Published online by Cambridge University Press: 19 March 2024
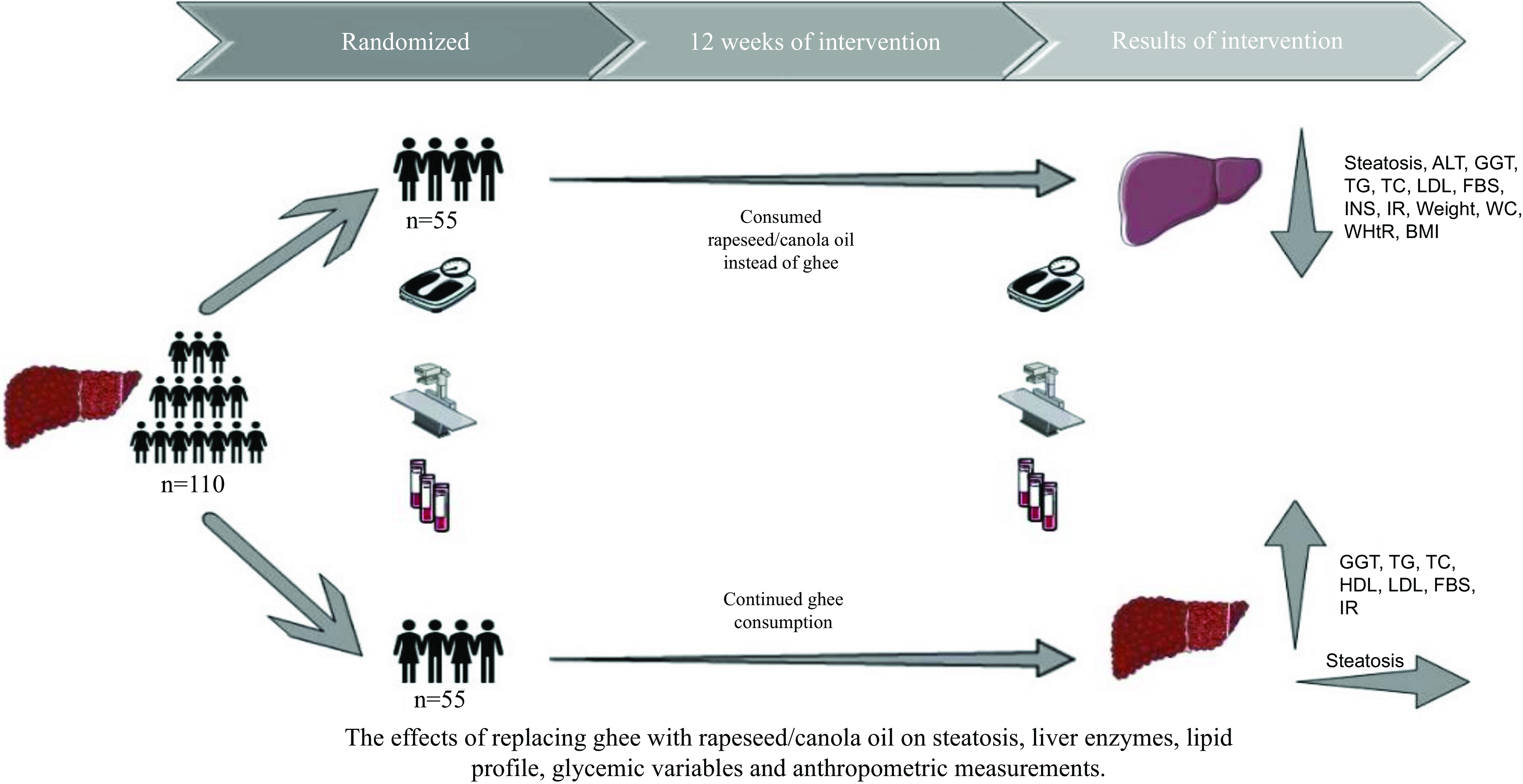
Non-alcoholic fatty liver disease (NAFLD), which is a prevalent hepatic condition worldwide, is expected to develop into the leading reason for end-stage fatty liver in the forthcoming decades. Incorporating rapeseed oil into a balanced diet may be beneficial in improving NAFLD. The goal of this trial was to evaluate the impact of substituting ghee with rapeseed oil on primary outcomes such as fatty liver and liver enzymes, as well as on secondary outcomes including glycaemic variables, lipid profile and anthropometric measurements in individuals with NAFLD. Over 12 weeks, 110 patients (seventy men and forty women; BMI (mean) 28·2 (sd 1·6 kg/m2); mean age 42 (sd 9·6) years), who daily consumed ghee, were assigned to the intervention or control group through random allocation. The intervention group was advised to substitute ghee with rapeseed oil in the same amount. The control group continued the consumption of ghee and was instructed to adhere to a healthy diet. Results showed a significant reduction in the steatosis in the intervention group in comparison with the control group (P < 0·001). However, a significant change in the levels of alanine aminotransferase (–14·4 μg/l), γ-glutamyl transferase (–1·8 μg/l), TAG (–39·7 mg/dl), total cholesterol (–17·2 mg/dl), LDL (–7·5 mg/dl), fasting blood glucose (–7·5 mg/dl), insulin (–3·05 mU/l), Homeostatic Model Assessment for Insulin Resistance (–0·9), Quantitative Insulin-Sensitivity Check Index (+0·01), weight (–4·3 kg), BMI (–0·04 kg/m2), waist (–5·6 cm) and waist:height ratio (–0·04) was seen in the intervention group. The consumption of rapeseed oil instead of ghee caused improvements in liver steatosis and enzymes, glycaemic variables and anthropometric measurements among individuals with NAFLD.