The maintenance of overall health and prevention of chronic diseases are closely linked to lifestyle and dietary factors. Indicators of cardiovascular health and metabolic function, such as blood pressure, lipid profile and glycaemic control, play a crucial role in this regard(Reference Arnett, Blumenthal and Albert1,Reference Yang, Jin and Zhang2) . Unfortunately, these parameters are often compromised in conditions such as hypertension, dyslipidaemia and diabetes. Such conditions significantly increase the risk of severe complications, including kidney failure, stroke and heart attack. According to the American Heart Association, CVD accounted for 874 613 deaths in the USA in 2019 and was the leading global cause of death, accounting for approximately 19·05 million deaths in 2020. Stroke was the second leading cause of death worldwide, causing 6·2 million deaths in 2019(Reference Tsao, Aday and Almarzooq3). Hypertension affects one in three adults worldwide and is a major risk factor for stroke, heart attack and kidney damage(Reference Organization4). Approximately one in three adults with diabetes have chronic kidney disease, which can lead to kidney failure and require dialysis or transplantation(Reference Koye, Magliano, Nelson and Pavkov5). It is therefore essential to adopt healthy habits and consume foods that can modulate these parameters and protect against cardiovascular and metabolic disorders(Reference Arnett, Blumenthal and Albert1,Reference Noce, Romani and Bernini6,Reference Locke, Schneiderhan and Zick7) .
Propolis is a natural substance that bees collect from various trees and plants to protect their hives from external threats. For centuries, propolis has been used in traditional medicine to treat various ailments such as colds, flu, sore throat and skin infections(Reference Šuran, Cepanec and Mašek8). This sticky substance consists of bioactive compounds such as flavonoids, phenolic acids and terpenes, which have been shown to possess potent medicinal properties such as antioxidant, anti-inflammatory, antimicrobial, anticancer and immunomodulatory effects(Reference Pasupuleti, Sammugam and Ramesh9). Propolis has been shown to modulate carbohydrate and lipid metabolism by stimulating insulin synthesis and β-cell proliferation(Reference Fuliang, Hepburn and Xuan10,Reference Shinohara, Ohta and Hayashi11) , inhibiting glucose production by the liver and glucose absorption by the gut(Reference Matsui, Ebuchi and Fujise12), preventing lipid peroxidation and the rise of TAG and LDL-cholesterol(Reference Fuliang, Hepburn and Xuan10,Reference Shinohara, Ohta and Hayashi11) , increasing HDL-cholesterol and enhancing cholesterol efflux from peripheral tissue(Reference Ichi, Hori and Takashima13,Reference Yu, Si and Song14) . Propolis may also influence blood pressure by inhibiting xanthine oxidase, chelating metal ions, regulating gene expression, suppressing cytokines, attenuating endothelial dysfunction and preventing platelet aggregation(Reference Braakhuis15–Reference Gogebakan, Talas and Ozdemir17).
Recently, a systematic review and meta-analysis by Karimian et al. (Reference Karimian, Hadi and Pourmasoumi18) evaluated the efficacy of propolis on markers of glycaemic control in adults with type 2 diabetes mellitus (T2DM). They found that propolis supplementation significantly reduced fasting blood glucose (FBG), and HbA1c, but had no effect on homeostatic model assessment for insulin resistance (HOMA-IR). However, this review did not include studies that assessed the effects of propolis on blood pressure and lipid profile, which are also important indicators of cardiovascular and metabolic health. Moreover, the quality of the included studies was low to moderate, and the heterogeneity among them was high. Additionally, the present review included more articles than the previous one and also considered more groups of participants rather than only diabetic patients. This may provide a more comprehensive and generalisable picture of the effects of propolis on cardiovascular and metabolic health in different populations and settings.
Several studies have investigated the effects of propolis consumption on blood pressure, lipid profile and glycaemic parameters in humans and animals. However, the results are inconsistent and conflicting. Some studies suggest that propolis can lower blood pressure(Reference Aoi, Hosogi and Niisato19), improve lipid profile(Reference Maddahi, Nattagh-Eshtivani and Jokar20) and reduce blood glucose levels(Reference Hallajzadeh, Milajerdi and Amirani21), while others report no significant effects or even adverse effects(Reference Gheflati, Dehnavi and Yazdi22–Reference Silveira, Teles and Berretta25). These discrepancies may be due to differences in the type, dose, duration and quality of propolis used, as well as the characteristics of the study population, the methods of measurement and the confounding factors.
Therefore, there is a need for a systematic review and meta-analysis to address these inconsistencies and provide a comprehensive and reliable assessment of the effects of propolis consumption on blood pressure, lipid profile and glycaemic parameters. This study may contribute to the growing body of knowledge on propolis as a natural product with diverse health benefits.
Methods
In order to conduct this meta-analysis, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines was employed(Reference Moher, Liberati and Tetzlaff26). The protocol for conducting this systematic review has already been registered in the PROSPERO database with the registration ID: CRD42023472448.
Search strategy
A thorough search of the literature was conducted up until September 2023 in PubMed, Scopus and Web of Science to identify relevant articles without any restrictions on language or timeline. A search strategy using these specific search terms was designed: (propolis) AND (intervention OR intervention* OR ‘controlled trial’ OR ‘randomized’ OR ‘randomised’ OR ‘randomly’ OR placebo OR ‘clinical trial’ OR ‘trial’ OR ‘randomized controlled trial’ OR ‘randomized clinical trial’ OR ‘RCT’ OR ‘blinded’ OR ‘double-blind’ OR ‘clinical trials’ OR ‘trials’ OR ‘Cross-Over’ OR parallel) (online Supplementary Table 1).
Eligibility criteria
All the articles included in the analysis fulfilled the following criteria: (1) They were randomised controlled trials that investigated the impact of propolis consumption on blood pressure, lipid profile (TAG, total cholesterol, LDL-cholesterol, HDL-cholesterol) and glycaemic parameters (FBG, fasting insulin, HbA1c and HOMA) in adults outcome measures. (2) The research was conducted on adults aged 18 years or older who received propolis as an intervention; (3) the interventions lasted at least 4 weeks; (4) the studies had a parallel or crossover design and (5) the studies reported the outcome measures at both the beginning and end of the intervention.
Exclusion criteria
After conducting a thorough analysis of the full-text articles, the articles that met the following criteria were excluded: (1) Studies that focused on animals, reviews, ecological or observational studies. (2) Studies conducted on individuals below the age of 18. (3) Studies that lacked randomisation, placebo groups or control groups.
Data extraction
Information related to this review was extracted from studies entered by two authors (H.B. and K.G.) independently. The following information was extracted: the first author’s name, publication year, study location and design, number of participants in each group, characteristics of the participants including gender, mean age and BMI, health status, the dosage of propolis used for intervention, intervention duration and the mean changes difference and standard deviation of outcomes for both intervention and control groups. Only the most recent data from each study at different time points were considered. Any disagreements were resolved through consultation.
Quality assessment
The quality of the included studies was assessed independently by two separate researchers (M.Sh.J. and K.G.) using the Cochran scoring framework(Reference Higgins and Green27). This framework included seven domains to evaluate the risk of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. Each domain was judged as ‘Low’, ‘High’ or ‘Unclear’ based on predefined criteria. Any discrepancies were resolved by the corresponding authors.
Data synthesis and statistical analysis
This meta-analysis utilised the random-effects model according to DerSimonian and Laird method to calculate the weighted mean differences (WMD) and sd of measures for both intervention and control groups(Reference DerSimonian and Laird28). The mean changes in cases of not reporting directly were calculated using the formula: mean change = final values – baseline values, and sd changes were calculated using the formula(Reference Borenstein, Hedges and Higgins29):
Standard errors, 95 % CI and interquartile ranges were converted to sd using the method of Hozo et al. (Reference Hozo, Djulbegovic and Hozo30).
The included studies varied in terms of types, doses and duration of intervention, as well as the location and health status of participants. Due to this heterogeneity, the random-effects model was used. The heterogeneity among the included trials was evaluated by performing Cochran’s Q test and measured using the I square (I 2)(Reference Higgins, Thompson and Deeks31). P < 0·05 or I2 > 40 % was considered as a significant heterogeneity between pooled studies(Reference Higgins, Thompson and Deeks31). Subgroup analyses were conducted in order to find the source of heterogeneity based on pre-planned criteria including country (Iran and non-Iran), gender (both sexes, only females), study duration (<12 weeks and 12 ≤), age (<50 years and 50<), baseline levels of BMI and propolis supplement dosage (<1000 and 1000 mg/d≤)(Reference Mitchell32). To evaluate the impact of individual studies on the overall estimation, a sensitivity analysis was performed(Reference Tobias33). The publication bias among the studies investigating the effect of propolis intake on each outcome was assessed using Egger’s regression test and the visually inspected funnel plot(Reference Egger, Smith and Schneider34). The linear and non-linear relationship between the changes in outcomes and the features of propolis supplementation (dose and duration) were assessed by performing meta-regression and fractional polynomial modelling, respectively(Reference Morton, Adams and Suttorp35,Reference Xu and Doi36) . Statistical analysis was conducted using STATA, version 11·2 (Stata Corp). In all the analyses performed, P values < 0·05 were considered statistically significant.
Results
Study selection
In Fig. 1, it can be seen that the search protocol initially yielded a total of 3686 studies. Out of these, 836 duplicates were identified and subsequently removed. Following this, an evaluation of the titles and abstracts based on inclusion criteria resulted in the exclusion of 2822 studies that were deemed irrelevant to the subject. A thorough assessment of the full text of twenty-eight studies led to the removal of six studies due to insufficient data reporting. Ultimately, a total of twenty-two studies met the criteria for inclusion in this meta-analysis.

Fig. 1. Flow chart of study selection for inclusion trials in the systematic review.
Study characteristic
Table 1 provides information on the inclusion of twenty-two studies in this meta-analysis, which involved a total of 1164 participants (intervention groups: 596, control groups: 577). All qualified articles included in this analysis were published between 2015 and 2023. The qualified studies were conducted in several countries including Japan(Reference Asama, Hiraoka and Ohkuma37,Reference Fukuda, Fukui and Tanaka38) , China(Reference Zhao, Pu and Wei24,Reference Gao, Pu and Wei39) , Iran(Reference Moayedi, Taghian and Jalali Dehkordi40–Reference Zakerkish, Jenabi and Zaeemzadeh51), Egypt(Reference El-Sharkawy, Anees and Van Dyke52), Chile(Reference Mujica, Orrego and Pérez53), Mexico(Reference Ochoa-Morales, González-Ortiz and Martínez-Abundis54), Brazil(Reference Silveira, Teles and Berretta25), Greece(Reference Tsamesidis, Egwu and Samara55) and France(Reference Sani, Cardinault and Astier56). Three studies were executed on females(Reference Moayedi, Taghian and Jalali Dehkordi40,Reference Maddahi, Nattagh-Eshtivani and Jokar43,Reference Abbasi, Bagherniya and Soleimani45) , and the others were conducted on both sexes(Reference Zhao, Pu and Wei24,Reference Silveira, Teles and Berretta25,Reference Asama, Hiraoka and Ohkuma37–Reference Gao, Pu and Wei39,Reference Tutunchi, Arefhosseini and Ebrahimi-Mameghani41,Reference Anvarifard, Anbari and Ostadrahimi42,Reference Sajjadi, Bagherniya and Soleimani44,Reference Afsharpour, Hashemipour and Khadem-Haghighian46–Reference Sani, Cardinault and Astier56) . The sample sizes varied across the studies, ranging from nine(Reference Sani, Cardinault and Astier56) to ninety-four(Reference Zakerkish, Jenabi and Zaeemzadeh51) participants. Out of the included studies, twenty-one had a parallel randomised controlled trial(Reference Zhao, Pu and Wei24,Reference Silveira, Teles and Berretta25,Reference Asama, Hiraoka and Ohkuma37–Reference Tsamesidis, Egwu and Samara55) design, while one had a crossover design(Reference Sani, Cardinault and Astier56). The intervention periods in the included trials ranged from 4 weeks(Reference Tsamesidis, Egwu and Samara55) to 48(Reference Silveira, Teles and Berretta25) weeks. The propolis type that was intervened was given as a solution in one study(Reference Mujica, Orrego and Pérez53), as a drop in another(Reference Tsamesidis, Egwu and Samara55) and as pills (tablets and capsules) in the other studies. The dosage of propolis supplement in the pill form in the included study ranged from 226·8(Reference Fukuda, Fukui and Tanaka38) to 2000 mg/d(Reference Sani, Cardinault and Astier56). The participants in these trials represented various populations, including healthy individuals(Reference Asama, Hiraoka and Ohkuma37,Reference Mujica, Orrego and Pérez53,Reference Tsamesidis, Egwu and Samara55) , and patients with type 2 diabetes(Reference Fukuda, Fukui and Tanaka38–Reference Moayedi, Taghian and Jalali Dehkordi40,Reference Afsharpour, Hashemipour and Khadem-Haghighian46,Reference Afsharpour, Javadi and Hashemipour47,Reference Samadi, Mozaffari-Khosravi and Rahmanian50–Reference El-Sharkawy, Anees and Van Dyke52,Reference Ochoa-Morales, González-Ortiz and Martínez-Abundis54) , type 2 diabetes and dyslipidaemia(Reference Moayedi, Taghian and Jalali Dehkordi40), insulin resistance(Reference Sani, Cardinault and Astier56), chronic kidney disease and proteinuria(Reference Silveira, Teles and Berretta25,Reference Anvarifard, Anbari and Ostadrahimi42) , metabolic syndrome(Reference Sajjadi, Bagherniya and Soleimani44), polycystic ovary syndrome(Reference Abbasi, Bagherniya and Soleimani45), non-alcoholic fatty liver disease (NAFLD)(Reference Tutunchi, Arefhosseini and Ebrahimi-Mameghani41,Reference Nikbaf-Shandiz, Tutunchi and Khoshbaten48,Reference Soleimani, Rezaie and Rajabzadeh49) and rheumatoid arthritis(Reference Maddahi, Nattagh-Eshtivani and Jokar43).
Table 1. Characteristic of included studies in meta-analysis

IG, intervention group; CG, control group; TB, triple blinded; DB, double blinded; SB, single blinded; PC, placebo controlled; CO, controlled; R, randomised; CKD, chronic kidney disease; NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus; PCOS, polycystic ovary syndrome; NR, not reported.
Quality assessment
In terms of the general risk of bias in the qualified articles, it was found that twenty-one studies had a low risk of bias(Reference Zhao, Pu and Wei24,Reference Silveira, Teles and Berretta25,Reference Asama, Hiraoka and Ohkuma37,Reference Fukuda, Fukui and Tanaka38,Reference Moayedi, Taghian and Jalali Dehkordi40–Reference Sani, Cardinault and Astier56) , and one article mentioned a high risk of bias(Reference Gao, Pu and Wei39). Details of the risk of bias assessment are presented in Table 2.
Table 2. Risk of bias assessment
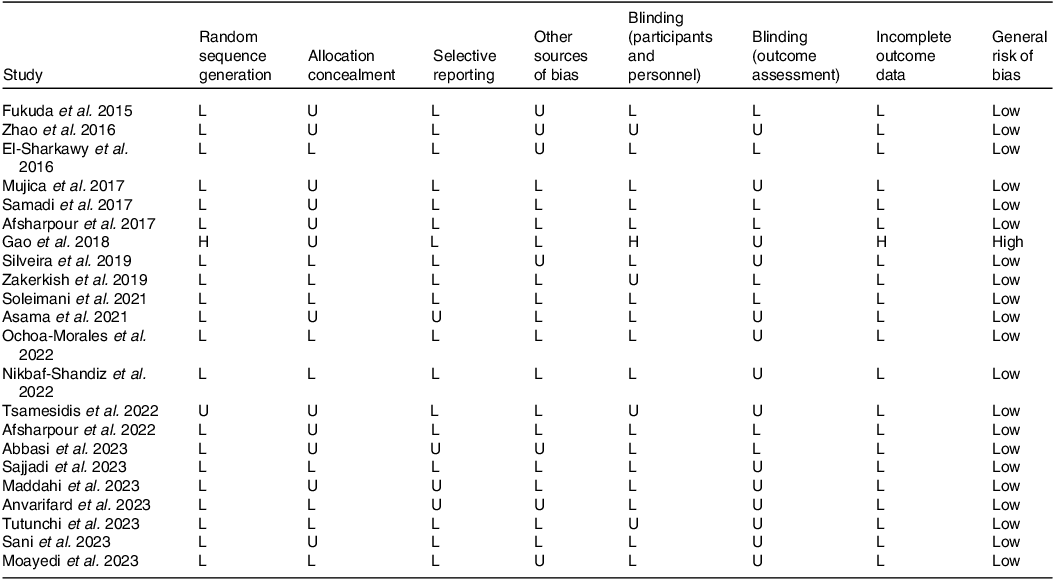
L, low risk of bias; H, high risk of bias; U, unclear risk of bias.
General low < 2 high risk.
General moderate = 2 high risk.
General high > 2 high risk.
Meta-Analysis
Effect of propolis consumption on lipid profile
Effect of propolis consumption on TAG
Assessing thirteen effect sizes indicated that propolis consumption led to a significant decrease in TAG levels compared with control groups (WMD: –10·44 mg/dl 95 % CI: –16·58, –4·31; P = 0·001) (Fig. 2(a)). Additionally, a low degree of heterogeneity was detected between the included trials (I 2 = 31·4 %, P = 0·13). In subgroup analysis, it was found that short-term (<12 weeks) propolis consumption or intervention with propolis among obese (BMI > 30) or non-diabetic participants failed to significantly decrease TAG (Table 3).
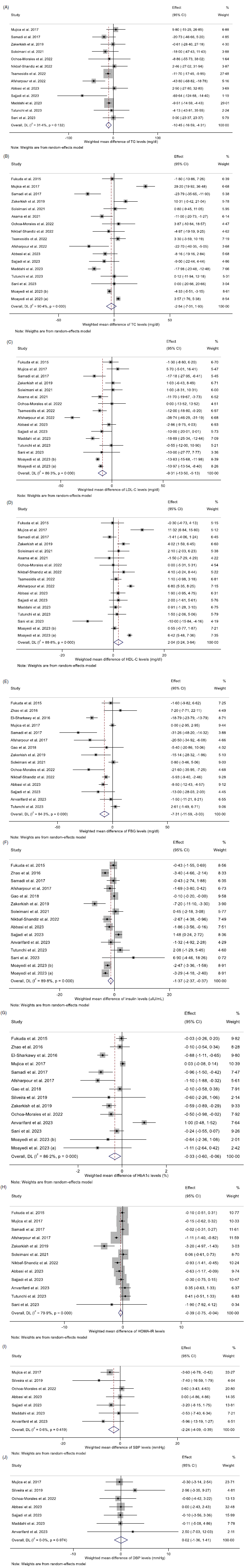
Fig. 2. Forest plot detailing weighted mean difference and 95 % CI for the effect of propolis intake on A) TAG (mg/dl); B) TC (mg/dl); C) LDL-cholesterol (mg/dl); D) HDL-cholesterol (mg/dl); E) FBG (mg/dl); F) fasting insulin (uIU/ml); G) HbA1c (%); H) HOMA-IR; I) SBP (mmHg); and J) DBP (mmHg). FBG, fasting blood glucose; HOMA-IR, homeostatic model assessment for insulin resistance; SBP, systolic blood pressure.
Table 3. Subgroup analyses of propolis consumption and cardiometabolic risk factors in adults
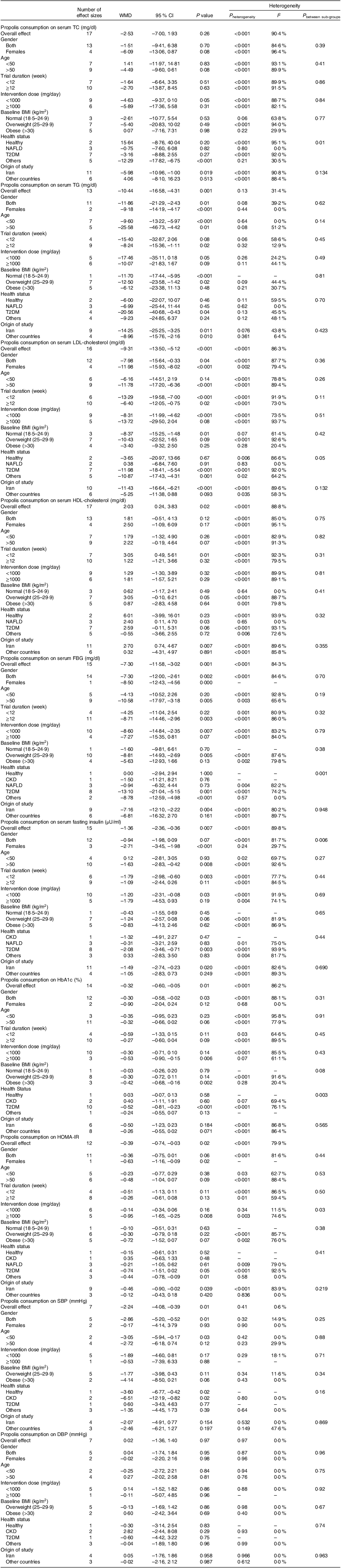
WMD, weighted mean differences; TC, total cholesterol; FBG, fasting blood glucose; HOMA-IR, homeostatic model assessment for insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; CKD, chronic kidney disease; NAFLD, non-alcoholic fatty liver disease; T2DM, type 2 diabetes mellitus.
Effect of propolis consumption on total cholesterol
Pooled data from seventeen effect sizes mentioned no significant impact of propolis intake on total cholesterol) (TC) levels compared with control groups (WMD: –2·53 mg/dl; 95 % CI: –7·00, 1·93; P = 0·26) (Fig. 2(b)). Moreover, a high degree of heterogeneity was observed among studies (I 2 = 90·4 %, P < 0·001). Subgroup analysis revealed that propolis consumption in the studies conducted in Iran had a significant reduction effect on TC levels (Table 3).
Effect of propolis consumption on LDL-cholesterol
The overall results from evaluating 16 effect sizes indicated a significant decrease in LDL-cholesterol levels following propolis consumption compared with control groups (WMD: –9·31 mg/dl; 95 % CI: –13·50, –5·12 mg; P < 0·001) (Fig. 2(c)). Moreover, a high degree of heterogeneity was observed among studies (I 2 = 86·3 %, P < 0·001). Subgroup analysis showed that propolis consumption with a high dosage (≥1000 mg/d) or propolis intake in the non-Iranian populations, people aged less than 50, individuals with NAFLD or healthy participants had no significant effect on LDL-cholesterol levels (Table 3).
Effect of propolis consumption on HDL-cholesterol
After evaluating seventeen effect sizes, it was found that propolis consumption showed a significant enhancing influence on HDL-cholesterol levels compared with control groups (WMD: 2·03 mg/dl; 95 % CI: 0·24, 3·83; P = 0·02) (Fig. 2(d)). In addition, a high between-studies heterogeneity was observed (I2 = 88·8 %, P < 0·001). Moreover, the results of the subgroup analysis demonstrated that short-term propolis consumption or propolis intake in the Iranian population as well as in NAFLD patients significantly increased HDL-cholesterol levels (Table 3).
Effect of propolis consumption on blood pressure
Effect of propolis consumption on systolic blood pressure
Combining seven effect sizes revealed that propolis consumption significantly reduced systolic blood pressure (SBP) compared with control groups (WMD: –2·24 mmHg 95 % CI: –4·08, –0·39; P = 0·01) (Fig. 2(i)). Furthermore, there was no significant heterogeneity among the included studies (I2 = 0·6 %, P = 0·41). Subgroup analysis demonstrated a significant reduction in SBP following propolis consumption in studies involving both sexes, conducted on individuals aged less than 50 years, healthy participants or patients with chronic kidney disease (Table 3).
Effect of propolis consumption on diastolic blood pressure
Pooling seven effect sizes showed that propolis intake had no significant effect on diastolic blood pressure (DBP) compared with control groups (WMD: 0·02 mmHg 95 % CI: –1·36, 1·40; P = 0·97) (Fig. 2(j)). Furthermore, no significant heterogeneity between the included studies was detected (I 2 = 0·0 %, P = 0·97). Subgroup analysis demonstrated that propolis consumption failed to alter DBP in all pre-defined subgroups (Table 3).
Effect of propolis consumption on glycaemic control
Effect of propolis consumption on fasting blood glucose
According to the results from fifteen studies, propolis consumption had a significant diminishing impact on FBG levels compared with control groups (WMD: –7·30 mg/dl; 95 % CI: –11·58, –3·02; P = 0·001) (Fig. 2(e)). Also, high heterogeneity was observed among the included studies (I 2 = 84·3 %, P < 0·001). Following the results of subgroup analysis, short-term (<12 weeks) or high-dose (≥1000 mg/d) propolis intake or intervention among individuals who were under 50 years, in participants with obesity (BMI > 30), normal BMI (18·5–24·9 kg/m2), NAFLD or chronic kidney disease, failed to diminish FBG significantly (Table 3).
Effect of propolis consumption on insulin
Pooling fifteen effect sizes demonstrated that propolis intake diminished fasting insulin levels significantly compared with control groups (WMD: –1·36 mU/ml; 95 % CI: –2·36, –0·36; P = 0·007) (Fig. 2(f)). Also, there was a high between-studies heterogeneity (I 2 = 89·8 %, P < 0·001). As a result, the outcomes of subgroup analysis revealed that propolis intake with a high dosage (≥1000 mg/d) or a long duration (≥12 weeks), as well as in the trials conducted on non-Iranian populations, both sexes, patients with NAFLD, chronic kidney disease or participants aged less than 50 years old, had no significant impact on fasting insulin levels.
Effect of propolis consumption on HbA1c
Analyzing fourteen overall effect sizes showed a significantly diminishing effect of propolis consumption on HbA1c compared with control groups (WMD: –0·32 %; 95 % CI: –0·60, –0·05; P = 0·01) (Fig. 2(g)). Also, there was a high between-studies heterogeneity (I 2 = 86·2 %, P < 0·001). Subgroup analysis demonstrated that propolis intake with a low dosage (<1000 mg/d) and in individuals aged less than 50, females, individuals with a BMI less than 30 kg/m2 or non-diabetic patients did not significantly affect HbA1c levels (Table 3).
Effect of propolis consumption on homeostatic model assessment for insulin resistance
Pooled data from twelve studies mentioned a significant decrease in HOMA-IR by propolis consumption (WMD: –0·39; 95 % CI: –0·74, –0·03; P = 0·02) (Fig. 2(h)). Also, a high between-studies heterogeneity was found (I2 = 79·9 %, P < 0·001). Moreover, the results of the subgroup analysis mentioned that high-dose (≥1000 mg/d) propolis consumption or intervention among female participants, or the Iranian population led to a significant reduction in HOMA-IR (Table 3).
Sensitivity analysis
To ascertain the impact of each study on the overall effect size, each included study was omitted from the analysis, respectively. By removing the studies Mujica et al. 2017 (WMD: 1·55 mg/dl, 95 % CI: –0·24, 3·35)(Reference Mujica, Orrego and Pérez53), Zakerkish et al. 2019 (WMD: 1·88 mg/dl, 95 % CI: –0·03, 3·80)(Reference Zakerkish, Jenabi and Zaeemzadeh51), Afsharpour et al. 2022 (WMD mg/dl: 1·67, 95 % CI: –0·20, 3·54)(Reference Afsharpour, Javadi and Hashemipour47) and Moayedi et al. 2023 (a) (WMD: 1·71 mg/dl, 95 % CI: –0·06, 3·48)(Reference Moayedi, Taghian and Jalali Dehkordi40), the overall result of HDL-cholesterol was altered significantly. Moreover, by excluding Mujica et al. 2017 (WMD: –4·63 mg/dl, 95 % CI: –8·69, –0·58)(Reference Mujica, Orrego and Pérez53), the pooled effect size for TC was significantly changed. Furthermore, by excluding Afsharpour et al. 2017 (WMD: –0·28, 95 % CI: –0·58, 0·02)(Reference Afsharpour, Hashemipour and Khadem-Haghighian46), Zakerkish et al. 2019 (WMD: –0·31, 95 % CI: –0·64, 0·01)(Reference Zakerkish, Jenabi and Zaeemzadeh51), Nikbaf-Shandiz et al. 2022 (WMD: –0·33, 95 % CI: –0·70, 0·04)(Reference Nikbaf-Shandiz, Tutunchi and Khoshbaten48) and Abbasi et al. 2023 (WMD: –0·36, 95 % CI: –0·75, 0·01)(Reference Abbasi, Bagherniya and Soleimani45), the overall result for HOMA-IR was significantly changed. Finally, the overall result for SBP was significantly altered by omitting the trials Mujica et al. 2017 (WMD: –1·55 mmHg, 95 % CI: –3·81, 0·69)(Reference Mujica, Orrego and Pérez53) and Sajjadi et al. 2023 (WMD: –2·07 mmHg, 95 % CI: –4·31, 0·16)(Reference Sajjadi, Bagherniya and Soleimani44). However, other outcomes were not significantly influenced by the quality of one study.
Publication bias
Upon examination of the funnel plots and conducting Egger’s test, it was observed that there is a notable publication bias in studies evaluating the effect of propolis consumption on HDL-cholesterol levels (P = 0·04). While among the studies examining other outcomes, no significant publication bias was detected (Fig. 3).

Fig. 3. Funnel plots for the effect of propolis intake on A) TAG (mg/dl); B) TC (mg/dl); C) LDL-cholesterol (mg/dl); D) HDL-cholesterol (mg/dl); E) FBG (mg/dl); F) fasting insulin (uIU/ml); G) HbA1c (%); H) HOMA-IR; I) SBP (mmHg); and J) DBP (mmHg). DBP, diastolic blood pressure; FBG, fasting blood glucose; HOMA-IR, homeostatic model assessment for insulin resistance; SBP, systolic blood pressure.
Non-linear dose–response analysis
A non-linear dose–response analysis was performed to investigate the non-linear relationship between changes in various outcomes and features of propolis supplementation (duration and dosage). The evaluation of the outcomes from the non-linear dose–response analysis revealed that there was no significant non-linear relationship between alterations in TC, TAG, LDL-cholesterol, HDL-cholesterol, FBG, insulin, HbA1c, HOMA-IR, SBP and DBP with the dosage and duration of propolis supplementation.
Meta-Regression analysis
Meta-regression analysis was performed to ascertain the linear relationship between changes in outcomes and propolis supplementation features (duration and dosage). The findings of the meta-regression test revealed that there was no significant linear relationship between the propolis supplementation features and changes in TC, TAG, LDL-cholesterol, HDL-cholesterol, FBG, insulin, HbA1c, HOMA-IR, SBP and DBP (online Supplementary Figs. 1–3).
GRADE analysis
This meta-analysis utilised the GRADE protocol to evaluate the quality of the evidence. The quality of evidence investigating the impact of propolis intake on LDL-cholesterol, HDL-cholesterol, FBG, insulin, HbA1c and HOMA was considered moderate. On the other hand, the quality of evidence for DBP was identified as high. Furthermore, trials assessing the effect of propolis on SBP and TAG had their evidence quality upgraded to very high levels (Table 4).
Table 4. GRADE profile of propolis consumption for cardiometabolic risk factors in adults

TC, total cholesterol; FBG, fasting blood glucose; HOMA-IR, homeostatic model assessment for insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure.
* There is high heterogeneity (I 2 > 75 %).
† There is no significant effect of propolis consumption.
Discussion
In the present GRADE-assessed systematic review and dose–response meta-analysis, the results showed that propolis consumption resulted in a significant reduction in the TAG, LDL-cholesterol, FBG, HbA1c, insulin, HOMA-IR and SBP in comparison with control or placebo groups. Furthermore, propolis consumption had a significant increasing effect on HDL-cholesterol levels. While propolis intake had no significant effect on TC and DBP levels. Based on the non-linear dose–response analysis, a significant reducing effect of propolis intakes on TAG, LDL-cholesterol, FBG and insulin levels was found in less than 1000 mg/d, and it was not significant at higher dosages; however, for HOMA-IR and HbA1c, doses of 1000 mg/d and higher had significant effects, and these effects were not significant for doses lower than 1000 mg/d.
In Karimian et al. systematic review and meta-analysis, it was shown that propolis intake in T2DM patients caused a significant decrease in FBG and HbA1c levels; however, its effect on HOMA-IR and fasting insulin was not significant(Reference Karimian, Hadi and Pourmasoumi18), the results of this study in terms of no effect on HOMA-IR and insulin are contrary to the results of present study, which is probably due to the low number of articles included in the study and the different doses of propolis used, as well as the fact that the patients are diabetic, also, the heterogeneity in their study was high and the source is unclear, as well as, in the sensitivity analysis, by removing some studies, our results changed, which shows that some studies can affect the overall results due to the low sample size, different study designs and the type of propolis used. In another study, Hallajzadeh et al. showed that glycaemic indices, including FBG, insulin, HbA1c and to some extent HOMA-IR, improved after consuming propolis, and the findings of this study confirmed the results of the present study(Reference Hallajzadeh, Milajerdi and Amirani21). Similar to the findings of the current study, in one interventional study, the consumption of propolis after 8 weeks (1500 mg/d) had significant effects on the improvement of FBG and HbA1c in T2DM patients(Reference Afsharpour, Javadi and Hashemipour57). A clinical trial study conducted by Samadi et al. showed that 12 weeks of propolis supplementation at a dose of 900 mg/d in T2DM patients significantly reduced FBG and HbA1c, while its effect on insulin and HOMA-IR was not significant(Reference Samadi, Mozaffari-Khosravi and Rahmanian50), subgroup analysis in current study showed that propolis in doses less than 1000 mg/d and in less than 12 weeks duration causes significant effects on reducing insulin levels, that is not similar to the results of this study, which is probably due to the type of propolis and geographical region that propolis was gathered and it is also likely that confounders were not adjusted for in this study. The results of a clinical trial study showed that the Chinese propolis supplementation at a dose of 900 mg/d for 18 weeks in diabetic patients had no significant effect on serum glucose, HbA1c and insulin(Reference Gao, Pu and Wei39), which is contrary to the results of the present study, that is probably due to the propolis type and geographical region and also the health conditions of the participants. Also, in a similar study, receiving Brazilian green propolis at a dose of 226·8 mg/d for 8 weeks in diabetic patients did not have a significant effect on FBG, HbA1c and HOMA-IR(Reference Fukuda, Fukui and Tanaka38), which does not confirm the results of the current study that the reason was due to the low dose of propolis and also kind and amount of propolis constituents in this study. It can be said that higher dosages of propolis supplementation, following long duration, might be necessary to observe definite effects of propolis on glycaemic indices in different conditions of health and illness of participants.
Although the mechanism of the effect of propolis on glycaemic indices has not been fully identified, it seems that one of the main probable mechanisms of propolis in improving glycaemic indices is increasing the activity of glucose transporter-4 in skeletal muscles, which increases glucose uptake, and on the other hand, propolis, by reducing the expression and activity of the glucose 6 phosphatase enzyme, causes decreasing effects on glycaemic indices(Reference Kang, Lee and Bae58,Reference Ueda, Hayashibara and Ashida59) . Also, due to its antioxidant properties, propolis can improve metabolic abnormalities and glycaemic indices and because of its bioactive compounds could elevate insulin production or/and increase cellular sensitivity response to insulin(Reference Mujica, Orrego and Pérez53,Reference Pahlavani, Malekahmadi and Firouzi60) .
The findings of the present study revealed that propolis intakes resulted in a significant reduction in the TAG, and LDL-cholesterol and had a significant increasing effect on HDL-cholesterol levels. In contrast, propolis had no significant impact on TC levels. Consistent with the findings of the current study, a meta-analysis study conducted by Salehi-Sahlabadi et al. on five randomised controlled trials showed that propolis consumption significantly reduced TAG and increased HDL-cholesterol levels, but had no significant effect on cholesterol and LDL-cholesterol levels(Reference Salehi-Sahlabadi, Chhabra and Rahmani23), and probably the reason that these effects on LDL-cholesterol were not significant (compared with current study) is the number of studies included in the analysis because in present study, between thirteen and seventeen effect sizes were evaluated for lipid profile. In another meta-analysis study, which included six studies, Gheflati et al. showed that propolis supplementation does not have a significant effect on TAG, TC, LDL-cholesterol, and HDL-cholesterol levels, and the findings of this study are somewhat contrary to the results of the current study(Reference Gheflati, Dehnavi and Yazdi22), which is probably due to the small number of included studies, because in their study, despite decreasing (TAG, TC, LDL-cholesterol) and increasing (HDL-cholesterol) effects, these effects were not significant, also, due to the small number of included studies, another reason for this inconsistency of the results may be the study designs and the doses of propolis used. However, in the sensitivity analysis we performed, three of the studies included in the Gheflati et al. study had a significant effect on overall outcome when excluded from the analysis. In an intervention study, propolis with a dose of 900 mg/d for 12 weeks in patients with T2DM, although it did not have a significant effect on reducing TC and LDL-cholesterol levels before and after the intervention, compared with the placebo group, it caused significant effects and also did not have significant impacts on TAG and HDL-cholesterol levels(Reference Samadi, Mozaffari-Khosravi and Rahmanian50). In one study conducted on women with rheumatoid arthritis, propolis supplementation (1000 mg/d for 12 weeks) significantly reduced TC, TAG and LDL-cholesterol levels compared with placebo after adjusting for confounders, but its effects on HDL-cholesterol levels were not significant(Reference Maddahi, Nattagh-Eshtivani and Jokar43), unlike present study, in this study the effects of propolis on TC levels were significant and the reason for this can be seen in the subgroup analysis of current study, because in the subgroup analysis, the propolis effects in overweight participants and women on TC levels were reduced, which is similar to this study. A probable mechanism for the useful impact of propolis on lipid profile indices may be that ATP-binding cassette transporters, which are related to HDL-cholesterol arrangement and peripheral tissue efflux, are communicated to a more prominent degree within the liver proteins after propolis supplementation(Reference Samadi, Mozaffari-Khosravi and Rahmanian50). Another possible mechanism is that the propolis flavonoids can diminish the biosynthesis of cholesterol by inhibiting the hepatic 3-hydroxy-3-methylglutaryl-CoA reductase and acyl CoA: cholesterol o-acyltransferase, which decreases the acyl CoA: cholesterol o-acyltransferase activity leads to low availability of cholesterol ester for VLDL-cholesterol packing, that can reduce the secretion of VLDL-cholesterol from the liver(Reference Bok, Lee and Park61–Reference Huang, Wu and Yan63). Another probable mechanism of the beneficial impacts of propolis on the lipid profile modification is related to sterol regulatory element binding transcription factor 1 responsive lipogenic genes, stearoyl-coenzyme A desaturase 1 and fatty acid-binding protein 5, which increases lipid oxidation and decrease its accumulation(Reference Hulver, Berggren and Carper64–Reference Listenberger, Han and Lewis66).
The results of the present study showed that propolis intakes lead to significant lowering effects on SBP, but its effects on DBP was not statistically significant. Two clinical trial studies showed that consumption of propolis (with a dose of 1000 mg/d in women with rheumatoid arthritis for 12 weeks and with a dose of 500 mg/d for 12 months in patients with chronic kidney disease) has not a significant effect on SBP and DBP(Reference Silveira, Teles and Berretta25,Reference Maddahi, Nattagh-Eshtivani and Jokar43) , which are contrary to the results of current study in terms of no effect on SBP, which is probably due to the variable doses and health conditions of the participants. However, in confirmation of the findings of the present study, Mujica et al. showed that oral administration of propolis solution (30 drops of 3 % propolis extract after 3 months) caused a significant decrease in SBP and no effect on DBP(Reference Mujica, Orrego and Pérez53). In another study, it was shown that propolis (500 mg, twice daily) improved blood pressure in healthy individuals after 8 weeks of intervention(Reference Khalaf and Thanoon67). It seems that the effects of propolis on blood pressure are probably due to its antioxidant and anti-inflammatory effects, which by reducing inflammation reduces vascular contraction, and by decreasing oxidative stress, improves the condition of atherosclerosis(Reference Khalaf and Thanoon67,Reference Fang, Sang and Yuan68) . Also, another mechanism of propolis in improving blood pressure can be due to its effects in inhibiting nitric oxide synthase, which can reduce catecholamines associated with high blood pressure(Reference Gogebakan, Talas and Ozdemir69); however, more studies with controlled doses and specific compositions of propolis are needed to evaluate its precise effects on blood pressure.
Generally in one comprehensive randomised controlled trial that was conducted on polycystic ovary syndrome women, 500 mg/d propolis supplement for 12 weeks was able to significantly reduce FBG, HOMA-IR and fasting insulin, but despite a significant reduction in the ratio of LDL-cholesterol/HDL-cholesterol had no significant effects on the levels of LDL-cholesterol, HDL-cholesterol, TC, TAG, SBP and DBP(Reference Abbasi, Bagherniya and Soleimani45). Considering the antioxidant, anti-inflammatory and antimicrobial effects of propolis with more than 300 effective compounds, its intake in safe doses seems to be effective in improving health and also in some chronic conditions(Reference Nattagh-Eshtivani, Pahlavani and Ranjbar70,Reference Fokt, Pereira and Ferreira71) .
From a clinical perspective, propolis consumption failed to have a clinically favourable effect on glycaemic parameters, lipid profile and blood pressure. Nevertheless, it is important to highlight that the statistically significant impact of propolis on glycaemic parameters, TAG, LDL-cholesterol, HDL-cholesterol and SBP was minor and may not have clinical significance. The minimal clinically important difference is defined as the minimum effect needed to generate clinically significant outcomes(Reference Cook72,Reference Bahari, Ashtary-Larky and Goudarzi73) . There is limited data on minimal clinically important difference for blood pressure and glycaemic and lipid profile, but multiple studies have indicated that minimal clinically important difference could be considered as 5 mmHg for SBP and DBP, >14 mg/dl for fasting blood glucose, >0·5 % for HbA1c, 10 % for LDL-cholesterol and HDL-cholesterol, and 30 % for TAG(Reference O’Brien, Stephen and Norton74–Reference Bradley, Kozura and Buckle76). Given that the WMD of the impact of propolis on SBP, HbA1c, FBG, TAG, LDL-cholesterol and HDL-cholesterol is less than the minimal clinically important difference, we can contemplate that the favorable effects of propolis on blood pressure, glycaemic parameters, and lipid profile are not clinically meaningful. Additional long-term and high-quality randomised controlled trials are necessary to further assess and validate the credibility of these findings.
To the best of our knowledge, the current GRADE-assessed systematic review and dose–response meta-analysis is one of the first comprehensive studies that evaluate the benefit of propolis effects on cardiometabolic markers in adults and also the general risk of bias was low in more than 95 % of the included studies, which indicates that the results can be more generalisable. Also, a subgroup analysis was performed to assess the effects of dose, gender, age, study durations and type of population on the changes in the cardiometabolic indices. However, the present study has some limitations, which should be considered when the data are interpreted. First, included articles have evaluated various types of propolis in different conditions, and it is unclear how much they differ in biological and bioactive components. Second, high heterogeneity because some studies were conducted on healthy participants and some on chronic conditions. Third, as most of the studies were done in Iran and Asia, possibly, the results could not be generalised to the general population of various areas, and these results should be interpreted with caution. Further studies in various populations or areas are recommended to achieve more definite results.
Conclusion
According to the findings of the present study, propolis intake can improve some cardiometabolic indices such as a significant reduction in the TAG, LDL-cholesterol, FBG, HbA1c, insulin, HOMA-IR and SBP. However, these effects were not clinically significant. Also due to the low significant side effects, it may be used as an auxiliary treatment in some chronic diseases and also as a health-promoting supplement in healthy participants in safe doses. Further, well-designed studies are required to confirm and elucidate all aspects of the findings of this study.
Acknowledgements
We would like to thank the Transplant Research Center, Mashhad University of Medical Sciences, for providing support in this manuscript.
The authors received no financial support for the research and/or publication of this article.
H. B. conceived and designed the research. S. T., K. G. and H. G. performed screening and data extraction. M. S. J. and H. B. analysed data. Z. N., N. P. and M. S. J. drafted the manuscript. N. P. and R. K. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Neither of the authors declared a conflict of interest.
All data generated or analysed during this study are included in this published article.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524002010










