Plasmacytoid dendritic cells (pDC) are a crucial subset of immune cells that act as the first line of defence against viral infection by producing large amounts of interferons (IFN)( Reference Trinchieri and Santoli 1 , Reference Siegal 2 ). IFN induce the expression of numerous interferon-stimulated genes (ISG) responsible for the inhibition of viral replication and spread( Reference Schneider, Chevillotte and Rice 3 ). Interferon-stimulated gene 15 (ISG15), a ubiquitin-like protein involved in protein modification, is one of the most highly induced ISG, and it has an indispensable role in antiviral immunity( Reference Schneider, Chevillotte and Rice 3 ). ISG15 can conjugate to viral proteins and inhibit replication and spread of viruses( Reference Lenschow, Lai and Frias-Staheli 4 – Reference Tang, Zhong and Zhu 7 ). It can also modulate host-derived proteins and enhance other antiviral systems( Reference Schneider, Chevillotte and Rice 3 , Reference Zhao, Collins and Hsiang 8 ). Furthermore, pDC and pDC-derived type I IFN act as inducers of antiviral factors and ‘commanders’ of antiviral immunity by controlling various immune factors such as T cells( Reference Luft, Pang and Thomas 9 – Reference Vanbervliet, Bendriss-Vermare and Massacrier 17 ), natural killer (NK) cells( Reference Gerosa, Gobbi and Zorzi 18 ) and B cells( Reference Jego, Palucka and Blanck 19 , Reference Poeck, Wagner and Battiany 20 ).
Probiotics are live micro-organisms present in intestinal flora or starter bacteria for dairy products that have beneficial effects on human health, and their diverse immunomodulatory effects of lactic acid bacteria (LAB) has been reported elsewhere( Reference Luis, Miriam and Julio 21 ). The protective function of LAB against viral infection has been drawing much attention. Seasonal influenza is one of the major epidemic viral infectious threats around the world. Some LAB were reported to be effective in protecting mice against influenza virus infection: intranasal administration of Lactobacillus pentosus S-PT84 activated protective immune responses( Reference Izumo, Maekawa and Ida 22 ), oral administration of Lactobacillus gasseri TMC0356 enhanced gut and respiratory immune responses( Reference Kawase, He and Kubota 23 ) and exopolysaccharides in yogurt fermented with Lacobacillus delbrueckii ssp. bulgaricus OLL1073R-1 also activated immune responses leading to influenza virus protection( Reference Nagai, Makino and Ikegami 24 ). Studies have reported that intake of LAB might be related to a reduction in pathogenesis of the common cold in humans: intake of yogurt fermented with L. delbrueckii ssp. bulgaricus OLL1073R-1 reduced the risk of pathogenesis of common cold in the elderly( Reference Makino, Ikegami and Kume 25 ) and Bifidobacterium longum BB536 reduced the incidence of influenza and fever in the elderly( Reference Namba, Hatano and Yaeshima 26 ). Gleeson et al.( Reference Gleeson, Bishop and Oliveira 27 ) suggested that Lactobacillus casei Shirota ingestion may reduce the frequency of upper respiratory tract infections in athletes. These reports are mainly based on up-regulation of the innate immune response, such as activity of NK cells and IgA secretion. However, previous reports do not discuss any mechanisms of how LAB affects antiviral immunity or antiviral function.
It is reported that normally LAB are not able to activate pDC directly( Reference Piccioli, Sammicheli and Tavarini 28 ). However, Lactococcus lactis JCM5805 (L. lactis JCM5805) is originally selected as a unique LAB strain, which has a significant impact on murine pDC in vitro and in vivo ( Reference Jounai, Ikado and Sugimura 29 ). This pDC stimulative activity of JCM5805 was confirmed using human peripheral blood mononuclear cells (PBMC)-derived pDC in vitro ( Reference Sugimura, Jounai and Oshio 30 ). We also confirmed that intake of JCM5805 increased the ability to produce IFN-α in response to CpG oligodeoxynucleotide stimulation, but the response to human influenza virus and the effects of antiviral factor are still unknown( Reference Sugimura, Jounai and Oshio 30 ). Furthermore, we previously explored the preventive effects and mechanism of oral administration of JCM5805 using a parainfluenza-infected model and found that an enhanced lung immune response could be elicited by oral intake of JCM5805, and it exerted protective effects against parainfluenza virus infection( Reference Jounai, Sugimura and Oshio 31 ).
In this study, a randomised placebo-controlled double-blind study was conducted to examine the effects of oral intake of JCM5805 on the pathogenesis of influenza-like illness and response against human influenza virus. Our results suggest that oral intake of JCM5805 was suggested to contribute to the inhibition of pathogenesis of influenza-like illness by stimulation of expression of the antiviral factor.
Methods
Volunteers
Volunteers in this study were recruited from Japanese who live in and around Tokyo (Tokyo, Kanagawa prefecture, Chiba prefecture and Saitama prefecture). All volunteers were healthy adults (30–59 years old) without serious illness (immune disease, hepatic disorder, renal disorder, cardiac disease, anaemia, including anamnesis), milk allergy and serious hay fever. Volunteers who had received the influenza vaccination within the previous 18 months, volunteers routinely taking supplements containing LAB or yogurt, pregnant women, lactating women and alcoholics were excluded. Furthermore, volunteers who were judged as unsuitable for the study by the medical doctor for other reasons were also excluded. This study was conducted at Shiba Palace Clinic in Tokyo. Informed consent was obtained before enrolling in this study. We explained sufficiently about the purpose of the test, parameters to be measured, test sample, exclusion criteria, possible risk and so on. Volunteers visited the medical doctor three times during the study: pre-blood test, before and after the intake period.
To determine the sample size, we simulated using data derived from our previous clinical test on thirty-eight volunteers( Reference Sugimura, Jounai and Oshio 30 ). As the results, of simulation using data of individual symptoms, more than eighty volunteers in each group were expected to detect differences between groups at 5 % significance level. Therefore, in this trial, we set the number of volunteers to 100 in each group.
A total of 214 volunteers from 297 candidates were selected on the basis of a pre-blood test (WBC, RBC, Hb, Ht, MCV, MCH, MCHC, PLT, Total-cho, TG, LDL-cho, HDL-cho, BUN, UN, CRE, UA, AST, GOT, ALT, GPT, γ-GT, γ-GTP, LD, LDH, CPK, CK, GLU) and a background questionnaire.
Selected volunteers were divided into two groups by computerised randomisation based on age and sex (Table 1): JCM5805 group (107 adults) and placebo group (107 adults). As one volunteer in the JCM5805 group who could not visit the doctor dropped out, the final number of subjects in the JCM5805 group was 106. In this study, any adverse events were not reported.
Table 1 Characteristics of volunteers in each group (Number of subjects, and mean values with their standard errors)

This study was approved by the clinical research ethics committee of Kirin Holdings Co. Ltd. Informed consents were obtained from all participants after explanation of the study, according to the Declaration of Helsinki.
Test samples
The yogurt beverage fermented by only JCM5805 (100 ml) and the placebo beverage without LAB (100 ml) were prepared by Koiwai Dairy Products. A volume of 100 ml of JCM5805 yogurt contained approximately 1×1011 colony-forming units. The compositions of yogurt beverage and placebo beverage are the same, except for L. lactis JCM5805: milk, powdered skim milk, milk peptide, granulated sugar, pectin, lactic acid, flavouring agent and water. In addition, they were similar in terms of nutritional compositions: 67 kcal, protein 3·2 g, lipid 0·7 g and carbohydrate 12 g.
Study design
This study was conducted as a randomised placebo-controlled double-blind study. Volunteers received JCM5805 yogurt beverage or placebo beverage daily, for 10 weeks (January 2013 to March 2013). During the intake period, volunteers recorded body temperature, daily questionnaires with regard to symptoms based on influenza-like illness and incidence of influenza or common cold after diagnosis by a medical doctor. Blood samples were collected before and after the intake period. Volunteers were prohibited from receiving food containing LAB during the test period. We registered this study to UMIN-CTR (University Hospital Medical Information Network Clinical Trials Registry). The registered ID is UMIN000017274.
Outcomes
Primary outcome is derived from daily questionnaire symptoms based on influenza-like illness. Secondary outcome is derived from immunological parameters using PBMC collected before and after the intake period – that is, pDC activity and response against human influenza virus A/H1N1 (A/PR/8/34).
Daily questionnaire symptoms based on influenza-like illness
Volunteers recorded the severity of symptoms of influenza-like illness, such as cough and feverishness, and common cold symptoms, such as sore throat, runny nose, nasal congestion, sneezing and headache. Volunteers were asked to choose their severity from five grades: (1) normal, (2) slight, (3) mild, (4) moderate and (5) severe. Influenza-like illnesses were defined as ‘an acute respiratory infection with measured fever of ≥38°C, and cough, with onset within the last 10 days’ by the WHO Global Epidemiological Surveillance Standards for influenza (2014). The severity of feverishness was derived from the subjective symptom.
We evaluated the cumulative incidence days of each severity. The cumulative incidence days are effective to evaluate both the duration and frequency of severe symptom onset comprehensively. At first, we compared the cumulative incidence days of each of the five grades between two groups using Wilcoxon rank sum test. This analysis was done to test whether there were significant differences between two groups in terms of both severity and duration together. Next, we compared the cumulative incidence days by separating two severities, ‘normal, slight, mild’ and ‘moderate, severe’, using χ 2 test. This analysis was done to test whether intake of JCM5805 affected the duration of severe symptom onset.
Fluorescence-activated cell sorting analysis
PBMC were stained with fluorescent dye conjugated to Abs: CD123-FITC (AC145), BDCA4-APC (AD-17F6) (Miltenyi Biotec), CD86-PE (IT2.2) (eBioscience) and HLA-DR-PerCP (L243) (BD Pharmingen). After staining, cells were washed twice with fluorescence-activated cell sorting (FACS) buffer (0·5 % bovine serum albumin in PBS buffer) and suspended in 4 % paraformaldehyde for FACS analysis. Data were collected by FACS Cant II (BD Biosciences) and analysed by FCS Express software (De Novo Software). pDC were defined as CD123+BDCA4+, and the activation markers on pDC were measured. To eliminate the influence of analytical error, volunteers whose data were outliers (mean±2 sd) were excluded from the analysis.
Immunological response to human influenza virus A/H1N1 (A/PR/8/34)
Human influenza virus A/H1N1 (A/PR/8/34) was propagated and inactivated by thermal denaturation (56°C for 60 min) at the National Institute of Infectious Diseases.
Immunological response against virus sensitisation was evaluated as follows: PBMC were cultured at a density of 1×106 cells/ml in RPMI medium in 48-well plates, for 24 h at 37°C, with 20 ng/ml inactivated virus particle. Total RNA was extracted using an RNeasy Micro Kit (Qiagen), and complementary DNA (cDNA) was prepared using an iScript cDNA synthesis kit (BioRad), according to the manufacturer’s protocol. Quantitative reverse transcription (qRT)-PCR was performed using SYBR Premix Ex Taq (TaKaRa) and LightCycler 480 (Roche). β-actin was used as the reference gene. The transcriptional level was normalised by placebo both before and after intake period in order to compare the transcriptional level between two groups without influence of storage term on the sample.
Primers for β-actin, IFN-α and ISG15 were originally designed as follows:
β-actin F (5'-gagcgggaaatcgtgcgtgacatt-3')
β-actin R (5'-tgcccaggaaggaaggctggaaga-3')
IFN-α F (5'-ctcccctgatgaatgcggactcca-3')
IFN-α R (5'-tgctctgacaacctcccaggcaca-3')
ISG15 F (5'-gcgggcaacgaattccaggtgt-3')
ISG15 R (5'-tcgcatttgtccaccaccagca-3')
Statistical analysis
The cumulative number of volunteers diagnosed with influenza or common cold infection during the trial was evaluated using a χ 2 test. The cumulative incidence days of each symptom were evaluated using the Wilcoxon rank sum test and a χ 2 test. The number of volunteers who marked on each scores were counted separately. The results from the biogenic markers were evaluated using a standard Student’s t test.
Results
Number of influenza and common cold infections and clinical symptoms of influenza-like illness
First, we evaluated the cumulative number of volunteers diagnosed with influenza or common cold by a medical doctor: fourteen volunteers were diagnosed in the placebo group and seven in the JCM5805 group (Table 2). The number of volunteers diagnosed with influenza by the doctor is one in each group. The cumulative numbers diagnosed with influenza or common cold were lower in the JCM5805 group; however, there was no statistical difference between the two groups (P=0·127, χ 2 test).
Table 2 Cumulative numbers of volunteers diagnosed with influenza or common cold infection during the trial*

* Comparison of the cumulative number of volunteers diagnosed with influenza or common cold by a medical doctor during the trial. Lactococcus lactis JCM5805 group: n 7420 (106 volunteers×70 d); and placebo group: n 7490 (107 volunteers ×70 d). The cumulative numbers were evaluated using the χ 2 test.
We also evaluated the effects of L. lactis JCM5805 on clinical symptoms using a daily questionnaire for symptoms of influenza-like illness and common cold symptoms. As noted in the ‘Daily questionnaire symptoms based on influenza-like illness’ section, volunteers chose the severity of each symptom from five grades, and the cumulative incidence days of each grade were compared between the two groups using the Wilcoxon rank sum test. The cumulative incidence days of cough and feverishness, which are defined as influenza-like illness by the WHO, were significantly lower in the JCM5805 group compared with the placebo group (Table 3, Fig. 1(a) and (b)). Furthermore, the sum of ‘severe’ and ‘moderate’ symptoms of cough, feverishness and sore throat in the JCM5805 group were significantly lower compared with the placebo group (Table 4). There was no significant difference between the two groups on the score for headache. Other symptoms such as runny nose, nasal congestion and sneezing were also analysed; however, 61 % of the volunteers claimed that they developed pollen allergy symptoms during the intake period; therefore, the data were regarded as ineffective. These results suggest that intake of L. lactis JCM5805 would have an ameliorating effect on the subjective symptoms of influenza-like illness.

Fig. 1. Comparison of the cumulative incidence days of symptoms associated with influenza-like illness and common cold. (a) Cough and (b) feverishness. The severity was evaluated on a scale of 1 to 5, as follows: (1) normal, (2) slight, (3) mild, (4) moderate and (5) severe. Lactococcus lactis JCM5805 group: n 7420 (106 volunteers×70 d); and placebo group: n 7490 (107 volunteers×70 d).
Table 3 Cumulative incidence days of each symptom and its grade during the trial*
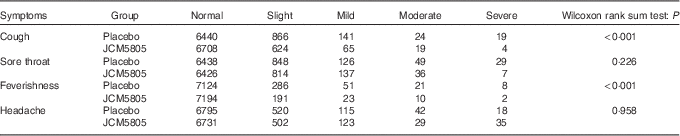
* The grades marked for each symptom were counted separately. The cumulative incidence days of each symptom were evaluated using the Wilcoxon rank sum test. Lactococcus lactis JCM5805 group: n 7420 (106 volunteers×70 d); and placebo group: n 7490 (107 volunteers×70 d).
Table 4 Cumulative incidence days of the grades as scores of severe–moderate and mild–normal in each group
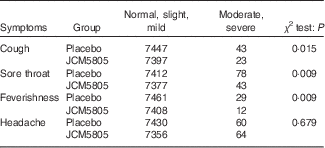
The number that volunteers marked for each symptom was counted separately. The cumulative incidence days of each symptom were evaluated using the χ 2 test. Lactococcus lactis JCM5805 group: n 7420 (106 volunteers×70 d); and placebo group: n 7490 (107 volunteers×70 d).
Effects of Lactococcus lactis JCM5805 on pDC activity in vivo
L. lactis JCM5805 was previously suggested to be a stimulator of pDC in mice and humans( Reference Piccioli, Sammicheli and Tavarini 28 , Reference Jounai, Ikado and Sugimura 29 ). We analysed the pDC status in this study using CD86 as an activation marker. No changes were observed in the placebo group between before and after the intake period. However, CD86 in the JCM5805 group tended to be increased after the intake period (P=0·126) (Fig. 2).

Fig. 2. Changes in CD86 expression level on plasmacytoid dendritic cells (pDC) before (![]() ) and after (
) and after (![]() ) the intake period. To evaluate pDC status, the expression level of CD86 was measured using fluorescence-activated cell sorting. To eliminate the influence of analytical error, volunteers whose data were outliers (mean±2 sd) were excluded from the analysis. As a consequence, the JCM5805 group and the placebo group consisted of ninety-eight and ninety-six volunteers, respectively. Numbers indicate median fluorescence intensity (MFI). Data are means, with their standard errors represented by vertical bars for each group, before and after the intake period. † Mean value was marginally significantly different from that before intake (P=0·13).
) the intake period. To evaluate pDC status, the expression level of CD86 was measured using fluorescence-activated cell sorting. To eliminate the influence of analytical error, volunteers whose data were outliers (mean±2 sd) were excluded from the analysis. As a consequence, the JCM5805 group and the placebo group consisted of ninety-eight and ninety-six volunteers, respectively. Numbers indicate median fluorescence intensity (MFI). Data are means, with their standard errors represented by vertical bars for each group, before and after the intake period. † Mean value was marginally significantly different from that before intake (P=0·13).
Effects of Lactococcus lactis JCM5805 intake on the immunological response against human influenza virus
To evaluate whether the antiviral immunity of volunteers could be affected by L. lactis JCM5805 consumption in accordance with the ameliorated symptoms of influenza-like illness, the transcriptional levels of IFN-α- and IFN-stimulated antiviral factor ISG15 were analysed. PBMC were cultured with heat-inactivated human influenza virus A/H1N1 (A/PR/8/34), and IFN-α and ISG15 transcriptional levels were determined by qRT-PCR.
No change was observed in the transcriptional level of IFN-α before the intake period between the two groups; however, IFN-α transcriptional levels tended to be higher in the JCM5805 group compared with the placebo group after the intake period (P=0·140) (Fig. 3(a)). The transcriptional level of ISG15 tended to be lower in the JCM5805 group compared with the placebo group before the intake period (P=0·054), but ISG15 was significantly higher in the JCM5805 group compared with the placebo group after the intake period (P=0·019) (Fig. 3(b)).

Fig. 3. Comparison of transcriptional levels of IFN-α and interferon-stimulated gene 15 (ISG15) in peripheral blood mononuclear cells cultured with inactivated influenza virus A/H1N1 (A/PR/8/34), between the JCM5805 group (![]() ) and the placebo group (
) and the placebo group (![]() ). (a) Relative IFN-α gene transcriptional level normalised by placebo. (b) Relative ISG15 gene transcriptional level normalised by placebo. Data were normalised using β-actin. Samples were excluded from the analysis if RNA extraction failed. JCM5805 group: n 102; and placebo group: n 105. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the placebo group: * P=0·05, ** P<0·05. † Mean value was marginally significantly different from that of the placebo group (P=0·14).
). (a) Relative IFN-α gene transcriptional level normalised by placebo. (b) Relative ISG15 gene transcriptional level normalised by placebo. Data were normalised using β-actin. Samples were excluded from the analysis if RNA extraction failed. JCM5805 group: n 102; and placebo group: n 105. Values are means, with standard errors represented by vertical bars. Mean value was significantly different from that of the placebo group: * P=0·05, ** P<0·05. † Mean value was marginally significantly different from that of the placebo group (P=0·14).
Discussion
We previously revealed that intake of JCM5805 can activate not only murine pDC but also human pDC both in vitro and in vivo ( Reference Jounai, Ikado and Sugimura 29 , Reference Sugimura, Jounai and Oshio 30 ). A double-blind human clinical trial conducted during the summer season using yogurt made with JCM5805 revealed that cell surface markers on serum pDC with activation status were significantly higher in the JCM5805 group compared with the placebo group( Reference Sugimura, Jounai and Oshio 30 ). The results of this study indicated that intake of JCM5805 would be able to inhibit the development of subjective symptoms associated with influenza-like illness. It was suggested that the clinical improvement would be related to a rise in the transcriptional level of the IFN-α gene and IFN-stimulated antiviral factor ISG15.
We analysed the cumulative incidence days of symptoms (cough, sore throat, feverishness and headache) using five grades to evaluate the severity, as well as incidence. Symptoms relating to influenza-like illness, such as ‘cough’ and ‘feverishness’, were significantly inhibited in the JCM5805 group compared with the placebo group. The cumulative number of volunteers diagnosed by a medical doctor to have influenza, influenza-like illness or common cold tended to be decreased in the JCM5805 group compared with the placebo group; however, there were no significant statistical differences between the two groups. The reason why the difference was not significant might be the low infection rate of middle-aged volunteers. It is generally known that children, the young and the elderly are more susceptible to infection, and inclusion of them in the trial would show the difference more clearly. These results suggested that intake of JCM5805 would have preventive effects on respiratory viral infection, including influenza.
The immunological response test using PBMC cultured with influenza A virus suggested that intake of JCM5805 would increase the transcriptional level of IFN-α in response to influenza virus stimulation. In our previous study, we demonstrated that JCM5805 could activate pDC to increase the transcriptional level of type I IFN (IFN-α, IFN-β), type III IFN (IFN-λ) and some interferon regulatory factor (IRF) genes (IRF3, IRF5, IRF7 and IRF8) in vitro. Furthermore, intake of JCM5805 could increase activation markers on pDC in vivo in humans( Reference Sugimura, Jounai and Oshio 30 ). pDC are known to be proficient cells producing type I IFN( Reference Trinchieri and Santoli 1 , Reference Siegal 2 ), and IRFs are key factors in the production of type I and type III IFN. Therefore, increased IFN-α reaction against human influenza virus ex vivo suggests that the antiviral potential would be elevated in vivo. Indeed, it was also shown in this study that the transcriptional level of ISG15 in response to influenza A virus infection was significantly higher in the JCM5805 group compared with the placebo group after the intake period. Type I IFN derived from pDC are known to induce numerous antiviral factors to restrict viral replication and spread( Reference Schneider, Chevillotte and Rice 3 ). Among them, ISG15 is one of the most important proteins in the inhibition of the influenza A virus. ISG15 protein can be conjugated to the NS1 protein of influenza virus A and inhibit its replication( Reference Lenschow, Lai and Frias-Staheli 4 – Reference Tang, Zhong and Zhu 7 ); in addition, hundreds of host-derived proteins are modulated by ISG15, including interferon-related factors( Reference Zhao, Denison and Huibregtse 32 ). For example, the stability of IRF3, which has an important role in inducing type I IFN, is increased by Herc5 via ISG15 modification( Reference Shi, Yang and Liu 33 ). PKR (protein kinase R), which inhibits translation of viral protein by phosphorylating eIF2α, is activated by ISG15 modification( Reference Okumura, Okumura and Uematsu 34 ). In addition, similar to cytokines, ISG15 secreted from immune cells activates IFN-γ production( Reference Bogunovic, Boisson-Dupuis and Casanova 35 ). Therefore, daily intake of certain LAB strains would be beneficial for the inhibition of viral infection due to ISG15 up-regulation.
In this study, we indicated that oral administration of JCM5805 could significantly decrease the symptoms of influenza-like illness, such as ‘cough’ and ‘feverishness’. Furthermore, we revealed that the transcription levels of IFN-α and ISG15, indicating responsiveness to influenza A virus, were up-regulated by administration of JCM5805. Further studies are required to understand the precise mechanisms and effect of JCM5805 on other immune systems.
Yogurt is widely accepted as healthy food these days, and it is well suited for daily intake. It is very valuable for us to promote immune function to prevent common cold and seasonal influenza by daily diet. However, yogurt form requires strict temperature control; therefore, it will be a significant step if the function will be obtained by various food forms. Indeed, the effects of JCM5805 are supposed to be not only dependent upon living LAB form, but also heat-killed LAB. We have demonstrated that oral administration of heat-killed JCM5805 could activate murine immune response and protect from viral infection( Reference Jounai, Ikado and Sugimura 29 , Reference Jounai, Sugimura and Oshio 31 ). Therefore, it is intriguing whether the heat-killed form also stimulates antiviral immunity to prevent common cold and seasonal influenza in humans.
Acknowledgements
The authors are grateful to Mr Masahiro Suwa and Ms Saeko Takahashi for preparing the yogurt samples used in this study.
Authors have no financial support or funding to report.
N. Y., D. F. and T. S. conceived and designed the experiments; H. T., T. S. and others performed the experiments; H. T., T. S. and others analysed the data; H. T. contributed reagents (influenza virus); and N. Y., D. F. and T. S. wrote the manuscript.
No conflicts of interest are declared by the authors.










