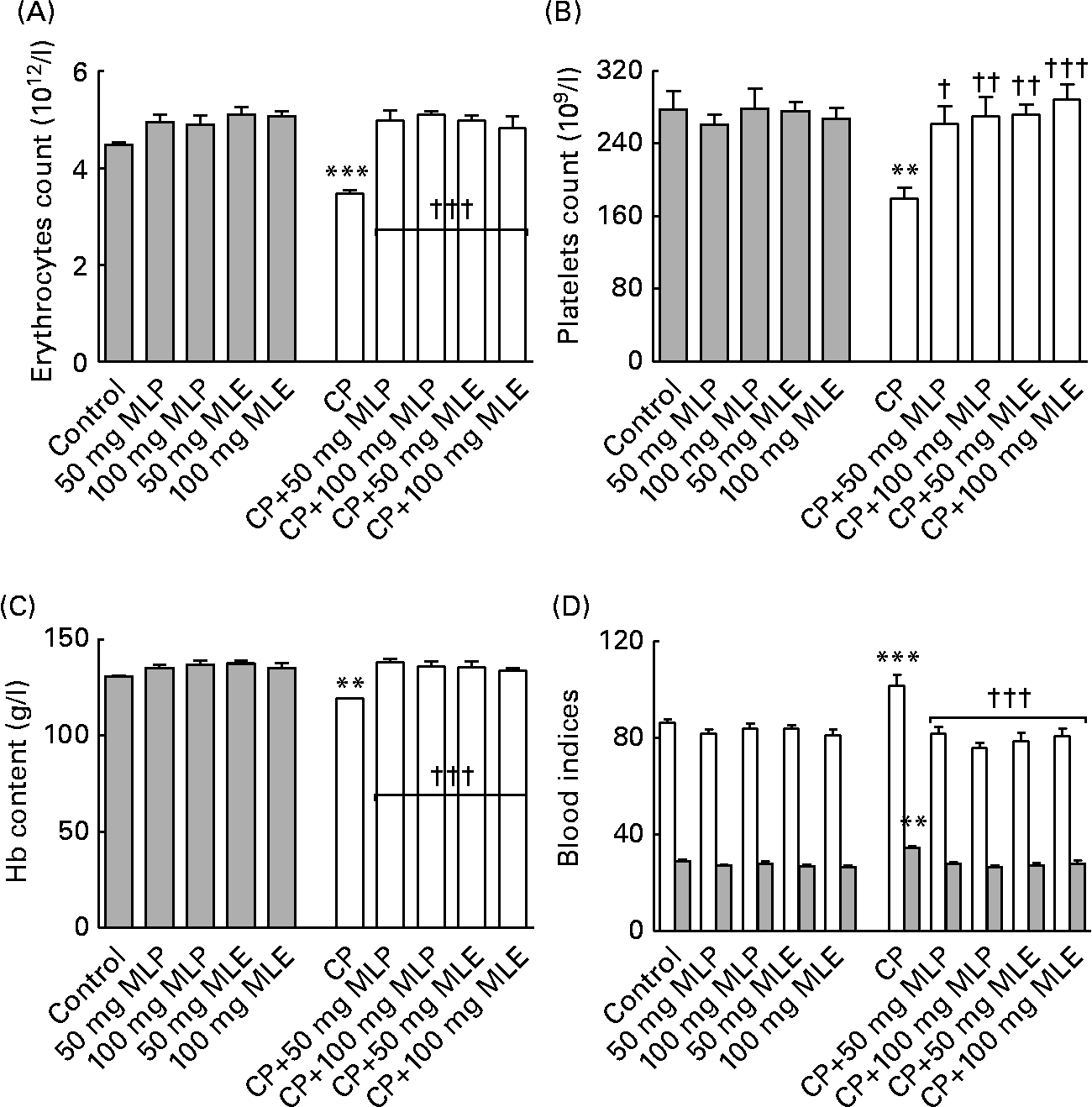
Fig. 5 (A) Erythrocytes count, (B) platelets count, (C) Hb content and (D) blood indices (□, mean corpuscular volume (fl); ![]() , mean corpuscular Hb (MCH, pg)) of cyclophosphamide (CP) and/or marjoram leaves-treated rats. The haematocrit value and MCH concentration did not significantly change among all groups. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from that of the control group: ** P < 0·01, *** P < 0·001. Mean values were significantly different from that of the CP-only-treated group: † P < 0·05, †† P < 0·01, ††† P < 0·001. MLE, marjoram leaf aqueous extract; MLP, marjoram leaf powder.
, mean corpuscular Hb (MCH, pg)) of cyclophosphamide (CP) and/or marjoram leaves-treated rats. The haematocrit value and MCH concentration did not significantly change among all groups. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different from that of the control group: ** P < 0·01, *** P < 0·001. Mean values were significantly different from that of the CP-only-treated group: † P < 0·05, †† P < 0·01, ††† P < 0·001. MLE, marjoram leaf aqueous extract; MLP, marjoram leaf powder.
Marjoram leaves alleviated the oxidative stress induced by cyclophosphamide in rats
The present study showed that treatment of rats with CP alone induced a very highly significant increase in serum MDA level (43 %, P < 0·001, t = 5·96, difference between means = − 0·14, 95 % CI − 0·22, − 0·06) and a significant decrease in the level of serum non-enzymic antioxidants (26–65 %, P < 0·05–0·001) and the activity of serum and erythrocyte enzymic antioxidants (11–46 %, P < 0·001), compared with the healthy control animals (Table 2). Low dose of MLP significantly alleviated (20–78 %, P < 0·01–0·001, compared with CP-only-treated rats) only the decrease in the activity of serum CAT and GST induced by CP in rats. The high dose of MLP significantly alleviated (17–86 %, P < 0·05–0·001, compared with CP-only-treated rats) the increase in serum MDA level as well as the decrease in serum total glutathione level and the activity of serum CAT and GST in CP-treated rats (Table 2). On the other hand, both doses of MLE significantly alleviated (9–184 %, P < 0·05–0·001, compared with CP-only-treated rats) the increase in the cellular toxicity and the decrease in the level/activity of all non-enzymic/enzymic antioxidants measured in serum and erythrocytes of animals that received CP (Table 2). These modulatory effects did not significantly change (P>0·05) by increasing the dose of either MLP or MLE. On the other hand, the modulatory effects of MLE on the increase in the cellular toxicity and the decrease in the activity of erythrocyte GPx were significantly higher (33–39 %, P < 0·05–P < 0·001) than that of MLP, especially at the high dose (Table 2).
Table 2 Levels of cellular toxicity and antioxidants in serum and erythrocytes of cyclophosphamide (CP) and/or marjoram leaves-treated rats
(Mean values with their standard errors)

MDA, malondialdehyde; GSH, reduced glutathione; GSSG, oxidised glutathione; CAT, catalase; GST, glutathione S-transferase; GPx, glutathione peroxidase; SOD, superoxide dismutase; MLP, marjoram leaf powder; MLE, marjoram leaf aqueous extract.
Mean values were significantly different from that of the control group: * P < 0·05, ** P < 0·01, *** P < 0·001.
Mean values were significantly different from that of the CP-only-treated group: † P < 0·05, †† P < 0·01, ††† P < 0·001.
Mean values were significantly different from that of the CP-treated group that received the corresponding dose of marjoram leaf powder suspension: ‡ P < 0·05, ‡‡ P < 0·01, ‡‡‡ P < 0·001.
Mean values were significantly different from that of the CP-treated group that received 50 mg/kg body weight of the same marjoram preparation: § P < 0·05, §§ P < 0·01, §§§ P < 0·001.
The percentages of changes of all parameters measured in the CP-only-treated group, the groups treated with CP plus either the low or high dose of MLP, and the groups treated with CP plus either the low or high dose of MLE were 453·5 (sem 116·4, P < 0·001), 216·3 (sem 53·8, P < 0·01), 74·4 (sem 24·3, P>0·05), 162·2 (sem 41·0, P>0·05) and 29·9 (sem 11·5, P>0·05), respectively, compared with the healthy control group. All of these results revealed that the utmost modulation on the serious side effects detected in the present study in CP-treated rats was induced by the high dose of MLE.
Beneficial and deleterious effects caused by marjoram leaves consumption in healthy rats
The present study showed that treatment of healthy rats with high dose of either MLP or MLE alone significantly increased (P < 0·05–0·001) the cellularity of thymus and spleen (22–45 %, Fig. 4(B)), serum total glutathione level, GSH:GSSG ratio and GST activity (27–160 %, Table 2). In addition, the low and high dose of MLE significantly increased the activity of serum CAT (23–52 %, P < 0·001) and erythrocyte superoxide dismutase (8–9 %, P < 0·05) in healthy animals (Table 2). Moreover, the low dose of MLE significantly increased the cellularity of thymus and spleen (28–41 %, P < 0·05–0·001, Fig. 4(B)), while the high dose of MLE significantly increased the delayed type of hypersensitivity response (26 %, P < 0·05, Fig. 3), bone marrow cellularity (46 %, P < 0·001, Fig. 4(B)) and erythrocyte GPx activity (20 %, P < 0·05, Table 2) in healthy animals. These beneficial effects significantly increased by increasing the dose of marjoram (P < 0·05–0·001) and were significantly higher in MLE (P < 0·05–0·001) in most cases. All other parameters measured in the present study did not significantly alter in healthy rats by both doses of either MLP or MLE (P>0·05). Moreover, food intake, body weight, blood total and differential leucocyte counts, haematocrit value and mean corpuscular Hb concentration were not significantly changed (P>0·05) in healthy rats that received either MLP or MLE, compared with the healthy control animals (data not shown). All of these results indicated that the marjoram doses used in the present study were considered safe.
Discussion
CP itself is an inactive cytostatic devoid of alkylating activity and must first undergo metabolic activation catalysed by the hepatic cytochrome P450 monoxygenase systems(Reference McCarroll, Keshava and Cimino1, Reference Haque, Bin-Hafeez and Parvez2, Reference Haque, Bin-Hafeez and Ahmad11, Reference Boddy and Yule32). Among the CP-reactive metabolites, phosphoramide mustard and acrolein are specially associated with the immunosuppressive action and oxygen radical formation of CP, respectively(Reference Bhatia, Kaur and Atif3, Reference Al-Yahya, Al-Majed and Gado14, Reference Boddy and Yule32). Acrolein also deprives the cells of its natural defence (GSH) against reactive oxygen species by interacting with its amino acid, cysteine(Reference Kehrer and Biswal33). Free radicals exert their toxic effects by acting on cellular macromolecules as DNA, membrane proteins and lipids(Reference Popov, Georgieva and Gadjeva15). The genotoxicity and chromosomal instability induced by many clastogenic agents are straight correlated with their ability to increase the oxidative stress(Reference Hussain, Hofseth and Harris34–Reference Lee, Lim and Lee36). Therefore, the oxidative stress arising from the overproduction of reactive oxygen species and the weakness in the antioxidant defence system by CP metabolites is involved in the pathogenesis of most of the side effects of CP.
The present study showed (probably for the first time) that both MLP and MLE (especially the high dose) significantly alleviated (P < 0·05–0·001) most of the side effects and toxicity of CP-treated rats including the increase in the structural and numerical chromosomal aberrations of bone marrow cells and serum MDA level, the deficiency in humoral and cellular immune responses and myelosuppression. The increase in the weight of lymphoid organs (thymus and spleen) induced by MLP and MLE in CP-treated animals was concomitant with the increase in their cellularity (Fig. 4). The modulatory effects of MLP and MLE on CP-induced myelosuppression were probably due to their ability to increase the mitotic index of bone marrow cells as shown in Fig. 2(B). The anticlastogenic and immunostimulatory activities of MLP and MLE shown in the present study may reduce the incidence of secondary tumours and infections, respectively, in cancer patients treated with CP or other chemotherapeutic agents. Other studies found that sweet marjoram extract and its component carvacrol induced apoptosis (programmed cell death) and showed antitumour activity against a number of human cancer cell lines and mouse N-ras transformed myoblast cells(Reference Lin, Liu and Chiang37–Reference Arunasree39). On the other hand, the antimicrobial activity of marjoram was attributed to ursolic acid, thymol and carvacrol(Reference El-Ashmawy, El-Nahas and Salama17, Reference Leeja and Thoppil40).
As shown in the present study (Table 2), one of the possible mechanisms for the beneficial effects of MLP and MLE in CP-treated animals was their ability to reduce CP-induced oxidative stress (as indicated by the decrease in the level of MDA, an indicator of lipid peroxidation and membrane damage) through reactivating the non-enzymic (GSH) and enzymic (CAT, GPx, GST, superoxide dismutase) antioxidant system. MDA can also react with deoxyadenosine and deoxyguanosine in DNA and form DNA adducts that are mutagenic(Reference Li, Henning and Zhang41). A number of GSH-inducing compounds have been found to be effective in reducing CP toxicity in animals(Reference Haque, Bin-Hafeez and Parvez2, Reference Bhatia, Kaur and Atif3, Reference Sharma, Trikha and Athar10, Reference Haque, Bin-Hafeez and Ahmad11, Reference Al-Yahya, Al-Majed and Gado14, Reference Ramadan, El-Beih and Abd El-Kareem16, Reference Manesh and Kuttan42). In addition, the toxic metabolites of CP, which are responsible for the drug urotoxicity, are mainly detoxified by GST(Reference Zhong, Huang and Yang43). Two previous studies also reported that the protective activity of sweet marjoram extracts and volatile oil against the clastogenic agents, hydroquinone and lead acetate, was mainly due to its ability to scavenge the free radicals(Reference El-Ashmawy, El-Nahas and Salama17, Reference Ghaly, Said and Abdel-Wahhab18). One of these two studies(Reference Ghaly, Said and Abdel-Wahhab18) did not investigate the antioxidant activity of sweet marjoram. In addition, the protective effect of sweet marjoram on the chromosomal aberrations induced by hydroquinone in bone marrow cells was weak, because the clastogenic activity of the hydroquinone itself was weak in the aforementioned study(Reference Ghaly, Said and Abdel-Wahhab18). The antioxidant activity of sweet marjoram was due to its high content of carnosol, carnosic acid, carvacrol, thymol and ursolic acid(Reference Vagi, Rapavi and Hadolin20, Reference Heo, Cho and Hong22, Reference Aydin, Basaran and Basaran44, Reference Hazzit, Baaliouamer and Faleiro45). Thymol and carvacrol also reduced the oxidative DNA damage in human lymphocytes(Reference Aydin, Basaran and Basaran44). Another compound, T3b, which was isolated from the methanol extract of sweet marjoram, showed strong antioxidant properties and may possess anticancer activity(Reference Jun, Han and Yu21). Its activity was approximately five times greater than that of α-tocopherol, an efficient natural ![]() scavenger(Reference Jun, Han and Yu21). Because free radicals are responsible for the pathogenesis of CP-induced dyslipidaemia, which is a well-known risk factor for CVD(Reference Ramadan, El-Beih and Abd El-Kareem16, Reference Mythili, Sudharsan and Sudhahar46), the antioxidant activity of sweet marjoram shown in the present study may also protect against hyperlipidaemic cardiomyopathy in CP-treated cancer patients. The anticlastogenic and immunostimulatory activities of sweet marjoram may also be attributed to its ability for DNA repair and mitogenic stimulation of the immunocompetent cells, respectively.
scavenger(Reference Jun, Han and Yu21). Because free radicals are responsible for the pathogenesis of CP-induced dyslipidaemia, which is a well-known risk factor for CVD(Reference Ramadan, El-Beih and Abd El-Kareem16, Reference Mythili, Sudharsan and Sudhahar46), the antioxidant activity of sweet marjoram shown in the present study may also protect against hyperlipidaemic cardiomyopathy in CP-treated cancer patients. The anticlastogenic and immunostimulatory activities of sweet marjoram may also be attributed to its ability for DNA repair and mitogenic stimulation of the immunocompetent cells, respectively.
In the present study, it was not determined whether the protection offered by the sweet marjoram leaves was related to a reduction in the efficacy of CP (by affecting the pharmacokinetics of this compound) or to a direct effect on the cells themselves. This topic needs further investigation and will be considered in the future. However, previous studies reported that an antioxidant dietary supplement can prevent the oxidation of biomolecules, including DNA, without decreasing the effectiveness of chemotherapeutic agents(Reference Ramadan, El-Beih and Badr13, Reference Popov, Georgieva and Gadjeva15, Reference Liu, Zhao and Zheng47, Reference Berger48). It was also reported that therapy with antioxidants concomitant with chemotherapy reduces the frequency and severity of side effects associated with many drugs in cancer patients, thereby allowing the treatment to be continued, since the toxicity of anticancer drugs is a frequent limitation to their extended use(Reference Borek49). Many plants were found to be effective in reducing or eliminating some of the undesirable side effects of CP in different animal models(Reference Haque, Bin-Hafeez and Parvez2–Reference Arafa4, Reference Bin-Hafeez, Ahmad and Haque7, Reference Sharma, Trikha and Athar10–Reference Ramadan, El-Beih and Abd El-Kareem16). Interestingly, Egyptian sweet marjoram leaves were found to be effective in the present study against most of the side effects caused by CP including genotoxicity, myelosuppression, immunosuppression and oxidative stress. The modulatory effects of marjoram leaves shown in the present study were dose dependent in most cases and much higher in MLE (21–23 % for all parameters taken together). Moreover, the high dose of MLE showed stronger immunostimulatory activity in healthy animals (Figs. 3 and 4(B)). The superior modulatory effects of MLE (especially the high dose) shown in the present study were probably due to its superior beneficial effect on the non-enzymic and enzymic antioxidant system (Table 2). No harmful effects were detected for sweet marjoram leaves on any of the parameters measured in the present study, indicating that the doses used in this study were considered safe. In general, people in different countries consume about 2·5–5 g/d of sweet marjoram leaves in culinary use, which was 4–5 times more than the dose used in the present study for animals. In conclusion, sweet marjoram leaves (especially in the form of a herbal tea) may be useful as an immunostimulant and in reducing genotoxicity in patients under chemotherapeutic interventions.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The authors thank Nadia M. S. Arafa (Department of Physiology, National Organization for Drug Control and Research, Giza, Egypt) for technical help/support in HPLC analysis. N. M. E.-B. and G. R. planned the study, designed all experiments, summarised, discussed and interpreted the results, and drafted the manuscript. M. M. Z. carried out the experiments and performed the statistical analysis with assistance from G. R. The authors have no potential financial conflict of interest.









