α-Ketoglutarate (AKG) is an intermediate in the citric acid cycle as well as a precursor of glutamate and glutamine(Reference Blachier, Boutry and Bos1), therefore potentially playing an important role in intestinal energy metabolism(Reference Baker2, Reference Burrin and Stoll3). Emerging evidence shows that, similar to glutamine(Reference Rhoads and Wu4), AKG can regulate gene expression and the mammalian target of rapamycin signalling pathway in the pig intestine(Reference Hou, Wang and Ding5). There is also a suggestion that AKG may have a sparing effect on glutamate and aspartate in cells by serving as a fuel source(Reference Junghans, Derno and Pierzynowski6, Reference Lambert, Filip and Stoll7), and these amino acids are important for arginine metabolism in young pigs(Reference Wu, Bazer and Davis8). Consistent with these previous reports, we have recently demonstrated that dietary supplementation with 1 % AKG improved small-intestinal histological morphology and absorptive function in weanling piglets challenged with lipopolysaccharide (LPS)(Reference Hou, Wang and Ding5). However, little is known about the molecular mechanisms responsible for the action of AKG on the small intestine. In the present study, we focused on small-intestinal energy status, because the gut has a high requirement for ATP to support intestinal integrity, function (including nutrient digestion and absorption) and health(Reference Blachier, Boutry and Bos1, Reference Burrin and Stoll3).
AMP-activated protein kinase (AMPK) is a serine/threonine protein kinase consisting of three subunits (α, β and γ) and widely exists in eukaryotic cells(Reference Stapleton, Mitchelhill and Gao9). AMPK is activated by phosphorylation of the α-subunit at threonine 172(Reference Aymerich, Foufelle and Ferré10) and serves as a sensor of cellular energy status(Reference Hardie11). AMPK phosphorylates and inactivates one of its downstream target enzymes, acetyl-CoA carboxylase (ACC)(Reference Dyck, Kudo and Barr12), which converts acetyl-CoA into malonyl-CoA (an inhibitor of fatty acid oxidation and a substrate for fatty acid synthesis). The present investigation tested the hypothesis that AKG could affect the expression of total AMPK and ACC proteins, phosphorylated levels of AMPK and ACC, substrate (fatty acid, glucose and glutamine) oxidation, as well as mucosal concentrations of ATP, ADP and AMP. The study presented here is a continuation of our previously published study(Reference Hou, Wang and Ding5).
Materials and methods
Experimental design
The animal protocol for the present study was approved by the Animal Care and Use Committee of Hubei Province. All piglets used in this experiment were born naturally at term (114 d of gestation). A total of eighteen crossbred (Duroc × Large White × Landrace, 7·25 (sd 0·23) kg) female pigs weaned at 21 (sd 1) d of age from two litters were assigned by weight and litter to one of three treatment groups: (1) non-challenged (control group, piglets fed the basal diet and receiving an intraperitoneal administration of sterile saline); (2) LPS-challenged control (LPS group, piglets fed the basal diet and receiving an intraperitoneal administration of Escherichia coli LPS); (3) LPS+1·0 % AKG (LPS+AKG group, piglets fed the basal diet supplemented with 1·0 % AKG and receiving an intraperitoneal administration of LPS). After removal from sows, piglets were housed individually in 1·20 × 1·10 m2 stainless-steel kennels and maintained in an environmentally controlled room (22–25°C)(Reference Hou, Wang and Ding5, Reference He, Kong and Wu13). During the experimental period, pigs had free access to drinking-water and a maize- and soyabean meal-based diet(Reference Hou, Wang and Ding5) to meet the National Research Council's (1998) requirements of nutrients for growing swine (Table 1).
Table 1 Composition and nutrient contents of the basal diet (air-dry basis)
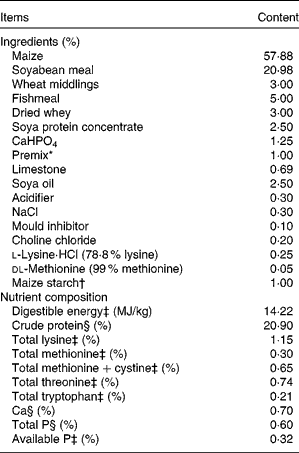
* Premix provided the following amounts of vitamins and trace minerals/kg of the complete diet: Fe, 100 mg (FeSO4.H2O); Cu, 150 mg (CuSO4.5H2O); Mn, 40 mg (MnSO4.5H2O); Zn, 100 mg (ZnSO4.7H2O); I, 0·5 mg (KI); Se, 0·3 mg (Na2SeO3.5H2O); retinol acetate, 10 800 IU (3·66 mg); cholecalciferol, 4000 IU (0·10 mg); dl-α-tocopheryl acetate, 40 IU (36·4 mg); vitamin K3, 4 mg; thiamin, 6 mg; riboflavin, 12 mg; pyridoxine, 6 mg; vitamin B12, 0·05 mg; biotin, 0·2 mg; folic acid, 2 mg; niacin, 50 mg; d-calcium pantothenate, 25 mg.
† In the α-ketoglutarate (AKG) diet, 1·0 % maize starch was replaced by 1·0 % AKG. All diets were isoenergetic.
‡ Calculated value.
§ Analysed value.
Each of the three treatment groups had six pigs. LPS was dissolved in sterile saline. In the AKG diet, 1·0 % maize starch in the basal diet was replaced by 1·0 % AKG (purity ≥ 99·8 %), and AKG (powder) was well mixed with the basal diet. All diets were isoenergetic. The dosage of 1 % AKG was chosen, because it could improve intestinal morphology and function in LPS-challenged pigs(Reference Hou, Wang and Ding5).
The first 3 d (days 0–3 post-weaning) was an adjustment period for weanling pigs to adapt to a solid food, and sample collection was performed in the following 16 d. On days 10, 12, 14 and 16 of the trial, overnight-fasted piglets in the LPS and LPS+AKG groups were administered intraperitoneally with LPS at 80 μg/kg body weight, whereas pigs in the control group were injected intraperitoneally with the same volume of the vehicle (sterile saline). LPS (E. coli serotype 055:B5; Sigma Chemical, Inc., St Louis, MO, USA) was dissolved in a sterile physiological solution (500 mg LPS/l saline). During days 0–10 of the trial (pre-challenge), all pigs were allowed free access to feed and drinking-water. In order to exclude the possible effects of LPS-induced food intake reduction on gastrointestinal characteristics of the pigs, during days 10–16 of the trial (post-challenge), the control and LPS+AKG piglets were fed the same amount of food/kg body weight as LPS piglets. On day 17 (24 h after the last LPS challenge or saline administration), all pigs were euthanised under anaesthesia with an intravenous injection of sodium pentobarbital (50 mg/kg body weight)(Reference Hou, Wang and Ding5).
Intestinal sample collection
The pig abdomen was opened immediately, and the whole gastrointestinal tract was immediately exposed(Reference Hou, Wang and Ding5). The small intestine was dissected free of the mesentery and sampled on a chilled stainless-steel tray. The 10 cm segments were cut at every distal duodenum, mid-jejunum and mid-ileum, respectively. The intestinal segments were opened longitudinally, and the contents were flushed with ice-cold PBS. Mucosal samples were rapidly collected by scraping using a sterile glass microscope slide, rapidly frozen in liquid N2, and then stored at − 80°C until analysis. All samples were collected within 10 min after the pigs were killed.
Determination of substrate oxidation in enterocytes
Jejunal enterocytes were isolated from the control, LPS-treated and LPS+AKG-treated pigs, using Ca-free Krebs bicarbonate buffer(Reference Wu, Knabe and Yan14). The viability of enterocytes, as assessed by trypan blue exclusion, did not differ among the treatment groups of pigs (92–94 %). Cells were incubated at 37°C for 30 min in a complete oxygenated (95 % O2/5 % CO2) medium (pH 7·4) containing 5 mm-d-glucose, 0·5 mm-oleic acid, 2 mm-l-glutamine and other amino acids at physiological levels found in the pig plasma(Reference Wu, Davis and Flynn15). The medium contained d-[U-14C]glucose, [1-14C]oleic acid or l-[U-14C]glutamine. Some media also included 2 mm-AKG+[U-14C]AKG. At the end of a 30 min period of incubation, 14CO2 was collected in 0·2 ml of NCS-II(Reference Li, Bazer and Gao16), and its radioactivity was determined using a Packard liquid scintillation counter(Reference Chen, Li and Wang17). The specific radioactivity of d-[U-14C]glucose, [1-14C]oleic acid, l-[U-14C]glutamine or [U-14C]AKG in the incubation medium was used to calculate the rates of CO2 production from d-[U-14C]glucose, [1-14C]oleic acid, l-[U-14C]glutamine or [U-14C]AKG, respectively(Reference Chen, Li and Wang17).
Determination of mucosal ATP, ADP and AMP
Frozen mucosal samples (50–100 mg) were homogenised with 1 ml pre-cooling 1·5 m-perchloric acid in an ice-bath(Reference Haynes, Li and Li18). The homogenates were centrifuged at 3000 g for 10 min at 4°C. An aliquot of the supernatant fluid (0·5 ml) was neutralised with 0·2 ml of 2 m-potassium carbonate in ice, and the solution was centrifuged at 3000 g for 10 min at 4°C. The supernatant fluid was stored at − 80°C until analysis using HPLC.
The chromatographic system consisted of the Waters Breeze HPLC system (Waters Corporation, Milford, MA, USA), including 1525 binary HPLC pumps, a 2487 Dual-λ Absorbance Detector, a 717 plus autosampler and Breeze system software, and a chromatographic column (Waters XBridge C18; 5 μm, 4·6 mm × 150 mm). The mobile phase (50 mm-K2HPO4–KH2PO4 buffer solution and methanol (chromatographic grade); 77:23, v/v; pH 7·0) was filtered through a 0·45 μm filter membrane and degassed 15 min before use. The detection wavelength was 260 nm, the column temperature was 35°C and the pump flow rate was 1·0 ml/min. The frozen sample was filtered through a 0·20 μm filter membrane after being thawed at room temperature, and the injection volume was 10 μl. Peaks were identified by their retention times using authentic standards (Sigma Chemical, Inc.). Total adenine nucleotide and adenylate energy charges (AEC) were calculated according to the following equation(Reference Atkinson19):
where TAN is the total adenine nucleotide.
Western blot analysis
Approximately 100 mg of the mucosal samples were quickly placed into the pre-cooling 2 ml centrifuge tubes, mixed with 1 ml of a pre-cooling mixture of radioimmunoprecipitation assay lysis buffer (SC-24948; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and phosphate inhibitor cocktail (P2850; Sigma Chemical, Inc.), vortexed for 30 s and homogenised in an ice-bath. The homogenate was centrifuged at 12 000 g for 15 min at 4°C. The supernatant fluid was divided into aliquots for Western blot and protein analyses. The concentration of protein was measured using a bicinchoninic acid protein assay kit (Sigma Chemical, Inc.), and then the supernatant fluid was diluted to the same concentrations. For measuring the levels of total and phosphorylated AMPK-α, total ACC and phosphorylated ACC-β, 2 × SDS sample buffer (2 ml of 0·5 mm-Tris, pH 6·8, 2 ml of glycerol, 2 ml of 10 % SDS, 0·2 ml of β-mercaptoethanol, 0·4 ml of a 4 % solution of bromophenol blue and 1·4 ml of water) was added to the diluted supernatant in a 1:1 ratio, boiled for 5 min and cooled on ice before use. Mucosal protein (150 μg/sample for AMPK and 90 μg/sample for ACC) was subjected to SDS-PAGE using 10 % (for AMPK) or 6 % (for ACC) polyacrylamide resolving gels and transferred to the polyvinylidene difluoride membranes in transfer buffer (25 mm-Tris base, 0·2 m-glycine and 20 % methanol, pH 8·5). Thereafter, the blotted membranes were washed with 25 ml Tris-buffered saline (TBS), which was prepared in 1 litre of 10 × TBS: 24·2 g Tris base and 80 g NaCl (pH adjusted to 7·6 with HCl). The membranes were blocked with 5 % non-fat dry milk in TBS/0·1 % Tween 20 (TBS-T) for 1 h at room temperature with slow agitation followed by three washes in TBS-T. The membranes were incubated overnight at 4°C either with anti-phospho-AMPKα-(Thr172) (no. 2535, rabbit mAb; Cell Signaling, Danvers, MA, USA) and anti-AMPKα (no. 2532; Cell Signaling) or with anti-phospho-ACC-β (Ser79) (no. 3661; Cell Signaling) and anti-ACC (no. 3662; Cell Signaling) with TBS-T containing 5 % bovine serum albumin, at a dilution of 1:1000. The membranes were washed three times with TBS-T and incubated with a secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG, 1:5000; Sigma Chemical, Inc.) at room temperature for 1 h. Blots were developed using an enhanced chemiluminescence substrate (SuperSignal®; Pierce Biotechnology, Rockford, IL, USA), visualised using an imaging system (Bio-Rad Versa Doc, Model-5000; Bio-Rad, Hercules, CA, USA) and quantified using Quantity One software.
Statistical analysis
Values are expressed as means and standard deviations. Experimental data were analysed by one-way ANOVA. Differences among treatment means were determined by Duncan's multiple range test. All statistical analyses were performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P ≤ 0·05 was taken to indicate significance.
Results
Oxidation of substrates
Rates of CO2 production from all carbons of AKG, glucose and glutamine as well as from the carbon 1 of oleic acid in pig enterocytes are shown in Table 2. Administration of LPS to pigs decreased (P < 0·05) the oxidation of all the four substrates. Dietary supplementation with AKG to the LPS-treated pigs partially ameliorated (P < 0·05) the reduction in the oxidation of AKG, glucose, glutamine and oleic acid in enterocytes.
Table 2 Oxidation of d-glucose, l-glutamine and oleic acid in the jejunal enterocytes* of lipopolysaccharide (LPS)-challenged weanling pigs receiving dietary supplementation with or without 1 % α-ketoglutarate (AKG)
(Mean values and standard deviations, n 6)
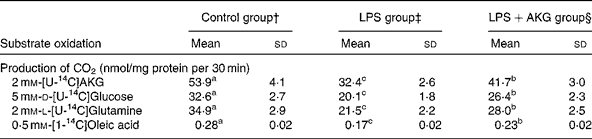
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Enterocytes were isolated from the jejunum of the control, LPS-treated and LPS+AKG-treated pigs.
† Control (non-challenged control), piglets fed a control diet and injected with sterile physiological saline.
‡ LPS (LPS-challenged control), piglets fed the same control diet and challenged with Escherichia coli LPS.
§ LPS+AKG (LPS+1·0 % AKG), piglets fed a 1·0 % AKG diet and challenged with LPS.
Concentrations of ATP, ADP and AMP in the small-intestinal mucosa
Data on the concentrations of ATP, ADP and AMP in the intestinal mucosa are summarised in Table 3. Compared with the control group, the concentrations of ATP, ADP and AMP in the duodenal mucosae of LPS piglets were decreased by 44 % (P < 0·05), 53 % (P < 0·05) and 42 % (P < 0·05), respectively. The LPS treatment also reduced (P < 0·05) the concentrations of ATP in the jejunal mucosae by 36 %, compared with the control piglets. In comparison with the LPS piglets, AKG supplementation increased (P < 0·05) the concentrations of ATP in the mucosae of the duodenum and jejunum by 44 and 25 %, respectively. The concentrations of ADP, AMP and total adenine nucleotide in the duodenal mucosa of LPS piglets were increased (P < 0·05) by 58, 18 and 44 %, respectively.
Table 3 Effects of dietary supplementation with 1 % α-ketoglutarate (AKG) on adenylate purines in the intestinal mucosa of weaned pigs challenged with lipopolysaccharide (LPS)
(Mean values and standard deviations, n 6)

TAN, total adenine nucleotide; AEC, adenylate energy charge.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Control (non-challenged control), piglets fed a control diet and injected with sterile physiological saline.
† LPS (LPS-challenged control), piglets fed the same control diet and challenged with Escherichia coli LPS.
‡ LPS+AKG (LPS+1·0 % AKG), piglets fed a 1·0 % AKG diet and challenged with LPS.
§ TAN = ATP+ADP+AMP.
∥ AEC = (ATP+0·5 ADP)/(ATP+ADP+AMP).
Compared with the control group, LPS piglets exhibited a decrease (P < 0·05) in AEC in the jejunum and ileum, and an increase (P < 0·05) in the ratio of AMP:ATP in the jejunum (P < 0·05) and ileum (Table 3). In comparison with the LPS piglets, dietary supplementation with 1 % AKG increased (P < 0·05) AEC but decreased (P < 0·05) AMP:ATP ratios in the mucosae of the jejunum and ileum (Table 3).
Phosphorylated levels for AMP-activated protein kinase and acetyl-CoA carboxylase
Phosphorylation at Thr72 for the α-isoform of AMPK is illustrated in Fig. 1. LPS challenge resulted in an increase (P < 0·05) in the ratio of phosphorylated AMPK-α:AMPK-α in the jejunal and ileal mucosae, compared with the control piglets. Relative to LPS piglets receiving no LPS, dietary supplementation with 1 % AKG decreased (P < 0·05) the phosphorylated AMPK-α:AMPK in the jejunum and ileum to the value observed for the control group. However, in the duodenum, the levels of phosphorylated AMPK-α did not differ (P>0·05) among the control, LPS and LPS+AKG piglets.

Fig. 1 Phosphorylation state of AMP-activated protein kinase-α (AMPK-α) in the small-intestinal mucosa of piglets. Mucosal protein extracts were separated by 10 % SDS-PAGE for the determination of the phosphorylation of AMPK-α (P) at threonine 172 and total AMPK-α (T). Values for phosphorylated AMPK-α were normalised for total AMPK-α. Values are means, with standard deviations represented by vertical bars (n 6). ![]() , Control (non-challenged control), piglets fed the basal diet and injected with sterile saline;
, Control (non-challenged control), piglets fed the basal diet and injected with sterile saline; ![]() , lipopolysaccharide (LPS) (LPS-challenged control), piglets fed the basal diet and challenged with Escherichia coli LPS;
, lipopolysaccharide (LPS) (LPS-challenged control), piglets fed the basal diet and challenged with Escherichia coli LPS; ![]() , LPS+α-ketoglutarate (AKG) (LPS+1·0 % AKG), piglets fed the basal diet supplemented with 1·0 % AKG and challenged with LPS. a,b Mean values within the same intestinal segment with unlike letters were significantly different (P < 0·05).
, LPS+α-ketoglutarate (AKG) (LPS+1·0 % AKG), piglets fed the basal diet supplemented with 1·0 % AKG and challenged with LPS. a,b Mean values within the same intestinal segment with unlike letters were significantly different (P < 0·05).
Phosphorylation at Ser79 for ACC-β and total ACC-β protein is illustrated in Fig. 2. Compared with the control group, the ratio of phosphorylated ACC-β:ACC-β in the duodenal, jejunal and ileal mucosae of LPS piglets was increased (P < 0·05) by 38, 49 and 40 %, respectively. Compared with the LPS group, dietary supplementation with AKG did not affect (P>0·05) the phosphorylated ACC-β:ACC-β in the small-intestinal mucosa of LPS piglets.

Fig. 2 Phosphorylation state of acetyl-CoA carboxylase (ACC) in the small-intestinal mucosa of piglets. Mucosal protein extracts were separated by 6 % SDS-PAGE for the determination of the phosphorylation of ACC-β (P) at Ser79 and total ACC (T). Values for phosphorylated ACC were normalised for total ACC. Values are means, with standard deviations represented by vertical bars (n 6). ![]() , Control (non-challenged control), piglets fed the basal diet and injected with sterile saline;
, Control (non-challenged control), piglets fed the basal diet and injected with sterile saline; ![]() , lipopolysaccharide (LPS) (LPS-challenged control), piglets fed the basal diet and challenged with Escherichia coli LPS;
, lipopolysaccharide (LPS) (LPS-challenged control), piglets fed the basal diet and challenged with Escherichia coli LPS; ![]() , LPS+α-ketoglutarate (AKG) (LPS+1·0 % AKG), piglets fed the basal diet supplemented with 1·0 % AKG and challenged with LPS. a,b Mean values within the same intestinal segment with unlike letters were significantly different (P < 0·05).
, LPS+α-ketoglutarate (AKG) (LPS+1·0 % AKG), piglets fed the basal diet supplemented with 1·0 % AKG and challenged with LPS. a,b Mean values within the same intestinal segment with unlike letters were significantly different (P < 0·05).
Discussion
There is growing interest in the nutritional regulation of mucosal inflammation in both clinical medicine and animal production, because the gastrointestinal tract is the first defence against diet-derived pathogens(Reference Wu20). AKG displays remarkable metabolic and regulatory versatility in the pig intestine(Reference Hou, Wang and Ding5, Reference Lambert, Filip and Stoll7), but little is known about its significance in gut signalling or function. The results of the present study indicate, for the first time to our knowledge, that AKG beneficially prevented LPS-induced alterations in substrate oxidation (Table 2), cellular energy status (Table 3) and protein levels for phosphorylated AMPK and ACC-β (Fig. 1) in the piglet small intestine.
LPS is a molecule found in the membrane of all gram-negative bacteria and can induce alterations in gastrointestinal oxygen metabolism(Reference Menguy21). In addition, LPS increased heat shock protein 70 expression in the pig small intestine(Reference Hou, Wang and Ding5), which is an indicative of oxidative stress in intestinal cells(Reference Sepponen and Poso22). Moreover, LPS can cause mitochondrial injury in the small intestine(Reference Lobo, Backer and Sun23). Extending these previous reports, we observed that LPS treatment reduced ATP concentrations and AEC, while altering the cellular energy status in piglet intestinal mucosae (Table 3). This is in keeping with our new findings that the intestinal oxidation of glucose, glutamine and fatty acids was reduced in response to LPS administration (Table 2). Thus, the LPS-challenged weanling piglets provide a useful animal model to develop nutritional interventions for improving energy status in the gut.
The energy charge of the adenyl pool is a better measure of the energy state of a tissue than the level of a single nucleotide(Reference Atkinson19, Reference McKnight, Satterfield and Jobgen24). ATP hydrolysis can increase the cellular concentration of ADP, which is converted by adenylate kinase (2 ADP ↔ ATP+AMP) to ATP and AMP(Reference Hardie11). Another novel and important finding of the present study is that AKG supplementation resulted in (1) increased ATP, (2) reduced AMP:ATP and (3) increased AEC in the pig small intestine (Table 3). These three lines of evidence indicate that dietary supplementation with AKG could modulate the adenine nucleotide pool and support the notion that AKG beneficially alleviates the LPS-induced damage of intestinal energy metabolism. The results reveal a hitherto unrecognised role for AKG in improving mitochondrial function in the small intestine. Lambert et al. (Reference Lambert, Stoll and Niinikoski25) reported that 80 % of dietary AKG was oxidised by the small intestine in young pigs, and the remaining AKG was utilised by other splanchnic tissues. Thus, dietary supplementation with AKG did not increase its concentrations in the porcine portal blood(Reference Lambert, Stoll and Niinikoski25). Because AKG is extensively metabolised in the first-pass by the intestinal mucosa and virtually does not enter the portal circulation(Reference Lambert, Stoll and Niinikoski25), we suggest that AKG exerts its nutritional benefits in weanling piglets primarily at the small-intestinal level. The possibility that AKG may also affect other splanchnic tissues cannot be ruled out.
AMPK activity in mammalian cells can be regulated by stimuli that affect cellular ATP levels(Reference Hardie11). For example, hypoxia, which occurs in the small intestine of LPS-treated animals(Reference Menguy21), leads to the activation of AMPK via an increase in the AMP:ATP ratio(Reference Evans, Mustard and Wyatt26). When activated, AMPK switches on catabolic pathways for ATP regeneration, such as the oxidation of glucose, amino acids and fatty acids, while switching off ATP-requiring pathways, such as fatty acid and TAG synthesis(Reference Winder and Hardie27). To our knowledge, this is the first study indicating that AKG may regulate AMPK signalling in the intestinal mucosa. At present, it is not known whether AKG directly or indirectly phosphorylates AMPK. AKG may act as an energy substrate or a signalling molecule, or both. It is also possible that the metabolism of AKG in enterocytes may regulate its intracellular concentration and thus its effects on cellular targets (e.g. AMPK). Either the elucidation of these mechanisms for the actions of AKG on the gut via AMPK activation and energy metabolism or the determination of whether ATP depletion in enterocytes may induce the activation of AMPK would require complex biochemical studies, and such studies are beyond the scope of the present study. However, emerging evidence shows that AMPK is an upstream molecule in the mammalian target of rapamycin signalling pathway(Reference Hong-Brown, Brown and Kazi28). Thus, we speculate that possibly through the mammalian target of rapamycin activation(Reference Hou, Wang and Ding5), AKG stimulates AMPK phosphorylation and oxidation of energy substrates in the intestinal mucosa (Table 2), thereby enhancing ATP supply and supporting cell function. Further studies are warranted to test this hypothesis.
Phosphorylated AMPK-α (active) AMPK could inhibit ACC activity in the skeletal muscle and liver by phosphorylating ACC-β (namely increasing the phosphorylated level of ACC)(Reference Carling29), but a role for ACC in enterocyte metabolism is unknown. Interestingly, there have been reports suggesting that, in colonic epithelial cells (colonocytes), conversion of acetyl-CoA to malonyl-CoA may play an important role in lipogenesis(Reference Andriamihaja, Chaumontet and Tome30, Reference Zambell, Fitch and Fleming31). ACC activation is a rate-controlling step in the conversion of acetyl-CoA to malonyl-CoA(Reference Jobgen, Fried and Fu32). Malonyl-CoA inhibits carnitine palmitoyl-CoA transferase-1, which transports long-chain fatty acyl-CoA from the cytoplasm into the mitochondria for oxidation(Reference Andriamihaja, Chaumontet and Tome30). Thus, a fall in malonyl-CoA concentration in response to the increased levels of phosphorylated AMPK and phosphorylated ACC may lead to increased oxidation of fatty acids. However, we found that LPS treatment reduced the oxidation of oleic acid in enterocytes (Table 2). This result may be explained by mitochondrial injury or dysfunction in the small intestine of endotoxin-challenged pigs, thereby impairing Krebs cycle activity and the mitochondrial electron transport system. Consistent with our finding is the report that LPS treatment reduced the activity of aconitase in rat myocardial tissue(Reference Mason and Stofan33). Aconitase is a target of NO(Reference Wu, Bazer and Davis34), and increased NO production by inducible NO synthase in response to LPS treatment could be a mechanism responsible for the reduced oxidation of substrates in the enterocytes of LPS-challenged pigs. Notably, oxidation of oleic acid in the enterocytes of LPS-treated pigs was increased by AKG supplementation, indicating the improvement of mitochondrial integrity and function.
In summary, dietary supplementation with AKG beneficially improves the energy status of the intestinal mucosa as well as AMPK activation and ACC inactivation in the intestinal mucosa of LPS-challenged pigs. These novel findings have important implications for the development of new interventions to ameliorate gut injury and dysfunction in animals. Further research is warranted to understand how AKG affects the AMPK signalling in the gut.
Acknowledgements
The present study was jointly supported by the National Natural Science Foundation of China (no. 30871801, 30901041, 30901040, 30928018), the Program for Innovative Research Groups of Hubei Provincial Natural Science Foundation (no. 2007ABC009), National Research Initiative Competitive Grants from the Animal Growth and Nutrient Utilization Program (no. 2008-35206-18764) of the USDA National Institute of Food and Agriculture, Texas AgriLife Research (H-8200), and the Thousand-People Talent program at China Agricultural University. Y. H. and G. W. designed the study and wrote the manuscript. K. Y., L. W., B. D., D. F., Y. L., H. Z., J. L. and Y. Li collected and analysed the experimental results. P. K. and Y. Y. participated in the experiments and revised the manuscript. All authors contributed to data interpretation and approved the final version of the manuscript. There is no conflict of interest that should be disclosed.







