Heart failure (HF) is well documented as a global health problem not only because it is a cause of increased morbidity and mortality, but also because it is responsible for high socio-economic costs( Reference Stewart, Jenkins and Buchan 1 , Reference Cook, Cole and Asaria 2 ). An epidemiological investigation in 2000 demonstrated that the morbidity due to HF was 0·9 % in China( Reference Weiwei, Runlin and Lisheng 3 ), and one in nine deaths in America was found to be related to HF( Reference Mozaffarian and Benjamin 4 ). Despite advances in the moderation of mortality, hospitalisation for HF is still frequent and re-admission rates continue to rise( Reference Roger 5 ).
Fe performs a crucial role in oxygen transport and storage because it is a key component of Hb( Reference Jankowska, von Haehling and Anker 6 , Reference Buratti, Gammella and Rybinska 7 ). Until recently, Fe deficiency (ID) was ignored despite it being a common and stand-alone co-morbidity in HF associated with poor physical activity and adverse outcome, independent of anaemia status( Reference Cohen-Solal, Leclercq and Deray 8 – Reference Klip, Comin-Colet and Voors 10 ). ID, the most common cause of anaemia, is becoming increasingly prevalent in HF patients, which is worth investigation and further discussion.
Recently, it has been recognised that the effect of anaemia treatment on HF patients using erythropoietin and Fe supplementation significantly improves cardiac function and quality of life (QoL)( Reference Cohen-Solal, Leclercq and Deray 8 ). A randomised controlled trial (RCT) carried out by Silverberg et al. ( Reference Silverberg, Wexler and Sheps 11 ) confirmed that erythropoietin and intravenous Fe treatment significantly enhanced cardiac function and reduced hospitalisation but without apparent increase in adverse events. Bolger et al. ( Reference Bolger, Bartlett and Penston 12 ) found in an observational study that intravenous Fe sucrose alone is a simple and safe therapy that increases the capacity for exercise and relieves symptoms in chronic HF patients.
However, the data produced in these studies were too underpowered to establish that Fe therapy provides clinical benefit in patients with heart failure who have reduced ejection fraction (HFrEF) having ID. Many further clinical trials have since been conducted, which claim to provide more detailed analysis to obtain a more accurate conclusion. Therefore, we carried out a meta-analysis to summarise the evidence from these RCT to evaluate the safety and efficacy of Fe therapy in patients with HFrEF having ID.
Methods
Search strategy
We performed a systematic literature search of PubMed, MEDLINE, EMBASE and the Cochrane Library databases, in addition to conference proceedings and the Clinical Trials database for ongoing and unpublished trials. The reference lists of all articles obtained through the search process were reviewed for additional trials. Specifically, the search query was ‘(“heart failure” OR “cardiac failure” OR “myocardial failure” OR “heart decompensation”) AND (“ferric” OR “ferrous” OR “iron”)’. The search was restricted to articles about humans and published up to December 2017.
Inclusion and exclusion criteria
The search results were limited to RCT. The inclusion criteria were as follows: (1) symptomatic stages of congestive heart failure (CHF) (New York Heart Association class II–IV) with ejection fraction (EF) lower than 45 %; (2) ID: serum ferritin lower than 100 ng/ml or serum ferritin between 100 and 299 ng/ml with transferrin saturation (TSAT) <20 %; (3) intervention: oral or intravenous Fe preparations and (4) control group: placebo or no treatment. Trials were excluded if they included patients under 18, pregnant women or patients with active bleeding. Trials that were still recruiting patients or without any available quantifiable data were excluded. There were no restrictions on publication status (published, conference proceedings or unpublished), publication year or language. Research results were independently screened by two reviewers (C. W. and S. Z.) using a structured literature tool (Endnote X7; Thomson Reuters). Any disagreements were resolved through consensus reached by discussion with a third researcher (F. Z.).
Data extraction and quality assessment
Two reviewers (C. W. and S. Z.) independently extracted the following data from the trials included in the review: first author’s family name, year of publication, number of participants, baselines, clinical end points and adverse events. Disagreements were resolved through discussion with a third researcher (F. Z).
The trials were assessed by examination of the following aspects: random sequence generation, allocation concealment, blinding of subjects and participants, blinding of outcome assessment and statistical analysis, integrity of follow-up visit, incomplete or selective outcome data reporting and baseline comparability. Each domain was graded as low risk, unclear risk (where there was lack of information or uncertainty over the potential for bias) or high risk (for bias according to the criteria specified in the Cochrane Handbook version 5.1.0.)( Reference Higgins and Green 13 , Reference Higgins, Altman and Gotzsche 14 ).
Definition of outcomes
The primary outcome measures were peak VO2/kg and change in distance for the 6 min walk test (6MWT) over baseline measurement.
Secondary outcomes included mortality, number of hospitalisations during follow-up, adverse effects related to treatment, Minnesota Living with Heart Failure Questionnaire (MLHFQ), European Quality of Life-5 Dimensions (EQ-5D) and Fe metabolism biomarkers, such as Hb levels, serum ferritin and TSAT.
Statistical analysis
Data analysis was performed using RevMan 5.3 (The Cochrane Collaboration). Dichotomous data were analysed by calculating the OR for each trial, with 95 % CI for the uncertainty of each result. Mean and standard deviation values were obtained for continuous variables, including calculating a weighted mean difference for variables on the same scale from different trials. A fixed effects model was used throughout the review, except in the event of significant heterogeneity between trials (I 2>50 %), in which case a random effects model was used in the analysis. Mean and variance from the median, range and size of sample were estimated when the mean and variance were not directly acquired. Publication bias was not assessed because of the limited number (below 10) of studies selected( Reference Higgins and Green 13 , Reference Hozo, Djulbegovic and Hozo 15 ).
Results
Search results and study characteristics
From the initial search, 3527 articles and abstracts (including duplicates) were extracted, with an additional six from other sources that were identified and retrieved for further review. The evaluation excluded 2898 of these, with seventy-nine selected for full screening. The study selection process, described using a preferred reporting items for systematic reviews and meta-analyses flow diagram( Reference Moher, Liberati and Tetzlaff 16 ), is displayed in Fig. 1. Finally, nine RCT performed between 2007 and 2017 were included in the meta-analysis.
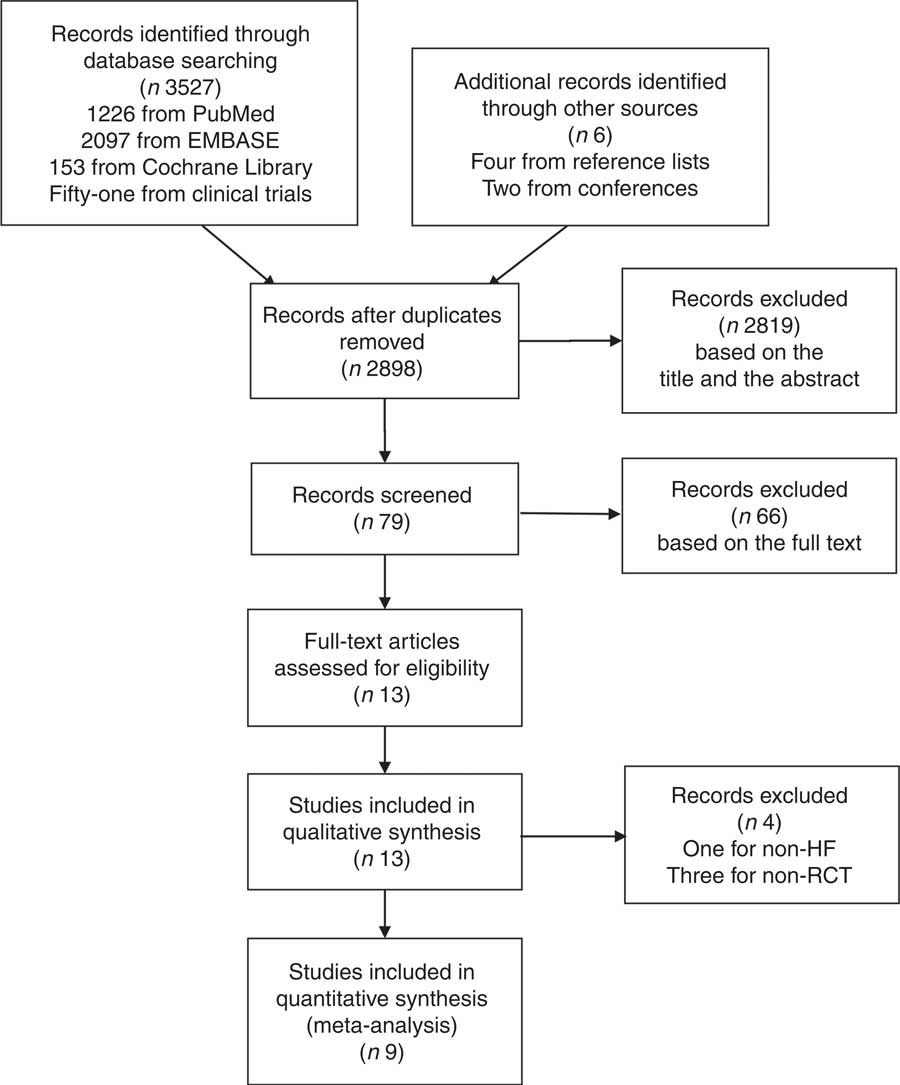
Fig. 1 Process of literature search and study selection. HF, heart failure; RCT, randomised control trial.
The characteristics of studies included in the review( Reference Anker, Comin Colet and Filippatos 17 – Reference Suryani, Raharjo and Sagita 25 ) are summarised in online Supplementary Table S1. All studies were prospective, double blinded RCT, except for one study that was open labelled( Reference van Veldhuisen, Ponikowski and van der Meer 24 ). The treatment group received a variety of doses of intravenous Fe, depending on baseline ferritin, with two studies using oral Fe preparations( Reference Lewis, Malhotra and Hernandez 20 , Reference Suryani, Raharjo and Sagita 25 ). The control group received a placebo or no treatment at all. It should be noted that in the study of Beck-da-Silva et al. ( Reference Beck-da-Silva, Piardi and Soder 19 ) there were two treatment groups, intravenous Fe and oral Fe preparation. Only safety and tolerability of intravenous Fe preparation were reported as outcomes in Arutyunov et al.’s study( Reference Arutyunov, Bylova and Ivleva 18 ). A total of 1374 patients with chronic HF with reduced EF and ID were included, of whom 789 were included in the treatment arm and 585 in the control group.
Risk of bias assessment
Two researchers evaluated the risk of bias of the studies included in the review, according to the Cochrane risk of bias assessment tool( Reference Higgins, Altman and Gotzsche 14 ). Online Supplementary Table S2 summarises the risk of bias results. No sufficient data were available to assess the risk of bias in the studies of Arutyunov et al. ( Reference Arutyunov, Bylova and Ivleva 18 ) and Suryani et al. ( Reference Suryani, Raharjo and Sagita 25 ).
Outcomes
Peak VO2
Three studies reported peak VO2/kg at the final follow-up. The studies were heterogeneous (I 2=87 %) and so a random effects model was used in the analysis, which demonstrated that Fe supplementation in the treatment group was associated with greater benefits to the peak VO2/kg in patients (Fig. 2(a)). Interestingly, this improvement was only observed in studies of intravenous Fe preparations, while oral Fe supplementation did not improve patient exercise tolerance.
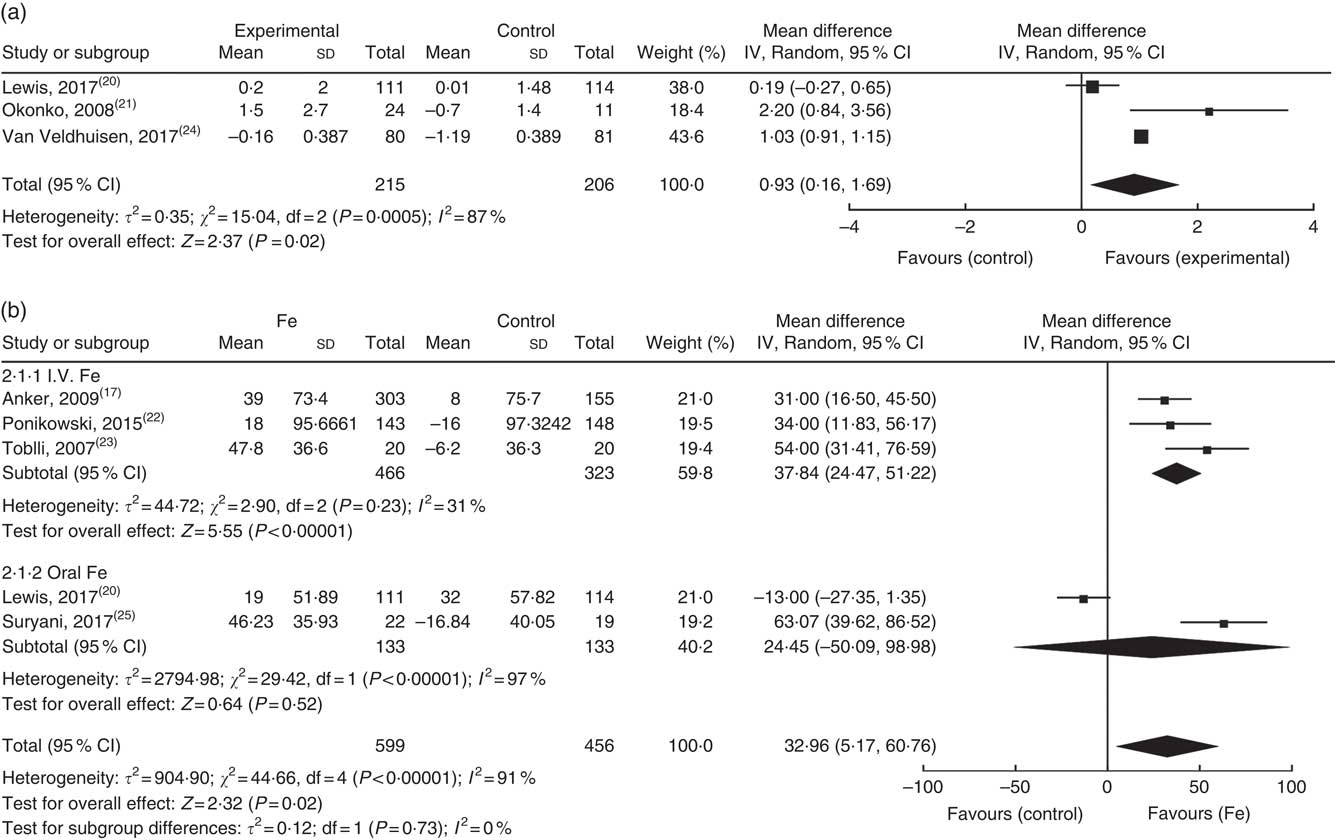
Fig. 2 Effect of exercise tolerance on: (a) peak VO2 (ml/kg per min); (b) sub-analysis of change in 6-min walk test distance compared with baseline.
6-min walk test
Five trials examined the effect of Fe on the 6MWT. The study published by Lewis et al. ( Reference Lewis, Malhotra and Hernandez 20 ) demonstrated that at the end of the 16-week follow-up, oral Fe supplementation had not increased the distance walked by patients in 6-min compared with baseline. The other four studies indicated that Fe supplementation (three intravenous and one oral) increased the 6-min walking distance compared with baseline. The overall heterogeneity was large (I 2=91 %) and analysis using a random effects model demonstrated that Fe supplementation failed to increase the distance of the 6MWT. Only when the Lewis et al. ( Reference Lewis, Malhotra and Hernandez 20 ) and Suryani et al. ( Reference Suryani, Raharjo and Sagita 25 ) studies were removed, the heterogeneity reduced significantly (I 2=31 %), and analysis using a fixed effects model demonstrated a significant increase in the distance of the 6MWT (Fig. 2(b)).
All-cause mortality and cardiovascular death
Seven studies reported the effect of Fe supplementation on cardiovascular death and all-cause mortality at the end of follow-up, with no apparent heterogeneity. Analysis by fixed effects model demonstrated that Fe supplementation failed to reduce all-cause mortality (OR: 0·70; 95 % CI 0·38, 1·28) (Fig. 3(a)). Three studies reported cardiovascular-related deaths with no apparent heterogeneity. Fixed-effects model analysis indicated that Fe supplementation did not reduce cardiovascular death in patients with chronic HF (OR: 0·81; 95 % CI 0·40, 1·64) (Fig. 3(b)).
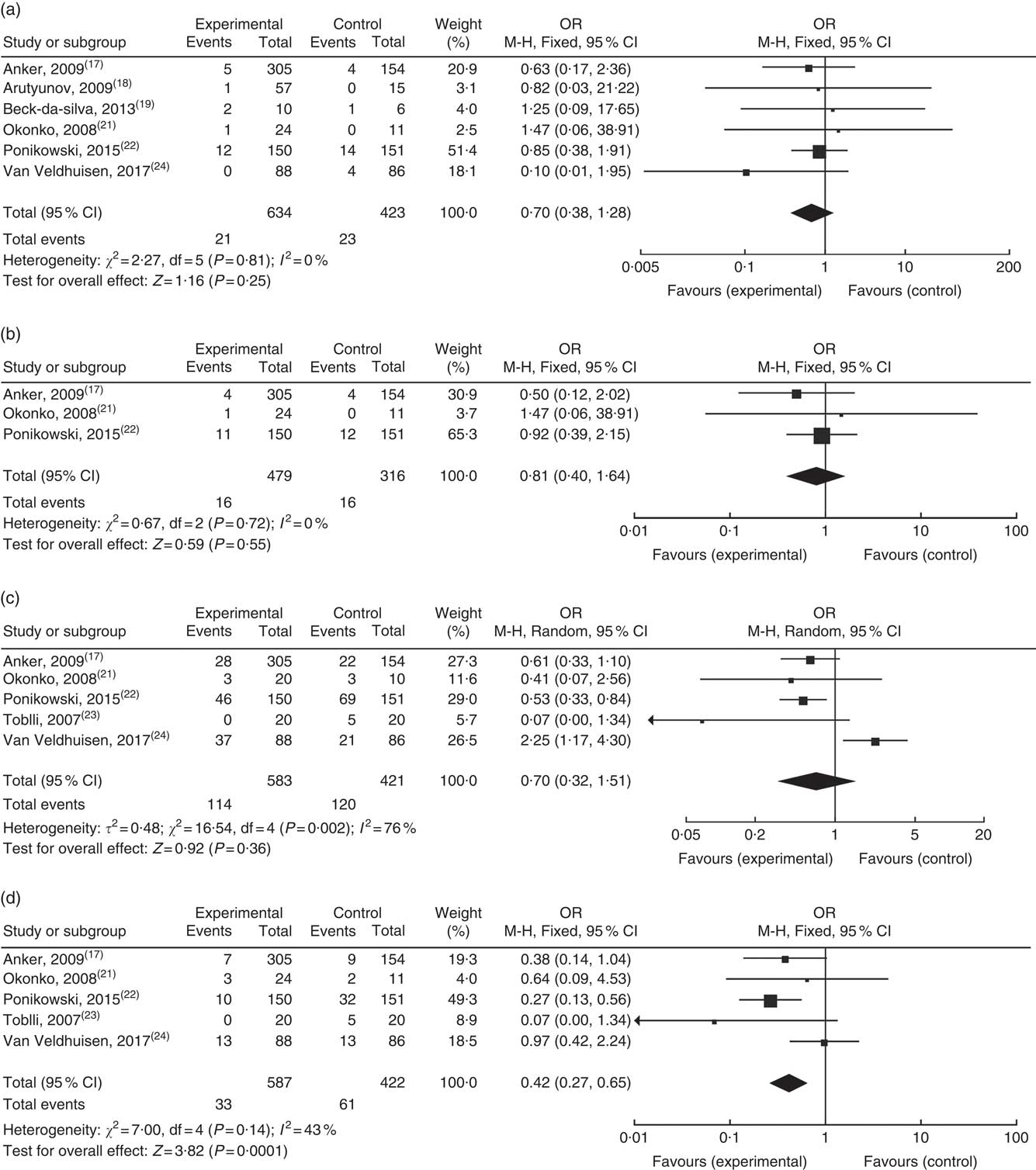
Fig. 3 Effect on: (a) all causes of death; (b) cardiovascular death; (c) re-hospitalisation and (d) re-hospitalisation for heart failure at the end of follow-up.
Hospitalisation
Five studies reported re-admission rate throughout the respective studies, indicating that intravenous Fe supplementation did not reduce the overall re-admission rate. Subgroup analyses were performed which indicated a significant decline in re-admission for HF (OR: 0·42; 95 % CI 0·27, 0·65) (Fig. 3(c) and (d)).
Serious adverse events
Six studies reported serious adverse reactions, with sixty-four patients in the treatment group and seventy-four patients in the control group having at least one serious adverse event. Analysis using a fixed effects model demonstrated that the treatment group exhibited a lower incidence of serious adverse reactions (OR: 0·58; 95 % CI 0·40, 0·83) (Fig. 4). No significant differences were observed in the adverse events, such as gastrointestinal dysfunction, nervous system adverse reaction or changes in skin mucosa.
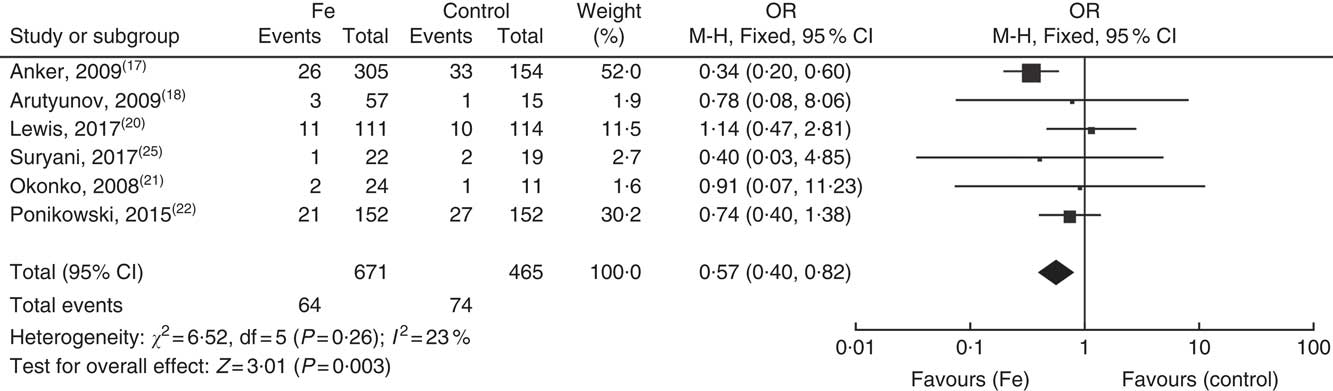
Fig. 4 Forest plot showing the serious adverse effects.
Quality-of-life parameters
Two trials reported the MLHFQ score, with their pooled results demonstrating a statistical improvement (–19·47; 95 % CI –23·36, –15·59). EQ-5D score was assessed by Anker et al. ( Reference Anker, Comin Colet and Filippatos 17 ) and Ponikowski et al. ( Reference Ponikowski, van Veldhuisen and Comin-Colet 22 ). Compared with the control group, the Fe therapy group had a higher score, indicating improved clinical status (online Supplementary Fig. S1).
Iron metabolism markers
Six trials( Reference Anker, Comin Colet and Filippatos 17 , Reference Lewis, Malhotra and Hernandez 20 , Reference Okonko, Grzeslo and Witkowski 21 , Reference Toblli, Lombraña and Duarte 23 , Reference van Veldhuisen, Ponikowski and van der Meer 24 , Reference Suryani, Raharjo and Sagita 25 ) examined the effects of Fe supplementation on the level of Fe metabolism-related markers. Available Fe metabolic markers were TSAT, Hb and ferritin. The trials included in this review indicated that Fe supplementation increased ferritin and TSAT except the study published by Lewis et al. ( Reference Lewis, Malhotra and Hernandez 20 ). Okonko et al. ( Reference Okonko, Grzeslo and Witkowski 21 ) suggested that Fe therapy improved ferritin and TSAT but did not increase Hb at the end of follow-up.
Cardiac and renal function
Four studies( Reference Anker, Comin Colet and Filippatos 17 , Reference Okonko, Grzeslo and Witkowski 21 – Reference Toblli, Lombraña and Duarte 23 ) reported the effects of Fe supplementation on cardiac function. These findings all demonstrated an improvement or a tendency of improvement in cardiac function at the end of follow-up, in addition to an improvement in QoL. There was no report of improvement in cardiac function through the use of oral Fe supplementation. Five studies( Reference Anker, Comin Colet and Filippatos 17 , Reference Beck-da-Silva, Piardi and Soder 19 – Reference Okonko, Grzeslo and Witkowski 21 , Reference Toblli, Lombraña and Duarte 23 ) reported the effects of Fe supplementation on renal function. Toblli et al. ( Reference Toblli, Lombraña and Duarte 23 ) and Anker et al. ( Reference Anker, Comin Colet and Filippatos 17 ) demonstrated that there was a significant improvement in renal function. Okonko et al. ( Reference Okonko, Grzeslo and Witkowski 21 ), Lewis et al. ( Reference Lewis, Malhotra and Hernandez 20 ) and Beck-da-Silva et al.( Reference Beck-da-Silva, Piardi and Soder 19 ) found no change in renal function over the duration of the respective studies. No decline in renal function was observed in any of the studies.
Sensitivity analysis
The analysis above demonstrated that heterogeneity was significantly reduced when the studies published by Lewis et al. ( Reference Lewis, Malhotra and Hernandez 20 ) and Suryani et al. ( Reference Suryani, Raharjo and Sagita 25 ), which analysed 6MWT distance and peak VO2/kg compared with the baseline, were removed. Eliminating the trials individually from the analysis had no relevant effect on the overall outcome. Recalculating the analysis using a random effects model instead of a fixed effects model did not change the overall outcome.
Discussion
In the present meta-analysis, we included available data on the treatment effect and safety of Fe supplementation in patients with HFrEF having ID. The analysis demonstrated that Fe supplementation increased peak VO2/kg and reduced re-admission for HF without increasing the incidence of serious adverse events but failed to reduce mortality. Unlike intravenous supplementation, oral Fe supplementation increased transferrin concentration and serum Fe levels but did not improve peak oxygen uptake, 6-min walking distance, N-terminal pro-hormone of brain natriuretic peptide, Kansas City Cardiomyopathy Questionnaire scores and failed to reduce HF re-admission or rates of death.
HF patients often have many co-morbidities that worsen their prognosis( Reference Ambrosy, Fonarow and Butler 26 , Reference Gheorghiade and Pang 27 ). Currently, recommendations for HF therapy include β-blockers, angiotensin-converting enzyme inhibitors, aldosterone receptor antagonists and diuretics, which perform a critical role in the management of HF( Reference Yancy, Jessup and Bozkurt 28 , Reference Ponikowski, Voors and Anker 29 ). However, despite the standard-of-care regimens, the mortality and re-admission rates for HF are still high, suggesting that the overall treatment strategy for patients with HF should consider other co-morbidities( Reference van Deursen, Urso and Laroche 30 ).
Fe plays a crucial role in many biological functions, including energy production, cell proliferation, oxygen delivery and storage (as a component of myoglobin) and oxidative metabolism in skeletal and heart muscles. Fe is an obligate element of Hb and many types of enzymes, participating in various cellular processes. ID may be detrimental to the cardiovascular system( Reference von Haehling, Jankowska and van Veldhuisen 31 ). Maeder et al. ( Reference Maeder, Khammy and dos Remedios 32 ) demonstrated that both Fe content in tissues and type 1 transferrin receptor expression in HF patients were reduced. Myoglobin is the primary oxygen-carrying and binding protein found in muscles. Mitochondrial function also requires Fe since it is a co-factor for many proteins involved in electron transfer and in ATP and energy production in cells.
ID describes a diminished concentration of Fe in stores and hence cannot meet the eventual demand. ID is among the most prevalent co-morbid condition in patients with CHD and chronic HF( Reference Jankowska, von Haehling and Anker 6 , Reference Ebner and von Haehling 33 ). Exercise tolerance is affected due to reduced oxygen storage in myoglobin, reduced energy efficiency and mitochondrial dysfunction when Fe storage is depleted( Reference Haas and Brownlie 34 ). Several observational studies have demonstrated that ID is associated with an increased mortality rate in CHF. Jankowska et al. ( Reference Jankowska, Rozentryt and Witkowska 35 ) found that ID was a poor prognostic factor in many patients with CHF, including hospitalisation and death. The 3-year survival rate was 59 % in patients with ID and 71 % for patients without ID (P=0·0006).
A previous meta-analysis (Avni et al.( Reference Avni, Leibovici and Gafter-Gvili 36 )) also showed that Fe repletion was associated with improved QoL parameters and safety for HF patients. Our study further confirmed that Fe supplementation can improve peak oxygen uptake and re-admission in patients with HFrEF, which was not reported in previous meta-analysis studies.
Lewis et al. ( Reference Lewis, Malhotra and Hernandez 20 ) published the first multicentre randomised controlled clinical trial exploring the effects and safety of oral Fe supplementation in patients with HFrEF having ID( Reference Lewis, Malhotra and Hernandez 20 ). There are several explanations for the failure of oral Fe supplementation to improve exercise tolerance in patients with HFrEF. The absorption of Fe from oral preparations is generally poor and, furthermore, up to 60 % of patients experience gastrointestinal side effects after its administration( Reference Anker and von Haehling 37 ). The absorption of Fe from the gastrointestinal tract may be limited by numerous foods and drugs, and intestinal mucosal oedema caused by systemic venous congestion in HF patients also results in a decrease in Fe absorption( Reference Sandek, Bjarnason and Volk 38 ). Hepcidin is a peptide hormone secreted by the liver that regulates Fe homoeostasis( Reference Ohno, Hanawa and Jiao 39 ). Hepcidin modulates Fe release by causing the degradation of Fe transporters( Reference Franchini, Montagnana and Lippi 40 , Reference Ganz 41 ). In a recent study by Jankowska et al. ( Reference Jankowska, Malyszko and Ardehali 42 ), systolic HF appeared to be the result of high levels of circulating hepcidin, which caused unnaturally high rates of Fe transporter degradation.
A variety of Fe complexes were assessed in the trials in this review, such as ferric carboxymaltose, Fe sucrose, etc. Although there is limited comparative data for IV Fe complexes, this review establishes that Fe supports improvement in many parameters in patients with HFrEF having ID.
In the sensitivity analysis, eliminating the study published by van Veldhuisen et al. ( Reference van Veldhuisen, Ponikowski and van der Meer 24 ) resulted in more apparent differences in total re-hospitalisation rates during the follow-up period, which may be due to non-cardiovascular reasons. This exclusion did not change the trend in re-hospitalisation following an analysis related to HF. After removing the study published by Lewis et al. ( Reference Lewis, Malhotra and Hernandez 20 ), Fe therapy resulted in a much greater increase in peak VO2/kg, while the numbers of serious adverse reactions did not change, also suggesting that oral Fe supplementation failed to improve the peak VO2, though not increasing the number of adverse reactions. The reasons mentioned above are likely to be the cause of this heterogeneity.
This review found that both oral and intravenous Fe supplementation did not increase the incidence of adverse reactions, such as gastrointestinal and nervous system disorders, and conversely, adverse effects in the treatment group were lower than that in the control group (OR: 0·58; 95 % CI 0·40, 0·83), suggesting the safety of Fe supplementation. Furthermore, Fe supplementation could even reduce the symptoms in patients with ID and HF.
There are some limitations in this review, that is, the inclusion of an open-label study and those of oral Fe supplementation, which may be sources of heterogeneity. In addition, total Fe dose varied, as did the duration of follow-up, which ranged from 16 to 52 weeks, so generalisation of the findings should be done with caution.
Despite these limitations, this meta-analysis suggests that patients with HFrEF having ID may benefit from Fe supplementation without increasing the incidence of adverse events. Additionally, a larger sample size, longer follow-up and multicentre prospective clinical studies are required to confirm whether Fe supplementation can reduce mortality and improve long-term prognosis. Whether oral Fe supplementation can improve the exercise tolerance of patients with HF and prognosis requires additional persuasive research.
The findings of this meta-analysis of RCT suggest that intravenous but not oral Fe supplementation can increase the peak VO2/kg and 6MWT and reduce re-admission for HF, without increasing the incidence of serious adverse events. This may provide a possible therapeutic target for patients with HFrEF having ID. Future clinical studies with larger sample sizes that focus on mortality are required.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (no. 81400303). The funder had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
C. W., S. Z. and F. Z. designed the study; S. Z., M. D. and K. H. collected the data; F. Z. performed the statistical analysis and drafted the manuscript; C. W., S. Z., F. Z., M. D. and K. H. critically revised the manuscript for important intellectual content and C. W. supervised the work and had primary responsibility for the final content. All authors read and approved the final manuscript. S. Z. and F. Z. did the literature search, screened the results, extracted and analysed data from included trials. M. D. and K. H. helped perform the analysis with constructive discussions. C. W. designed the study, performed the research, analysed data and wrote the paper. This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451900014X







