In recent years, n-3 PUFA supplementation has been associated with several health benefits. The n-3 fatty acids DHA (22 : 6n-3) and EPA (20 : 5n-3), found primarily in seafood, are essential constituents of cell membranes, and are critical for normal brain function(Reference Das1–Reference Su3). DHA is highly concentrated in membrane phospholipids and is important in neuronal membrane stability, neuroplasticity, signal transduction and neurotransmission(Reference Salem, Litman and Kim4, Reference Valenzuela5). EPA, although comprising only a small percentage of total brain fatty acid composition, is important in balancing immune and inflammation functions because the eicosanoids produced from EPA are anti-inflammatory(Reference Farooqui, Ong and Horrocks6).
There is increasing evidence that n-3 PUFA are involved in mood disorders. Epidemiological studies have shown that fish consumption is inversely associated with depression(Reference Edwards, Peet and Shay7–Reference Tanskanen, Hibbeln and Tuomilehto9). In addition, depressed patients show several alterations in n-3 PUFA, and particularly DHA, compared with healthy controls(Reference Edwards, Peet and Shay7, Reference Adams, Lawson and Sanigorski10–Reference Peet, Murphy and Shay12). There is also evidence that n-3 PUFA are of therapeutic benefit as an adjunctive treatment in depression(Reference Nemets, Stahl and Belmaker13–Reference Su, Huang and Chiu15). Meta-analyses of clinical trials(Reference Freeman, Hibbeln and Wisner16, Reference Lin and Su17) have shown a moderate anti-depressant effect of DHA and/or EPA, in addition to anti-depressant medication, after 4–16 weeks of treatment. However, there was substantial heterogeneity among trials, and several double-blind randomised controlled trials(Reference Marangell, Martinez and Zboyan18–Reference Silvers, Woolley and Hamilton20) found no beneficial effect of n-3 PUFA on depression.
Pregnancy and the post-partum period provide an excellent opportunity to examine the relationship between n-3 PUFA and depression. Pregnancy leads to several changes in PUFA status, including a depletion of maternal plasma DHA under normal dietary conditions(Reference Al, van Houwelingen and Kester21, Reference Holman, Johnson and Ogburn22) that persists after delivery(Reference Otto, van Houwelingen and Badart-Smook23, Reference Al, van Houwelingen and Hornstra24). This suggests that normal dietary intake may be insufficient during the perinatal period. During pregnancy, maternal DHA is selectively transferred to the fetus to support optimal fetal development, and after birth, breast milk provides DHA to the infant. Mothers may be at higher risk for post-partum depression when they become depleted of n-3 PUFA, and especially of DHA(Reference Hibbeln and Salem25). Depression is quite common during pregnancy and in the post-partum period. A large longitudinal cohort study found a combined prevalence of depression of 25 % during pregnancy and post-partum, with higher prevalence during pregnancy than during post-partum(Reference Evans, Heron and Francomb26). In terms of post-partum depression, a meta-analysis of fifty-nine studies reported a prevalence rate of 13 %(Reference O'Hara and Swain27).
Increased dietary intake of n-3 PUFA results in increased n-3 levels in maternal plasma and breast milk(Reference Makrides, Neumann and Gibson28), which might play a role in preventing or ameliorating depressive symptoms during pregnancy and the post-partum period. A meta-analysis of cross-national epidemiological data showed that lower seafood consumption and lower DHA content in mother's milk were associated with higher rates of post-partum depression(Reference Hibbeln29). Several studies support an association between low n-3 intake from seafood or low n-3 PUFA status and increased risk of depressive symptoms during pregnancy(Reference Golding, Steer and Emmett30) or in the post-partum period(Reference De Vriese, Christophe and Maes31), but results are mixed(Reference Browne, Scott and Silvers32–Reference Strom, Mortensen and Halldorsson34). In recent years, several intervention studies have been published. The question we attempt to answer in the present systematic review and meta-analysis is whether treatment with n-3 PUFA prevents or reduces symptoms of depression during and directly after pregnancy.
Methods
Inclusion criteria for the systematic review and meta-analysis were as follows: intervention with at least one n-3 PUFA or fish oil supplement; intervention period at least 4 weeks; mood or depression as a primary or secondary outcome measure using validated instruments on at least one occasion at the end of or after the intervention period. Participants had to be pregnant or post-partum women, either depressed or non-depressed. Furthermore, studies had to be placebo-controlled, double-blinded and randomised.
The initial search aimed to identify all reports of n-3 or fish oil interventions during pregnancy or post-partum with mood or depression being measured at least once; study quality was assessed after the search. Databases searched for this review included Embase.com, Medline, PubMed, PsycINFO, Web of Science, World Health Organization Reproductive Health Library and the Cochrane's Central Register of Controlled Trials, until December 2009. Search terms included a wide range of synonyms for perinatal (pregnan* or prenatal or antenatal or perinatal or postnatal or peripartum or post-partum); fish fatty acids (fish or DHA or docosahexaenoic acid or EPA or eicosapentaenoic acid or α-linolenic acid (ALA) or α-linolenic acid or omega-3 fatty acid or n-3 fatty acid); randomised controlled trials (supplemen* or randomised or RCT or trial or intervention or treatment); depression (depress* or mood) both in Medical Subject Heading (MeSH) or in index terms and text words. Reference lists of included studies and relevant reviews were searched, and reviews and meta-analyses concerning the treatment of perinatal depression were screened for additional relevant studies. Furthermore, attempts were made to locate unpublished material by searching conference abstracts and clinical trial registers for unpublished ongoing research (http://www.controlled–trials.com; http://www.wombatcollaboration.net). Authors of original reports were contacted to ask them for additional information if needed.
Meta-analysis
For most studies, the pre- to post-treatment effect sizes were calculated by subtracting the average post-treatment score from the average pre-treatment score and dividing the result by the pooled standard deviations of both groups or by using the average difference between the pre- and post-treatment scores in both groups. The standardised mean difference corrected for bias (Hedges's g) was used (with the correction factor J: 1 − (3/(4 × df − 1))). Several studies reported more than one outcome measure for depression, e.g. Edinburgh Postnatal Depression Scale (EPDS), Beck Depression Inventory (BDI), Hamilton Depression Rating Scale (HAM-D) and Montgomery–Åsberg Depression Rating Scale. We selected the EPDS because it is the most appropriate scale for this population. We selected the BDI from Mattes et al. (Reference Mattes, McCarthy and Gong35) as the EPDS was not used in that study. Each study was represented by only one effect size in the meta-analysis. When available, intention-to-treat (ITT) data were used in the meta-analysis. When means or mean differences and standard deviations were not reported, we used other statistics (i.e. P value) to compute the effect sizes, which applied to one study(Reference Krauss-Etschmann, Shadid and Campoy36). To calculate the pooled mean effect sizes, we used the computer program Comprehensive Meta-analysis (version 2.2.021; Biostat, Englewood, NJ, USA). The pooled mean effect sizes using both the fixed- and random-effects models were computed. In the random-effects model, the included studies are seen as a sample drawn from a population of studies, resulting in wider 95 % CI. As an indicator of homogeneity, the Q-statistic was calculated, and as an indicator of heterogeneity, the I 2-statistic (with 0 % indicating no, 25 % indicating low, 50 % indicating moderate and 75 % indicating high heterogeneity) was calculated. Selection bias was visually examined using the funnel plot.
Results
The literature search resulted in 508 citations. Relevant reviews were screened for potentially relevant references. The majority of these citations and references were excluded in the first screening phase. A total of eleven intervention reports were retrieved for detailed evaluation in the second screening phase. Of these eleven intervention papers, four were additionally excluded due to our inclusion and exclusion criteria(Reference Chiu, Huang and Shen37–Reference Marangell, Martinez and Zboyan40), and seven randomised, placebo-controlled, double-blind trials were included in the meta-analysis(Reference Mattes, McCarthy and Gong35, Reference Krauss-Etschmann, Shadid and Campoy36, Reference Doornbos, van Goor and Dijck-Brouwer41–Reference Su, Huang and Chiu45). For three studies, depression was not the primary outcome measure(Reference Mattes, McCarthy and Gong35, Reference Krauss-Etschmann, Shadid and Campoy36, Reference Doornbos, van Goor and Dijck-Brouwer41). All included studies used marine-derived n-3 PUFA interventions; some used fish oil(Reference Mattes, McCarthy and Gong35, Reference Krauss-Etschmann, Shadid and Campoy36, Reference Rees, Austin and Parker44), some DHA(Reference Doornbos, van Goor and Dijck-Brouwer41, Reference Llorente, Jensen and Voigt43), and some a combination of DHA and EPA(Reference Freeman, Davis and Sinha42, Reference Su, Huang and Chiu45). Therefore, the remainder of the present paper focuses on these fatty acids. Fig. 1 shows a detailed flow chart of the results of the literature search.
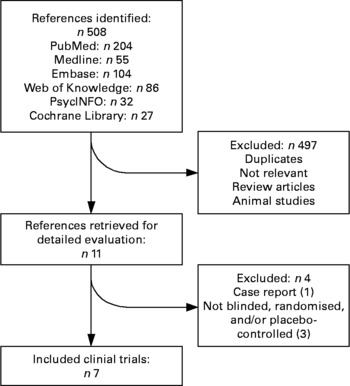
Fig. 1 Flow chart of the results of the literature search.
Description of included studies
A detailed outline of the intervention studies that were included in the meta-analysis is presented in Table 1. In the study by Doornbos et al. (Reference Doornbos, van Goor and Dijck-Brouwer41), apparently healthy pregnant women received either DHA (220 mg), DHA+arachidonic acid (220 mg each) or placebo daily from enrolment (weeks 14–20 of pregnancy) until 3 months after delivery. Depression was assessed with the EPDS in weeks 16 and 36 of pregnancy and 6 weeks post-partum. Erythrocyte fatty acid analysis was performed at enrolment and in week 36 of pregnancy. A total of 182 women were included in the trial; 111 participants completed all measurements. n-3 PUFA levels in erythrocytes were significantly higher in the supplemented groups. EPDS scores of 12 or higher were found in eight women (6·7 %) in week 36 of pregnancy and in seven women (5·9 %) at 6 weeks post-partum. Doornbos et al. (Reference Doornbos, van Goor and Dijck-Brouwer41) reported median EPDS and delta EPDS scores, as the data were skewed. For the meta-analysis, we used the mean delta EPDS scores (completers only) provided by the authors, as delta scores tend to be more normally distributed. Only data of the DHA group and the placebo group were included in the meta-analysis.
Table 1 Outline of the included studies

FO, fish oil; 5-MTHF, methyltetrahydrofolic acid (folate); AA, arachidonic acid; ITT, intention-to-treat; Max., maximum; BDI, Beck Depression Inventory; EPDS, Edinburgh Postnatal Depression Scale; SCID, Structured Clinical Interview for DSM Disorders; HAM-D, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; PP, per protocol.
In the study by Mattes et al. (Reference Mattes, McCarthy and Gong35), ninety-eight pregnant women with allergic disease, but otherwise healthy, were recruited before 20 weeks of pregnancy. The participants received either a daily supplement of 4 g fish oil (56 % DHA and 27·7 % EPA) or placebo from week 20 of pregnancy until delivery. Depression was measured with the BDI at 20 weeks of gestation and in the first week after delivery. Blood samples for fatty acid analyses were collected at 20 weeks of gestation and immediately after delivery. Complete data were available for seventy-five participants. At 20 weeks of gestation (before dietary intervention), 22·2 % of all participants had a BDI score of 10 or higher. Fish oil supplementation was associated with a significant increase in n-3 PUFA levels and the n-3:n-6 ratio, together with a proportional fall in n-6 PUFA levels (reported in Dunstan et al. (Reference Dunstan, Mori and Barden46)). Mean delta BDI scores (completers only), provided by the authors, were used in the meta-analysis.
In the study by Su et al. (Reference Su, Huang and Chiu45), pregnant women with DSM-IV major depressive disorder, onset between weeks 16 and 32 of gestation, were given gelatin capsules containing either n-3 PUFA (2·2 g EPA+1·2 g DHA) or placebo for 8 weeks. After a single-blind placebo run-in of 1 week, four participants who had a decrease of 20 % or more in HAM-D scores did not proceed to the randomisation phase. The HAM-D, EPDS and BDI were completed before the placebo run-in, at baseline and at weeks 2, 4, 6 and 8 of the intervention period. Blood samples for n-3 fatty acid analysis were taken before the placebo run-in and at week 8 of the intervention. Of the thirty-six included participants, twenty-four completed all measurements. n-3 Supplementation induced a significant increase in the erythrocyte DHA level, but not in the EPA level. Mean pre- and post-EPDS scores (baseline and week 8) presented in the paper (ITT) were used in the meta-analysis. The ITT population included all participants who had been evaluated on more than two visits.
In the study by Freeman et al. (Reference Freeman, Davis and Sinha42), pregnant (12–32 weeks) and post-partum (within 6 months of childbirth) women with major depressive disorder (DSM-IV criteria) and EPDS score 9 or higher, received either n-3 fatty acids (1·1 g EPA+0·8 g DHA) or maize oil (placebo; with 1 % fish oil for blinding purposes) for 8 weeks. Moreover, all patients received six 30 min sessions of individual psychotherapy during the trial. Depression was assessed with the HAM-D and EPDS at baseline and every 2 weeks during the treatment period. Of the fifty-nine participants, fifty-one completed at least two assessments. Mean pre- and post-EPDS scores (baseline and week 8; pregnant and post-partum women combined) presented in the paper were used in the meta-analysis, including all patients who completed baseline and at least one follow-up assessment.
In the study by Rees et al. (Reference Rees, Austin and Parker44), women in their third trimester of pregnancy or up to 6 months post-partum, with a current episode of depression or dysthymia, were treated with fish oil (6 g; 27·3 % DHA, 6·9 % EPA, 80 mg vitamin E) or placebo for 6 weeks. Blood samples for plasma fatty acid analyses were taken at baseline and at the end of the study. EPDS, HAM-D and Montgomery–Åsberg Depression Rating Scale data were collected weekly. Of the twenty-six women who entered the study, twenty-one completed all the measurements. Mean pre- and post-EPDS scores (baseline and week 6; pregnant and post-partum women together) presented in the paper were used in the meta-analysis. The ITT population included all subjects who started the study (at least baseline session).
In a four-arm study by Krauss-Etschmann et al. (Reference Krauss-Etschmann, Shadid and Campoy36), apparently healthy pregnant women received fish oil (500 mg DHA and 150 mg EPA); 400 μg methyltetrahydrofolic acid; both or placebo from week 22 of gestation until delivery. Plasma fatty acid analyses were performed at baseline (gestation week 20), gestation week 30 and at delivery. Depression was measured with the EPDS at delivery and/or at 2 months post-partum; this is not clearly described in the paper, and the authors did not provide additional information on repeated requests. Of the 311 participants enrolled, 270 completed the study. The fish oil supplementation increased maternal DHA and EPA during the supplementation period. Because the EPDS data are not included in the paper and the authors declined to provide these data, this study was included in the meta-analysis with a P value of 1 (as it is mentioned in the paper that no statistically significant group difference was found). A separate sensitivity analysis was run without these data. In the meta-analysis, the DHA and DHA+methyltetrahydrofolic acid groups were compared with the placebo and methyltetrahydrofolic acid groups.
In the study by Llorente et al. (Reference Llorente, Jensen and Voigt43) apparently healthy pregnant women received either 200 mg/d of DHA or placebo for 4 months, starting within a week of delivery. Plasma fatty acids were measured shortly before delivery and 4 months after delivery. The BDI was completed at baseline, 3 weeks, 2 months and 4 months after delivery. Of the 138 women enrolled, eighty-nine completed all measurements. Plasma DHA levels after 4 months were significantly higher in the DHA group than in the placebo group. Mean pre- and post-BDI scores (completers only; baseline and 4 months post-partum) presented in the paper were used in the meta-analysis.
Characteristics of study quality are shown in Table 2. Although all studies were randomised, double-blinded and placebo-controlled, the quality of the included studies was not always optimal. Placebo type was not always mentioned, and it was not always clear whether the placebo matched the active treatment in terms of dose, appearance, smell and flavour. Moreover, only one study mentioned evaluating whether the blinding was adequate. It is important to verify adequate blinding because n-3 supplements can have a fishy aftertaste, which may reduce the success of blinding. The number of participants was low in most studies. In one study, which was the largest study, depression was presumably measured on only one occasion(Reference Krauss-Etschmann, Shadid and Campoy36). In contrast with Consolidated Standards of Reporting Trials guidelines, ITT analyses were presented in only one of the seven studies(Reference Rees, Austin and Parker44). Two other papers(Reference Freeman, Davis and Sinha42, Reference Su, Huang and Chiu45) presented their analyses as ITT analyses (Table 1), but closer inspection revealed that, in these studies, only participants who completed at least two(Reference Freeman, Davis and Sinha42) or more than two(Reference Su, Huang and Chiu45) visits were included. It is also important to note that most studies measured a change in mood or depressive symptoms, but not a change in the diagnostic status of perinatal depression.
Table 2 Methodological quality characteristics of the included studies

EPDS, Edinburgh Postnatal Depression Scale.
Meta-analysis
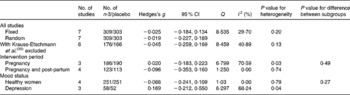
We could compare the EPA and/or DHA pre- to post-treatment depression change in seven studies, totalling 612 subjects (Fig. 2). A fixed-effect meta-analysis on all contrasts was conducted (Fig. 2 and Table 3) resulting in a mean pooled effect size of − 0·03 (95 % CI − 0·18, 0·13; P = 0·76) and − 0·02 (95 % CI − 0·23, 0·19; P = 0·86) using a random-effects model. The hypothesis of homogeneity was not rejected because a non-significant Q value was found (Q = 8·54, P = 0·20; I 2 = 29·7). The effect sizes and 95 % CI of the included studies are plotted in Fig. 2, which shows that the 95 % CI of one study did not overlap with the CI of the pooled mean effect size. When only the EPDS was used as the outcome measure, the pooled mean effect size was 0·02 (n 450; 95 % CI − 0·17, 0·21; P = 0·83 using a fixed-effects model). Repeating the analyses while excluding the trial by Krauss-Etschmann et al. (Reference Krauss-Etschmann, Shadid and Campoy36) resulted in a similar pre- to post-treatment effect size (Hedges's d = − 0·05; n 342; 95 % CI − 0·26, 0·17; P = 0·68; Table 3). Therefore, the pooled mean effect size was non-significant and indicated no or a small pre- to post-treatment decrease in the perinatal depression. The pooled effect size of the three trials in depressed patients showed some indication of effectiveness (effect size 0·17; 95 % CI − 0·21, 0·55), though not statistically significant (Table 3).
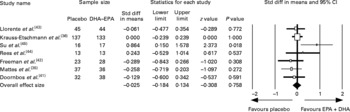
Fig. 2 Standardised effect sizes of n-3 fatty acids DHA and EPA compared with that of placebo oil and 95 % CI of the included studies and the pooled effect size. Std diff, standardised difference.
Table 3 Meta-analyses of studies examining the effects of n-3 fatty acids DHA and EPA (i.e. fish oil) on perinatal depressive symptoms: overall results and subgroup analyses
The funnel plot (Fig. 3) indicated no strong evidence for the presence of publication bias or systematic heterogeneity. Although the positive study by Su et al. (Reference Su, Huang and Chiu45) was an outlier, the other studies fitted a rather symmetric inverted funnel shape.
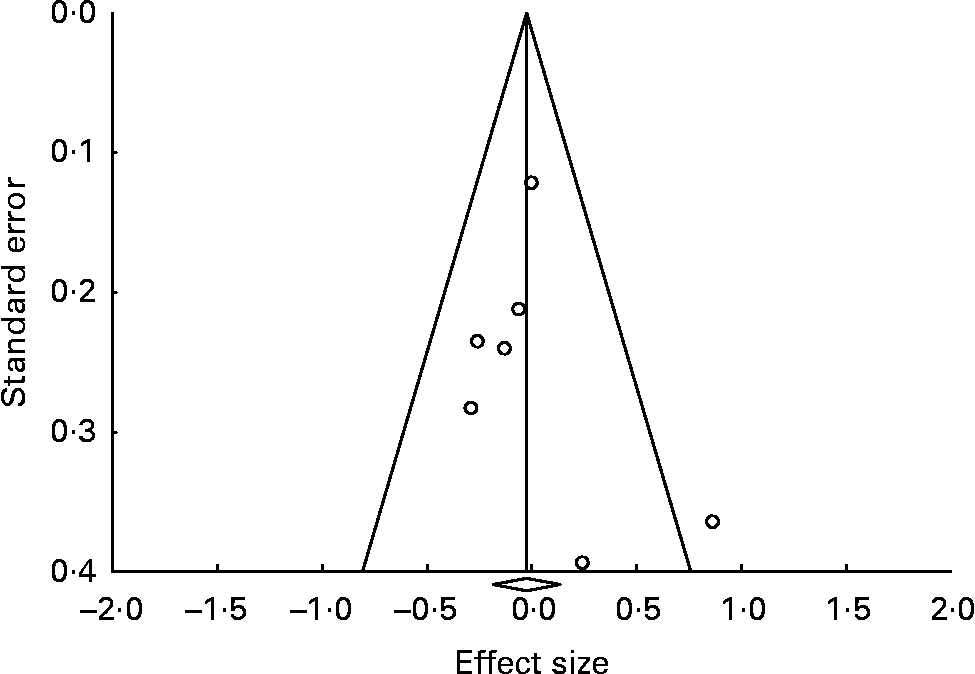
Fig. 3 Funnel plot (publication bias assessment) of the effect sizes (Hedges's g) according to their standard errors. ![]() , Drawn at the pooled effect size; —, expected 95 % CI for a given standard error, assuming limited between-study heterogeneity.
, Drawn at the pooled effect size; —, expected 95 % CI for a given standard error, assuming limited between-study heterogeneity.
Discussion
The meta-analysis showed no beneficial effect of n-3 PUFA over placebo on symptoms of perinatal depression. The pre- to post-treatment effect sizes were consistently close to zero, indicating no significant change in depressive symptoms during fish oil, EPA and/or DHA administration, except for one study(Reference Su, Huang and Chiu45). In this study(Reference Su, Huang and Chiu45), prenatally depressed Taiwanese women received a relatively large dose of DHA and, especially, EPA daily for 8 weeks. This was the only study in which a single-blind placebo run-in of 1 week was used; the participants who showed a decrease in the HAM-D score of 20 % or more were excluded. Because only one randomised double-blind placebo-controlled study reported a beneficial effect of n-3 supplementation on perinatal depression, it is important to replicate this finding using a larger study population and also in other ethnic populations.
The results of the present meta-analysis are not in line with two previous meta-analyses on the efficacy of n-3 PUFA for unipolar major depression, which found n-3 PUFA to be superior to placebo treatment(Reference Lin and Su17, Reference Freeman, Hibbeln and Wisner47). It should be noted, however, that significant heterogeneity was observed in the latter two meta-analyses. Meta-analyses in which samples other than depressed patients were also included(Reference Appleton, Hayward and Gunnell48) (and recent updates(Reference Rogers, Appleton and Kessler19, Reference Appleton, Rogers and Ness49)) found little evidence for a beneficial effect of n-3 PUFA on depressed mood. However, in two of these meta-analyses(Reference Appleton, Hayward and Gunnell48, Reference Appleton, Rogers and Ness49), a separate analysis including only trials that enrolled populations with diagnosed depressive illness did show a beneficial effect of n-3 PUFA supplementation on depressed mood, although substantial heterogeneity remained. This indicates that a possible effect may be restricted to depressed populations, and that there may be as-yet unknown moderators of the effect within depressed populations.
Of the individual studies included in this meta-analysis, most showed limitations in methodological quality. In fact, several authors mention limitations or advise caution in the interpretation of results(Reference Mattes, McCarthy and Gong35, Reference Doornbos, van Goor and Dijck-Brouwer41–Reference Su, Huang and Chiu45). In the study by Doornbos et al. (Reference Doornbos, van Goor and Dijck-Brouwer41), reported limitations included the relatively small sample size caused by a high drop-out and the relatively low DHA dosage. In addition, the EPDS was used to measure perinatal depression, which is a self-report questionnaire that is developed as a screening tool and not as an instrument to assess the effects of interventions. This latter concern is also mentioned by Llorente et al. (Reference Llorente, Jensen and Voigt43). Su et al. (Reference Su, Huang and Chiu45) point out that interpretation of the results is complicated by the high discontinuation rate and the lack of information about compliance, which might have biased the results. In the study by Freeman et al. (Reference Freeman, Davis and Sinha42), both groups showed significant improvement. The psychotherapy intervention may have obscured any differences in effect between treatment groups. Furthermore, the small number of subjects was a limitation of that study. In the study by Rees et al. (Reference Rees, Austin and Parker44), it is possible that the large placebo response and/or spontaneous remissions may have masked any beneficial effect of the n-3. Furthermore, the small sample size may also be a reason that this trial should not be viewed as definitive. Thus, the quality and study sizes of research so far have been far less than optimal.
Besides the limitations that were mentioned by the authors, the following limitations further complicate the interpretation of the results of the present meta-analysis. First, the number of included studies is low. Second, there are large differences among the included studies in terms of treatment (dose, duration, EPA and/or DHA), outcome measure and population (depressed or healthy participants, pregnant and/or post-partum). Heterogeneity, although not statistically significant, is therefore of concern. Due to the low number of included studies, it was not possible to compare subsets of studies. Third, most studies measured a change in the mood or depressive symptoms but not a change in the clinical diagnosis of depression. Fourth, not all studies were designed to address perinatal depression, and consequently these studies may have been underpowered to detect differences in the depression measures. Fifth, per-protocol analyses instead of ITT analyses were used in most studies, which may have increased the chance of finding treatment effects. The fact that a treatment effect was not found suggests that EPA and/or DHA treatment is not effective in treating and/or preventing perinatal depression. Finally, in the studies with non-depressed participants(Reference Mattes, McCarthy and Gong35, Reference Krauss-Etschmann, Shadid and Campoy36, Reference Doornbos, van Goor and Dijck-Brouwer41, Reference Llorente, Jensen and Voigt43), baseline depressive symptoms were already low. This, combined with a small sample size, has probably resulted in insufficient power to detect small-to-moderate treatment effects in most studies. In conclusion, although currently available data indicate no beneficial effect of n-3 supplementation on the perinatal depression, it may at this stage be too early to draw conclusions.
The available evidence appears to suggest that EPA and/or DHA supplementation is more likely to be beneficial in treating existing symptoms of perinatal depression than in preventing perinatal depression in healthy populations, as is the case for the treatment of depression in general. Sample size may also be an issue; as most included studies were small, it may have been difficult to detect preventative effects. Moreover, the higher severity of depression at baseline also increases statistical power, as the scales used (i.e. BDI, HAM-D and EPDS) are designed to be sensitive in clinically depressed patients and are not very sensitive in detecting changes in the non-pathological range. Future research should include participants with relatively high levels of depressive symptoms (or at high risk of depressive symptoms). It is unclear whether DHA or EPA or their combination may be more effective. The positive study used 2·2 g EPA+1·2 g DHA daily, suggesting that a high dose of EPA may be important, but in this study, the intervention induced an increase in the erythrocyte DHA level but not in the EPA level. Future studies should provide a complete profile of the oils used, and blood samples should be taken to evaluate the biochemical effects of the intervention. The intervention should be sufficient in dose and duration, and should start when the natural decline in n-3 PUFA during pregnancy occurs. Sample sizes should be large enough to detect small-to-moderate effect sizes. A lead-in phase as suggested by Thase(Reference Thase50) may be helpful to exclude placebo responders and to increase power. Fish consumption should be controlled or included in the analysis, as this variable has the potential to confound the results. Compliance should be monitored, and blinding success should be verified. Furthermore, future studies should provide ITT in addition to per-protocol data. If depressed patients are included, a structured clinical interview should be used to confirm the diagnosis. If self-report questionnaires are used, attention should be paid to the severity of the symptoms because it may be difficult to detect treatment effects in populations with mild symptoms. More preclinical research may be needed to determine the mechanism of action and dose–response characteristics.
In conclusion, on the basis of these findings, EPA and/or DHA cannot be considered to be an empirically supported treatment for perinatal depression as yet. However, the limitations in study quality complicate this interpretation. Well-controlled and larger studies of longer duration are necessary to assess the efficacy of the DHA and EPA in pregnant patients with a major depressive disorder or at high risk for developing depression (e.g. with a history of depression).
Acknowledgements
The authors' responsibilities were as follows: L. A. W. J. performed the literature search and drafted the manuscript; E. J. G. was involved in the conception and design of the study, the statistical analysis and the provision of significant advice; A. J. W. V. d. D. helped in the study supervision, provision of significant advice; all the authors contributed to the data interpretation, critical review and revision of the manuscript. This work was supported by a NWO-VICI grant (no. 453-06-005) to A. J. W. V. d. D., who has also received research support (€10 000) from Minami Nutrition NV, Antwerp, Belgium, which produces n-3 capsules. The other authors report no conflicts of interest.








