In ruminants, successful embryo implantation and survival is dependent on efficient recognition of the embryo by the endometrium and the continuous secretion of progesterone (P4) by the corpus luteum (CL)( Reference Spencer, Burghardt and Johnson 1 , Reference Bazer, Spencer and Ott 2 ). In cows, interferon-τ (IFNT) is the main anti-luteolytic pregnancy recognition signal secreted by the elongating conceptus between days 15 and 28 of gestation. IFNT prevents the regression of the CL through the suppression of endometrial oxytocin-induced luteolytic pulses of PGF2α synthesis( Reference Bazer, Spencer and Ott 2 – Reference Xiao, Murphy and Sirois 4 ). Particularly, it has earlier been reported that the establishment of pregnancy may also depend on increases in uterine PGE2 synthesis( Reference Arosh, Banu and Kimmins 5 – Reference Pratt, Butcher and Inskeep 7 ) and on conceptus-secreted PG( Reference Lewis and Waterman 8 ). Consequently, variations in uterine and conceptus PGE2 and PGF2α synthesis, along with variations in the PGE2:PGF2α ratio, may affect embryo implantation and survival.
Prostaglandins are the derivates of PUFA. Arachidonic acid (AA), an n-6 fatty acid (FA), leads to the production of series 2 PG (including PGF2α and PGE2), whereas EPA, an n-3 FA, leads to the production of series 3 PG( Reference Mattos, Staples and Thatcher 9 ). The same elongase and desaturase enzymes can convert the main sources of dietary n-6 FA (linoleic acid (LA)) and n-3 FA (α-linolenic acid (ALA)) to AA and EPA, respectively( Reference Mattos, Staples and Thatcher 9 , Reference Schmitz and Ecker 10 ). Therefore, competition between n-3 and n-6 FA occurs for the synthesis of PG, which may decrease the production of series 2 PG when there is an increased availability of dietary n-3 FA( Reference Mattos, Staples and Thatcher 9 ). Indeed, it has been demonstrated that n-3 FA can reduce PGF2α secretion from bovine endometrial cells in vitro ( Reference Mattos, Guzeloglu and Badinga 11 ). Also, feeding cows with ALA and LA supplements decreased( Reference Lessard, Gagnon and Petit 12 ), and increased( Reference Robinson, Pushpakumara and Cheng 13 ), respectively, circulating series 2 PG metabolites in vivo. In addition to the above-mentioned effects on elongase and desaturase enzymes, it has earlier been reported that n-3 and n-6 FA can modulate gene expression( Reference Schmitz and Ecker 10 ). For example, diets enriched in EPA and DHA, both n-3 FA found in fish oil, modulate bovine endometrial gene expression of PG endoperoxide synthase (PGFS) and PGE synthase (PGES) in a manner that favours the establishment and maintenance of pregnancy( Reference Coyne, Kenny and Childs 14 – Reference Bilby, Guzeloglu and MacLaren 16 ).
Flaxseed contains about 55 % of total FA in the form of ALA and is known to have positive effects on fertility. For example, cows fed whole flaxseed have fewer pregnancy losses and lower plasma concentrations of series 2 PG than cows fed sunflower seed, which is rich in LA( Reference Ambrose, Kastelic and Corbett 17 , Reference Petit, Germiquet and Lebel 18 ). Moreover, cows fed with a flaxseed supplement have higher conception and lower embryo mortality rates than cows fed Megalac (rich in SFA) or micronised soyabeans (rich in LA)( Reference Petit and Twagiramungu 19 , Reference Petit and Benchaar 20 ).
Apart from its important ALA content, flaxseed is also one of the richest sources of plant lignans. Indeed, it contains high amounts of secoisolariciresinol diglucoside, which is mainly converted within the cows’ rumen into the mammalian lignan metabolite enterolactone (ENL)( Reference Gagnon, Cortes and da Silva 21 ). This lignan presents antioxidant activity and acts as a selective oestrogen receptor agonist( Reference Landete 22 ), and thus could be responsible for some of the reproductive benefits observed when flaxseed is fed to cows. However, the effects of ENL on reproductive performances have been poorly explored so far.
At the time of peri-implantation, the expression of several endometrial genes is modulated to favour embryo implantation and development( Reference Bauersachs, Mitko and Ulbrich 23 – Reference Klein, Bauersachs and Ulbrich 25 ). To better understand the mechanisms behind the positive effects of flaxseed on the fertility of dairy cows, we first performed a transcriptomic study comparing endometrial tissue transcripts of pregnant (day 17) dairy cows fed a supplement of 10 % whole flaxseed with those fed a control diet( Reference Palin, Beaudry and Vallée 26 , Reference Palin, Brochu-Gaudreau and Small 27 ). Among all differentially expressed transcripts, genes involved in the synthesis of PG such as PGE synthase (PTGES) and aldo–keto reductase family 1, member B1 (AKR1B1, a PGF synthase) were identified. Genes known to be regulated by IFNT were also identified (e.g. 2′,5′-oligoadenylate synthetase 1, 40/46 kDa (OAS1)( Reference Short, Geisert and Helmer 28 ) and ISG15 ubiquitin-like modifier (ISG15))( Reference Pru, Austin and Haas 29 ) along with genes known or suspected to have a critical role in embryo survival (e.g. dickkopf homologue 1 (DKK1)( Reference Zhang and Lai 30 ) and Zn finger protein 36, C3H type-like 1 (ZFP36L1))( Reference Stumpo, Byrd and Phillips 31 ). As whole flaxseed was fed to cows in these previous experiments, it was not possible to determine whether differences in gene expression were due to the presence of n-3 FA, flax lignans or both in the diet. Moreover, these studies provided information on gene expression levels in the whole endometrium with no distinction among cell types. It has earlier been demonstrated that epithelial cells (EC) and stromal cells (SC) from the cow endometrium exhibit specific functional properties, such as greater production of PGF2α by EC and of PGE2 by SC( Reference Fortier, Guilbault and Grasso 32 ). Therefore, the present in vitro study was undertaken to identify the effects of n-3 FA (ALA), n-6 FA (LA) and ENL (mammalian lignan) on two bovine endometrial cell types, the SC and EC. More precisely, the objectives of the present study were to determine the effects of different LA:ALA ratios and of ENL on the mRNA abundance of selected genes having important roles in embryo implantation and survival and in the synthesis of PGE2 and PGF2α in bovine endometrial primary cell culture.
Materials and methods
Endometrial primary cell isolation and culture
Uteri were collected from multiparous Holstein cows at stage 1 of the oestrous cycle (days 1–4) at the local slaughterhouse and transported on ice to the laboratory. A total of twelve cows were used to perform the experiments described in the present study. Uteri were selected based on their healthy and normal appearance and the presence of a corpus haemorrhagicum and a degraded CL from the previous cycle on the ovaries( Reference Ireland, Murphee and Coulson 33 , Reference Arosh, Parent and Chapdelaine 34 ). Endometrial EC and SC were isolated using methods described in Fortier et al. ( Reference Fortier, Guilbault and Grasso 32 ) and Xiao & Goff( Reference Xiao and Goff 35 ) with the following modifications. The cervix was removed and both horns were rinsed in sterile PBS (1·37 m-NaCl, 27 mm-KCl, 100 mm-Na2HPO4, 18 mm-KH2PO4, pH 7·4). The myometrium was removed by dissection and the uterine horns were carefully inverted with a long hook to expose the epithelium. Each horn was then cut into four to five sections that were each tied up at both ends. The tissue was then digested for 1 h 30 min at 37°C in Hanks’ buffer saline solution (HBSS) without Ca and Mg (Invitrogen), supplemented with 0·3 % (v/v) trypsin (Invitrogen) and an antibiotic-antimycotic 1 × solution (ABAM) (Invitrogen), to collect luminal epithelial cells (LEC). This was followed by a second digestion for 1 h 15 min at 37°C in a new HBSS solution containing 0·06 % (v/v) trypsin, 0·06 % (w/v) collagenase type 1 (Invitrogen) and ABAM to collect SC and glandular epithelial cells (GEC). After each digestion, the sections of the horns were rubbed lightly with the non-cutting edge of a cell lifter and rinsed twice in HBSS to free the cells. Right after the LEC suspension (first digestion) and the SC and GEC suspension (second digestion) were obtained, 10 % (v/v) fetal bovine serum (FBS) (Invitrogen) was added to inhibit trypsin activity. To purify LEC from the contamination by SC, the LEC suspension was centrifuged at 200 g for at least 7 min to collect LEC clumps (SC remaining in the supernatant). LEC pellets were washed three times with HBSS, pooled and then suspended in 30 ml of culture medium: Roswell Park Memorial Institute (RPMI)-1640 with l-glutamine and 25 mm-HEPES (Invitrogen) supplemented with 10 % (v/v) FBS and 100 μg/ml of Primocin (InvivoGen). To increase the total yield of EC, the SC and GEC suspension was filtered through a 40 μm BD Falcon cell strainer (BD Biosciences). GEC clumps were recovered by backwashing the strainer with HBSS, whereas the SC passed through the strainer. The GEC suspension was then centrifuged at 200 g for 5 min. The pellets were washed one to two times with HBSS, pooled and then suspended in 20 ml culture medium. To eliminate the remaining contaminating SC from the EC, LEC and GEC were plated in 92 mm × 16 mm Petri dishes (10 ml/dish) and incubated at 37°C with 5 % CO2 for 3 h. The culture media containing the floating EC clumps were then collected delicately, thus leaving the contaminating SC adhered to the dish. The filtered SC suspension was centrifuged at 60 g for 5 min to further eliminate contaminating EC, and the supernatant was then centrifuged at 1000 g for 10 min to recover SC. The SC pellets were then resuspended in 4 ml erythrocyte lysis buffer (154 nm-NH4Cl, 10 mm-KHCO3 and 0·1 mm-EDTA)( Reference Ramsay 36 ) for 7 min at room temperature and centrifuged at 1000 g for 5 min. The SC pellets were washed twice in HBSS, pooled and suspended in 20 ml culture medium.
Cell counts were assessed using trypan blue staining (BioWhittaker) for the three purified cell types. The SC were plated in Costar six-well TC-treated plates (Corning) at 5 × 105 viable cells per well. At 3 h after SC plating, the culture medium was changed to get rid of the remaining contaminating EC clumps. LEC and GEC were pooled and plated in Corning CellBIND six-well plates (Corning) at a minimum of 4·5 × 105 viable cells per well. Cell isolation from one uterus provides enough cells to seed about thirty wells (six-well plates) for SC and thirty wells for EC. Both SC and EC (includes LEC+GEC) were cultured at 37°C with 5 % CO2 until 95–100 % confluence (6–8 d) in 2 ml culture medium supplemented with 10 ng/ml of P4 (Sigma-Aldrich) except for the cells with no hormones (CO). For the first 3 d of EC culture, FBS concentration was increased to 20 % (v/v). The culture medium was changed every 2–3 d. The purity of cell cultures was determined using an inverted microscope, with cell morphology between SC and EC being distinctive (i.e. cuboidal or columnal for EC and fibroblast-like shapes for SC). At the end of the cell treatments, the average purity of SC and EC was estimated to be 97 and 95 %, respectively. In the present study, decision was made to use a monolayer cell-culture system in order to avoid cell proliferation inhibition associated with the use of Matrigel coating( Reference Arnold, Kaufman and Seppälä 37 – Reference Mackintosh, Schubert and Healy 39 ).
Cytotoxicity assay
To determine the cytotoxic effects of the different treatments (Table 1) on endometrial cells, preliminary experiments were conducted. Treatment of cells with ENL, recombinant ovine interferon-τ (roIFNT), or P4 had no effect on endometrial cell viability. However, when using a total FA concentration of 100 μm for the different LA:ALA ratios studied, as previously reported for bovine immortalised endometrial (BEND) cells( Reference Caldari-Torres, Rodriguez-Sallaberry and Greene 40 ), cytotoxicity was observed in SC for all ratios that contained ALA, whereas in EC, cytotoxicity was observed in the LA:ALA ratio of 25 (FA25) and ∞ (FA∞). Therefore, to avoid the cell toxicity of FA, preliminary trials were conducted in order to determine the optimal total FA concentration to be used in cell cultures. Concentrations of 0, 1, 10, 20, 30, 50 and 100 μm-ALA were first tested in duplicate on confluent SC (6–7 d). Cytotoxicity was estimated as reported previously( Reference Paupoo, Zhu and Wang 41 ) using crystal violet staining to quantify cell number in monolayer cultures after a 24 h period of exposure to FA. Briefly, the culture medium was aspirated and the cells were fixed with 10 % buffered formalin for 10 min. Formalin was then removed and the cells were stained with a solution of 1 % crystal violet in 70 % ethanol, for 15 min at room temperature. The wells were rinsed with distilled water to remove excess staining, and cell death was evaluated visually under an inverted microscope. Crystal violet stains the nuclei of live cells that remained attached to the surface of the six-well plate. For SC, the number of adherent cells was decreased in the wells that were exposed to ALA concentrations ≥ 30 μm, whereas the wells containing ALA concentrations of 1, 10 and 20 μm had no effect on cell number. For EC, there was no effect on adherent cell number in wells containing FA concentrations ≤ 50 μm, regardless of the LA:ALA ratio, after a 24 h period of exposition to FA. Based on these observations, a concentration of 20 μm total FA was chosen for both cell types (Table 1).
Table 1 Description of the experimental treatments used in primary bovine endometrial cell cultures
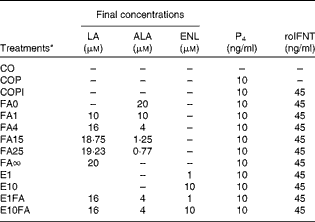
LA, linoleic acid (n-6); ALA, α-linolenic acid (n-3); ENL, enterolactone; P4, progesterone; roIFNT, recombinant ovine interferon-τ; CO, control cells with no hormones; COP, control cells treated with P4; COPI, control cells treated with progesterone and roIFNT; FA, fatty acids; FA0, LA:ALA ratio of 0; FA1, LA:ALA ratio of 1; FA4, LA:ALA ratio of 4; FA15, LA:ALA ratio of 15; FA25, LA:ALA ratio of 25; FA∞, LA:ALA ratio of ∞; E1, ENL 1; E10, ENL 10; E1FA, ENL 1+FA4; E10FA, ENL 10+FA4.
* The FA ratios represent the n-6:n-3 ratio (LA:ALA).
Product preparation for cell treatments
LA and ALA were purchased from Cayman Chemical. In total, six FA stock solutions, each having a different LA:ALA (n-6:n-3) ratio (Table 1), were prepared at a total FA concentration of 20 mm. FA were dissolved in pure ethanol purged with N2 and kept in darkness under N2 atmosphere at 4°C. For ENL (Sigma-Aldrich), two stock solutions of 1 and 10 mm were prepared using pure ethanol purged with N2. These solutions were kept under N2 atmosphere at 4°C. For P4, 20 μg/ml of stock solution was prepared in culture medium and kept in darkness. Finally, 180 μg/ml of roIFNT (RayBiotech) stock solution were prepared on ice according to the manufacturer's instructions. It has earlier been demonstrated that bovine endometrial cells show similar responses to roIFNT and recombinant bovine IFNT( Reference Parent, Villeneuve and Alexenko 42 ). The P4 and roIFNT were used to mimic the day-17 gestational situation of dairy cows( Reference Spencer, Johnson and Bazer 3 , Reference Arosh, Banu and Kimmins 5 ).
Endometrial primary cell treatments
Upon reaching confluence (6–7 d), the culture medium was removed and each well was rinsed twice with 1 ml HBSS. The cells were then incubated for 24 h at 37°C with 5 % CO2 in 2 ml of a freshly prepared RPMI-1640 culture medium with l-glutamine and 25 mm-HEPES and without FBS( Reference Mattos, Guzeloglu and Badinga 11 ) that contained 100 μg/ml of Primocin, a final concentration of 0·2 % ethanol (v/v), 10 ng/ml of P4 ( Reference Spencer, Johnson and Bazer 3 , Reference Weems, Lee and Weems 43 ) (except for the CO treatment) and the appropriate treatment (Table 1). The LA:ALA ratios of 4, 15 and 25 were chosen because they correspond to physiological serum concentrations found in dairy cows fed 10 % flaxseed (rich in ALA) in the diet (DM basis), Megalac/normal control diet and 10 % sunflower seed (rich in LA) in the diet, respectively( Reference Lessard, Gagnon and Petit 12 , Reference Petit, Germiquet and Lebel 18 ). The 1 μm-ENL treatment corresponds to the sum of ENL and ENL-glucuronide concentrations found in the serum of dairy cows receiving flax hulls and oil( Reference Gagnon, Cortes and da Silva 21 ). The effect of ENL was also evaluated in combination with a LA:ALA ratio of 4 (FA4) in order to mimic an in vivo situation of cows fed with 10 % flaxseed supplements( Reference Lessard, Gagnon and Petit 12 , Reference Petit, Germiquet and Lebel 18 , Reference Gagnon, Cortes and da Silva 21 ) and to detect potential synergetic effects between ENL and FA. The culture medium was then removed and each well was rinsed twice with 1 ml HBSS. Cells were incubated for an additional 24 h in 2 ml of a freshly prepared RPMI-1640 culture medium with l-glutamine and 25 mm-HEPES and without FBS, containing 100 μg/ml of Primocin, 10 ng/ml of P4 (except for the CO treatment) and 45 ng/ml of roIFNT( Reference Guzeloglu, Michel and Thatcher 44 ) (except for the CO and CO with P4 (COP) treatments). P4 and roIFNT were added to the cell-culture media in order to mimic the hormonal conditions of pregnancy during the peri-implantation period. At the end of roIFNT incubation, the culture medium was immediately collected from each well to measure the concentrations of PGE2 and PGF2α. Total RNA was extracted from the cells using the NucleoSpin RNA/Protein Kit (Macherey-Nagel), according to the manufacturer's instructions. For both cell types, each treatment was performed in triplicate and repeated four times (i.e. four cows for validation of the endometrial cell-culture system, four cows to study the effects of FA ratios and four cows for ENL experiments). As the CO and COP treatments were prepared only to assess the effects of P4 and roINFT in our cell-culture system, the COPI treatment was used as the reference to compare the treatments and analyse the results.
PG concentrations in culture media
For both cell types, PGF2α and PGE2 concentrations were measured in culture medium from each treatment, after a 24 h incubation period, using PGF2α and PGE2 Enzyme Immunoassays kits (Assay Designs), following the manufacturer's instructions. Culture media obtained from duplicates were pooled for each treatment. Intra- and inter-assay CV were 3·74 and 6·21 % for PGF2α and 4·12 and 5·12 % for PGE2, respectively. The PGE2:PGF2α ratio was also measured for each treatment.
Quantitative RT-PCR analyses of selected genes
For cell cultures of SC and EC, reverse transcription of extracted RNA and real-time quantitative PCR analyses of selected genes were performed as described in Farmer et al. ( Reference Farmer, Palin and Gilani 45 ). The complementary DNA samples obtained from duplicate cell treatments were pooled. Amplification of selected genes and reference genes (RG) (glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peptidylpropyl isomerase A (PPIA) and polyubiquitin) in bovines was performed using bovine-specific primer sequences (Table 2). Genes were selected from a previous study where endometrial tissue transcripts in dairy cows were hybridised to a two-species complementary DNA microarray. This microarray was developed in our laboratory for profiling transcripts in sows and dairy cows, which are believed to be associated with embryo survival during the peri-implantation period( Reference Palin, Beaudry and Vallée 26 , Reference Palin, Brochu-Gaudreau and Small 27 ). The endometrial tissue samples of that previous study were collected on day 17 of pregnancy from dairy cows fed a control diet or a diet supplemented with 10 % flaxseed, thus resulting in in vivo gene modulation by a diet rich in n-3 FA and lignans.
Table 2 Primer sequences used for quantitative RT-PCR

GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F, forward; R, reverse; PPIA, peptidylpropyl isomerase A; AKR1B1, aldo–keto reductase family 1, member B1; ANXA2, annexin A2; CTGF, connective tissue growth factor; CXCL10, chemokine (C–X–C motif) ligand 10; CYP39A1, cytochrome P450, family 39, subfamily A, polypeptide 1; DKK1, dickkopf homologue 1; FOS, FBJ murine osteosarcoma viral oncogene homologue; GJA1, gap junction protein, α 1, 43 kDa; IFIT3, interferon-induced protein with tetratricopeptide repeats 3; IRF6, interferon regulatory factor 6; ISG15, ISG15 ubiquitin-like modifier; OAS1, 2',5'-oligoadenylate synthetase 1, 40/46 kDa; PARM1, prostate androgen-regulated mucin-like protein 1; PTGES, PGE synthase; PTGS2, PG endoperoxide synthase 2; TGFB1, transforming growth factor, β1; ZPFP36L1, Zn finger protein 36, C3H type-like 1.
PCR amplification was performed in triplicate, and standard curves were established in duplicate for each gene. Standard curves were composed of serial dilutions of complementary DNA pools from the same cell type (SC or EC) and were used to obtain the relative mRNA abundance of selected genes using the standard curve method as described by Applied Biosystems( 46 ). Selection of the best RG for normalisation was made according to the geNorm software (http://medgen.ugent.be/genorm/), which finds the optimum RG out of a group of candidate genes( Reference Vandesompele, De Preter and Pattyn 47 ). For both EC and SC cultures, a normalisation factor was calculated with the geometric mean of all three RG. This normalisation factor was then used as a RG. Selected genes and RG amplifications were run in separate assays. For each experimental sample, the mRNA abundance of selected genes relative to that of the RG was determined from the corresponding standard curves. Mean values were calculated from triplicate amplifications and relative quantity ratios were then obtained by dividing the relative quantity unit of selected genes by those of RG. These values were used to perform statistical analyses.
Statistical analyses
Statistical analyses were performed using the MIXED procedure of SAS (SAS Institute, Inc., 2002). The relative mRNA abundance data collected from SC and EC culture experiments were first analysed globally for all treatments (i.e. thirteen experimental treatments; see Table 1) using a one-way ANOVA followed by multiple comparisons of all treatments with the COPI treatment with a Dunnett adjustment. Then, a separate analysis for the effect of the different FA ratios on the relative mRNA abundance of selected genes was performed using a one-way ANOVA followed by an all-pairwise multiple comparison with a Tukey correction. The relative mRNA abundance of selected genes in primary cell cultures treated with ENL were also separately analysed according to a 3 × 2 factorial arrangement with ENL (0, 1 or 10 μm) and FA (no added FA or 20 μm-FA4) concentrations as the main effects. Baseline (COPI) PG concentrations varied considerably between the different cell-culture experiments, but similar responses to the treatments were observed among the four cows. Therefore, PG data were analysed using Friedman's test on rank-transformed data with the same three steps as those described above for relative mRNA abundance data. Finally, to study the effects of P4 and roIFNT on endometrial cells, the relative mRNA abundance data for the CO, COP and COPI treatments were analysed separately using a one-way ANOVA, followed by an all-pairwise multiple comparison with a Tukey correction. Statistical significance was set at P≤ 0·05 and tendencies at 0·05 < P≤ 0·1.
Results
Validation of the endometrial cell-culture system
To validate the cell-culture system, we evaluated cell response to the hormonal treatments that mimic the hormonal conditions of pregnancy during the peri-implantation period. This was achieved by comparing the relative mRNA abundance of selected genes in bovine endometrial cells treated with P4 (COP), P4 and roIFNT (COPI) or with no hormone (CO). Among these treatments, P4 and roIFNT had no effect on the gene expression levels of AKR1B1, connective tissue growth factor (CTGF), cytochrome P450, family 39, subfamily A, polypeptide 1 (CYP39A1), FBJ murine osteosarcoma viral oncogene homologue (FOS), gap junction protein, α1, 43 kDa (GJA1), interferon regulatory factor 6 (IRF6), PTGES and PG endoperoxide synthase 2 (PTGS2) in either SC or EC (results not shown). In EC, the transcript levels of chemokine (C–X–C motif) ligand 10 (CXCL10) and DKK-1 were below the detection limit as measured by the quantitative PCR technique. Significant results are shown in Fig. 1. In SC, significant increases in relative mRNA abundance were observed for CXCL10 (P< 0·05), interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) and OAS1 (P< 0·01), ISG15 and prostate androgen-regulated mucin-like protein 1 (PARM1) (P< 0·001) genes in cells treated with P4 and roIFNT (COPI) when compared with those treated with P4 only (COP) or with no hormone (CO) (Fig. 1(A)). There was a significant increase in the relative mRNA abundance of annexin A2 (ANXA2; P< 0·05) in cells treated with P4 and roIFNT (COPI) compared with those treated with P4 only (COP). Moreover, there was a tendency for an increase in the relative mRNA abundance of DKK1 in cells treated with P4 (COP v. CO: 0·05 < P< 0·1), whereas a further increase was observed in cells exposed to P4 and roIFNT (COPI v. CO: P< 0·05). In EC, there was a significant increase in the mRNA abundance of ISG15 (P< 0·05), IFIT3 and OAS1 (P< 0·01), and PARM1 (tendency, 0·05 < P< 0·1) in cells treated with P4 and roIFNT (COPI) compared with those not treated with hormones (CO) or P4 (COP) (Fig. 1(B)).
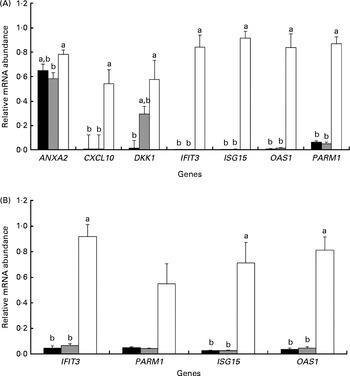
Fig. 1 Relative mRNA abundance of selected genes in primary endometrial cell cultures treated with progesterone and recombinant ovine interferon-τ (roIFNT). Genes with significant differences or tendencies in relative mRNA abundance between the three treatments are presented. Data represents relative mRNA abundance mean values with their standard errors of four cell-culture experiments performed in triplicate. a,bMean values with unlike letters were significantly different (P≤ 0·05). The mean of all the three reference genes polyubiquitin, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and PPIA (peptidylpropyl isomerase A) was used for normalisation. The hormonal treatments were as follows: CO (■), control cells without progesterone and roIFNT; COP (![]() ), control cells treated with progesterone; COPI (□), control cells treated with progesterone and roIFNT. ANXA2, annexin A2; CXCL10, chemokine (C–X–C motif) ligand 10; DKK1, dickkopf homologue 1; IFIT3, interferon-induced protein with tetratricopeptide repeats 3; ISG15, ISG15 ubiquitin-like modifier; OAS1, 2′,5′-oligoadenylate synthetase 1, 40/46 kDa; PARM1, prostate androgen-regulated mucin-like protein 1. (A) SC, stromal cells and (B) EC, epithelial cells (includes luminal and glandular epithelial cells).
), control cells treated with progesterone; COPI (□), control cells treated with progesterone and roIFNT. ANXA2, annexin A2; CXCL10, chemokine (C–X–C motif) ligand 10; DKK1, dickkopf homologue 1; IFIT3, interferon-induced protein with tetratricopeptide repeats 3; ISG15, ISG15 ubiquitin-like modifier; OAS1, 2′,5′-oligoadenylate synthetase 1, 40/46 kDa; PARM1, prostate androgen-regulated mucin-like protein 1. (A) SC, stromal cells and (B) EC, epithelial cells (includes luminal and glandular epithelial cells).
Effects of different fatty acid ratios and enterolactone on the relative mRNA abundance of selected genes in bovine endometrial cells
Selected genes (see Table 2) were previously identified as differentially expressed genes in the endometrial tissues of cows that were fed a 10 % flaxseed supplement compared with those fed a control diet( Reference Palin, Beaudry and Vallée 26 , Reference Palin, Brochu-Gaudreau and Small 27 ). To identify which flaxseed component (flax oil (n-3), lignans (ENL) or both) influenced the endometrial mRNA abundance of the genes studied, different n-6:n-3 FA ratios and ENL concentrations were assayed in two bovine endometrial cell types (SC and EC). The global analysis of the relative mRNA abundance data of all treatments containing P4 and roIFNT (see Table 1) revealed a trend (0·05 < P< 0·1) for an overall treatment effect for the IFIT3 gene in SC only (results not shown). Furthermore, multiple comparisons with the COPI treatment revealed that the treatment of SC with 10 μm-ENL (E10) decreased the mRNA abundance of IFIT3 (P< 0·05; results not shown).
To further investigate how the different n-6:n-3 FA ratios modulate the mRNA abundance of selected genes in both endometrial cell types, an all-pairwise multiple comparison analysis was performed separately (Fig. 2). In SC (Fig. 2(A)), analysis of all FA ratios revealed a significant overall treatment effect for the transcript abundance of CXCL10 and IFIT3 (P< 0·05) and a tendency for the transcript abundance of FOS and CYP39A1 (0·05 < P< 0·1). Moreover, the all-pairwise multiple comparison analysis revealed that the relative mRNA abundance of FOS was the highest in cells treated with LA and ALA at a ratio of 15 (FA15), and significant differences were observed when compared with the FA0, FA1 and FA25 treatments (P< 0·05; Fig. 2(A)). The CXCL10 and CYP39A1 transcripts responded similarly to the addition of FA at different ratios. Indeed, the highest and lowest mRNA levels of CXCL10 and CYP39A1 were observed, respectively, with the FA4 and FA25 treatments (P< 0·05). The mRNA abundance of IFIT3 decreased with increasing LA:ALA ratios, and the lowest mRNA abundance was observed with the FA∞ treatment, which was significantly different from the FA0, FA1, FA4 and FA15 treatments (P< 0·05).

Fig. 2 Relative mRNA abundance of selected genes in bovine primary endometrial cell cultures treated with fatty acids (FA) at different ratios (linoleic acid (LA):α-linolenic acid (ALA)). Upon reaching confluence, cells were incubated for 24 h with appropriate treatments, followed by an additional 24 h of incubation in a fresh culture medium without FA (see Table 1). Genes with significant overall differences or tendencies for relative mRNA abundance between the six treatments are presented. In stromal cells (SC), a global analysis of all FA ratios revealed a significant treatment effect on the transcript abundance of CXCL10 (chemokine (C–X–C motif) ligand 10) and IFIT3 (interferon-induced protein with tetratricopeptide repeats 3) (P< 0·05) and a tendency for the transcript abundance of FOS (FBJ murine osteosarcoma viral oncogene homologue) and CYP39A1 (cytochrome P450, family 39, subfamily A, polypeptide 1) (0·05 < P< 0·1). In epithelial cells (EC), there was a significant overall effect of the different LA:ALA ratios on the mRNA abundance of PTGES (PGE synthase) and PTGS2 (PG endoperoxide synthase 2) (P< 0·05) and tendencies were observed for the mRNA abundance of AKR1B1 (aldo–keto reductase family 1, member B1), FOS, CYP39A1 and IRF6 (interferon regulatory factor 6) (0·05 < P< 0·1). Values are means of four cell-culture experiments performed in triplicate, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P≤ 0·05). The mean of all the three reference genes polyubiquitin, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and PPIA (peptidylpropyl isomerase A) was used for normalisation. The cell treatments were as follows: FA0 (LA:ALA ratio of 0; ![]() ), 20 μm-ALA; FA1 (
), 20 μm-ALA; FA1 (![]() ), 10 μm-LA+10 μm-ALA; FA4 (
), 10 μm-LA+10 μm-ALA; FA4 (![]() ), 16 μm-LA+4 μm-ALA; FA15 (
), 16 μm-LA+4 μm-ALA; FA15 (![]() ), 18·75 μm-LA+1·25 μm-ALA; FA25 (
), 18·75 μm-LA+1·25 μm-ALA; FA25 (![]() ), 19·23 μm-LA+0·77 μm-ALA; FA∞ (
), 19·23 μm-LA+0·77 μm-ALA; FA∞ (![]() ), 20 μm-LA. (A) SC and (B) EC (includes luminal and glandular epithelial cells).
), 20 μm-LA. (A) SC and (B) EC (includes luminal and glandular epithelial cells).
In EC (Fig. 2(B)), there was an overall effect of the different LA:ALA ratios on the mRNA abundance of PTGES and PTGS2 (P< 0·05), and tendencies were observed for AKR1B1, FOS, CYP39A1 and IRF6 genes (0·05 < P< 0·1). The two PG synthase genes AKR1B1 and PTGES responded similarly to the addition of FA at different ratios. Indeed, these two genes had the lowest mRNA abundance in cells treated with FA25, which was significantly different from those treated with FA0, FA1, FA4 and FA∞ (P< 0·05). For PTGES, mRNA abundance was lower in cells treated with FA15 than in those treated with FA1 and FA∞ (P< 0·05). The mRNA abundance of CYP39A1 was lower in cells exposed to FA25 than in those exposed to FA4, FA15 and FA∞ (P< 0·05). Also, the mRNA abundance of CYP39A1 was lower in cells treated with FA1 than in those treated with FA4 and FA∞ (P< 0·05). The mRNA abundance of FOS and IRF6 was found to be lowest in cells exposed to FA1, which was significantly different from those exposed to FA4, FA15 and FA∞ (P< 0·05). The mRNA abundance of PTGS2 was found to be highest in EC treated with FA15, which was significantly different from those exposed to FA0 (P< 0·05) and FA1, FA4, FA25 and FA∞ (P< 0·01).
To determine whether ENL, alone or in combination with FA4, can modulate the relative mRNA abundance of selected genes in primary endometrial cell cultures, an additional analysis was performed according to a 3 × 2 factorial arrangement of treatments. Significant results are presented in Table 3. In SC, the presence of ENL significantly decreased the mRNA abundance of AKR1B1, DKK1, IFIT3, IRF6, PTGES and ZFP36L1 and tended to reduce the mRNA abundance of OAS1 and transforming growth factor, β1 (TGFβ1). Regardless of the presence or absence of FA in cell-culture media, SC that were cultured with E10 had lower mRNA abundance than those exposed to 0 μm-ENL for the following genes: AKR1B1 (0·474 v. 0·544, P< 0·05); DKK1 (0·430 v. 0·584, P< 0·05); ZFP36L1 (0·502 v. 0·668, P< 0·05); IFIT3 (0·591 v. 0·777, P< 0·01); IRF6 (0·446 v. 0·587, P< 0·05); PTGES (0·357 v. 0·542, P< 0·001). Moreover, a significant difference was observed between 10 and 1 μm-ENL for the mRNA abundance of AKR1B1 (0·474 v. 0·542, P< 0·05). A significant interaction between FA and ENL (P< 0·05) was obtained for AKR1B1, with the lowest mRNA abundance being observed when E10 was combined with 20 μm-FA4. There was a significant effect of FA on the mRNA abundance of PTGS2 (P< 0·038), with higher levels being observed in cells treated with FA4 than in those with no added fat (0·595 v. 0·499, 0·05 < P< 0·1). Finally, there was a trend observed for the effect of FA on the mRNA abundance of TGFβ1 (0·05 < P< 0·1), with higher mRNA levels being observed in SC that had no added fat compared with those exposed to 20 μm-FA4.
Table 3 Effects of enterolactone (ENL), alone or in combination with a linoleic acid (LA):α-linolenic acid (ALA) ratio of 4:1, on the expression of the selected genes in endometrial stromal (SC) and epithelial (EC) cells‡ (Mean values with their standard errors)

FA, fatty acids; COPI, control cells treated with progesterone and recombinant ovine interferon-τ; E1, 1 μm-ENL; E10, 10 μm-ENL; FA4, 16 μm-LA:4 μm-ALA ratio of 4; E1FA, 1 μm-ENL+FA4; E10FA, 10 μm-ENL+FA4; AKR1B1, aldo–keto reductase family 1, member B1; DKK1, dickkopf homologue 1; IFIT3, interferon-induced protein with tetratricopeptide repeats 3; IRF6, interferon regulatory factor 6; OAS1, 2’,5’-oligoadenylate synthetase 1, 40/46 kDa; PTGES, PGE synthase; PTGS2, PG endoperoxide synthase 2; TGFB1, transforming growth factor, β1; ZFP36L1; Zn finger protein 36, C3H type-like 1; ANXA2, annexin A2; CTGF, connective tissue growth factor; GJA1, gap junction protein, α 1, 43 kDa; ISG15, ISG15 ubiquitin-like modifier; PARM1, prostate androgen-regulated mucin-like protein 1.
* P< 0·05.
† 0·05 < P< 0·1.
‡ Results were obtained from four cell-culture experiments performed in triplicate and compared with a 3 × 2 factorial arrangement. All culture media contained 10 nm-progesterone and 50 nm-recombinant ovine interferon-τ.
In EC (Table 3), the presence of ENL in culture media significantly increased the mRNA abundance of ANXA2 (P< 0·05) and tended to increase the mRNA abundance of ZFP36L1. In contrast, the transcript abundance of IFIT3 significantly decreased (P< 0·01) and the mRNA abundance of GJA1 tended to decrease when ENL was included in cell-culture media. When comparing the different ENL concentrations, the E10 treatment decreased the mRNA abundance of IFIT3 when compared with the 0 μm-ENL treatment (0·679 v. 0·853, P< 0·01). There was a tendency for an ENL × FA interaction for the mRNA abundance of PTGS2 in EC, with the highest level being observed for the E1 treatment, without added FA. Moreover, the mRNA levels of PTGS2 for the E1 treatment (independently of FA) were higher than those found for the E0 (0·546 v. 0·429, P= 0·01) and E10 (0·546 v. 0·420, P <0·01) treatments. The addition of 20 μm-FA4 in culture media significantly decreased the mRNA abundance of PARM1 (P< 0·05), IFIT3 (P< 0·01), OAS1 (P< 0·01) and TGFβ1 (P< 0·001), and tended to reduce the mRNA abundance of CTGF, GJA1 and ISG15 (0·05 < P< 0·1).
Effects of different fatty acid ratios and enterolactone on PG concentrations in bovine endometrial cells
In addition to the relative mRNA abundance of selected genes, PGE2 and PGF2α concentrations were assessed in the culture media of SC and EC, with series 2 PG playing a key role in the embryo implantation process. The global analysis of PGE2 and PGF2α concentrations for all the treatments (thirteen experimental treatments; see Table 1) revealed an overall treatment effect in SC and EC (P< 0·001) and an overall treatment effect for the PGE2:PGF2α ratio in EC only (P< 0·01) (results not shown). Multiple comparisons (Fig. 3) then revealed that the treatment of SC with FA4 (P< 0·001), FA15 (P< 0·05) and FA∞ (P< 0·01) significantly increased PGE2 concentrations when compared with those that were not treated with LA and/or ALA (COPI). Significant increases in PGF2α concentrations were observed when SC were treated with FA4 (v. COPI, P< 0·01) and FA∞ (v. COPI, P< 0·01), whereas the FA0 treatment decreased PGF2α concentrations (FA0 v. COPI, P< 0·01). In EC, PGE2 concentrations increased with the FA4, FA15, FA25 and FA∞ treatments when compared with the COPI treatment (P< 0·001). The FA15 (v. COPI, P< 0·05), FA25 (v. COPI, P< 0·05) and FA∞ (v. COPI, P< 0·001) treatments all increased PGF2α concentrations. On the contrary, EC treated with FA0 (v. COPI, P< 0·001) and FA1 (v. COPI, P< 0·05) significantly decreased PGF2α concentrations. Finally, the PGE2:PGF2α ratio was increased with the FA0 treatment (v. COPI, P< 0·01) in EC only.

Fig. 3 Effects of the different fatty acid ratios (linoleic acid (LA):α-linolenic acid (ALA)) on PGE2 and PGF2α concentrations measured in the culture media of stromal (SC; left panels) and epithelial (EC; right panels) endometrial cells. Upon reaching confluence, cells were incubated for 24 h with appropriate treatments, followed by an additional 24 h of incubation in fresh culture medium without fatty acids (see Table 1). Values are means of four cell-culture experiments performed in duplicate, with their standard errors represented by vertical bars. PG data were analysed using Friedman's test on rank-transformed data followed by multiple comparisons of each treatment with the control cells treated with progesterone and recombinant ovine interferon-τ containing no added fatty acids (COPI). Mean value was significantly different from that of the COPI treatment: * P≤ 0·05, ** P≤ 0·01, *** P≤ 0·001. When performing a global analysis of PG concentration data, there was an overall treatment effect for PGE2 and PGF2α concentrations in SC and EC (includes luminal and glandular epithelial cells) (P< 0·001) and for the PGE2:PGF2α ratio in EC (P< 0·01). Fatty acid ratios: 0, 20 μm-ALA; 1, 10 μm-LA+10 μm-ALA; 4, 16 μm-LA+4 μm-ALA; 15, 18·75 μm-LA+1·25 μm-ALA; 25, 19·23 μm-LA+0·77 μm-ALA; ∞, 20 μm-LA.
To further investigate how the different LA:ALA ratios affect PGE2 and PGF2α secretion in both endometrial cell types, an all-pairwise multiple comparison analysis was performed separately (Fig. 4). In the culture media of SC, PGE2 and PGF2α concentrations were affected in the same way by the different LA:ALA ratios, with lower PG concentrations being observed in cells treated with LA and ALA at the ratios of 0 and 1 when compared with those treated with FA at all the other ratios (P< 0·05). In SC, no significant difference in the PGE2:PGF2α ratio was found among the different FA ratios. In EC, the lowest PGE2 and PGF2α concentrations were found in cells treated with LA and ALA at the ratios of 0 and 1, which were significantly different from those treated with FA at all the other ratios (P< 0·05). PGF2α concentrations measured in the culture media of EC increased gradually with increasing LA:ALA ratios. The PGE2:PGF2α ratios were not affected by the treatment with LA and ALA at the different ratios.

Fig. 4 Effects of the different fatty acid ratios (linoleic acid (LA):α-linolenic acid (ALA)) on PGE2 and PGF2α secretion in stromal (SC; upper panels) and epithelial (EC; lower panels) (includes luminal and glandular epithelial cells) endometrial cells. Upon reaching confluence, cells were incubated for 24 h with appropriate treatments, followed by an additional 24 h of incubation in fresh culture medium without fatty acids (see Table 1). Values are means of four cell-culture experiments performed in duplicate, with their standard errors represented by vertical bars. PG data were analysed using Friedman's test on rank-transformed data followed by all-pairwise multiple comparisons. Mean values with unlike letters were significantly different (P≤ 0·05). The cell treatments were as follows: FA0 (LA:ALA ratio of 0), 20 μm-ALA; FA1, 10 μm-LA+10 μm-ALA; FA4, 16 μm-LA+4 μm-ALA; FA15, 18·75 μm-LA+1·25 μm-ALA; FA25, 19·23 μm-LA+0·77 μm-ALA; FA∞, 20 μm-LA.
To determine whether ENL, alone or in combination with FA4, can modulate PGE2 and PGF2α secretion in primary endometrial cell cultures, an additional analysis was performed according to a 3 × 2 factorial arrangement of treatments (Table 4). In SC, there was an overall effect of ENL and FA on PGE2 (P< 0·05) and PGF2α (ENL, P< 0·01; FA, P< 0·05) concentrations, whereas no effect on the PGE2:PGF2α ratio was observed. When performing an all-pairwise analysis on PGE2 concentrations, there was a tendency for lower PGE2 concentrations in cells treated with E10 compared with those treated with 0 μm-ENL (598·16 v. 841·57 pg/ml, 0·05 < P< 0·1) or 1 μm-ENL (598·16 v. 708·20 pg/ml, 0·05 < P< 0·1). Similarly, lower PGF2α concentrations were found in SC treated with E10 compared with those treated with 0 μm-ENL (23·58 v. 39·31 pg/ml, P< 0·01) or with 1 μm-ENL (tendency, 23·58 v. 30·23, 0·05 < P< 0·1). For both PGE2 and PGF2α concentrations, the interaction between ENL and FA tended to be significant (0·05 < P< 0·1).
Table 4 Effects of enterolactone (ENL), alone or in combination with a linoleic acid (LA):α-linolenic acid (ALA) ratio of 4:1, on PGE2 and PGF2α synthesis in endometrial stromal (SC) and epithelial (EC) cells‡ (Mean values and lower and upper values)
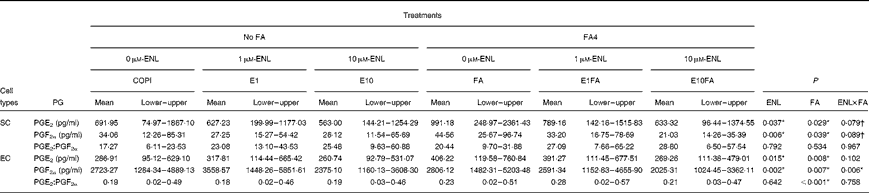
FA, fatty acids; COPI, control cells treated with progesterone and recombinant ovine interferon-τ; E1, 1 μm-ENL; E10, 10 μm-ENL; FA4, 16 μm-LA:4 μm-ALA ratio of 4; E1FA, 1 μm-ENL+FA4; E10FA, 10 μm-ENL+FA4.
* P< 0·05.
† 0·05 < P< 0·1.
‡ Results were obtained from four cell-culture experiments performed in duplicate. The baseline (COPI) of PG concentrations varied considerably between the different cell-culture experiments, but similar responses to the treatments were observed among the four cows. Therefore, the results were analysed using non-parametric Friedman's test and compared with a 3 × 2 factorial arrangement. All culture media contained 10 nm-progesterone and 50 nm-recombinant ovine interferon-τ.
In EC, there was an overall effect of ENL and FA on PGE2 (ENL, P< 0·05; FA, P< 0·01) and PGF2α (ENL and FA, P< 0·01) concentrations and an effect of FA on the PGE2:PGF2α ratio (P <0·001). There was a significant effect of the ENL × FA interaction (P< 0·01) on PGF2α concentrations, with the lowest concentration being observed when E10 was combined with FA (FA4). The all-pairwise analysis for the ENL factor revealed lower PGE2 concentrations in cells treated with E10 compared with those treated with 1 μm-ENL (265·00 v. 354·54 pg/ml, P< 0·05) or 0 μm-ENL (tendency: 265·00 v. 346·57 pg/ml, 0·05 < P< 0·1). The lowest PGF2α concentrations were obtained with the addition of E10 compared with the treatments with 0 μm-ENL (2200·21 v. 2764·70 pg/ml, P< 0·01) and 1 μm-ENL (2200·21 v. 3074·96 pg/ml, P< 0·01). The presence of FA (FA4) in cell-culture media significantly increased PGE2 concentrations (P< 0·01) and the PGE2:PGF2α ratio (P= 0·01), whereas PGF2α concentrations were decreased when both ENL and FA were present.
Discussion
The present study aimed to enhance the understanding on how transcription levels of selected genes and series 2 PG secretion vary under the treatment of LA and ALA at different ratios and/or ENL concentrations in bovine endometrial cells. Genes that were selected in the present study were previously identified as endometrial transcripts differentially expressed at day 17 of pregnancy, when dairy cows were fed 10 % flaxseed in the diet( Reference Palin, Beaudry and Vallée 26 , Reference Palin, Brochu-Gaudreau and Small 27 ). Moreover, they were selected based on their known or suspected roles in embryo survival and PG synthesis.
In vivo, the bovine elongating conceptus secretes IFNT to signal its presence to the endometrium, and P4 is a pregnancy hormone that plays a key role in the remodelling of the endometrium during the peri-implantation period( Reference Spencer, Johnson and Bazer 3 , Reference Pru, Austin and Haas 29 ). To mimic the peri-implantation period in the present in vitro model, endometrial cultured cells were exposed to P4 and roIFNT. The addition of P4 alone had little effect on the mRNA abundance of the genes studied in SC and EC. However, the addition of P4 and roIFNT (COPI) successfully induced the expression of several genes in both cell types. In accordance with the present results, it was previously observed that early pregnancy and/or IFNT can induce the transcription levels of CXCL10 ( Reference Gray, Abbey and Beremand 48 ), DKK1 ( Reference Bauersachs, Ulbrich and Gross 24 ), IFIT3 ( Reference Mansouri-Attia, Aubert and Reinaud 49 ), ISG15 ( Reference Hansen, Austin and Johnson 50 ) and OAS1 ( Reference Schmitt, Geisert and Zavy 51 ) in the ruminant endometrium. Therefore, these similarities validate our in vitro cell-culture system. In the present study, we observed a modest increase in the mRNA abundance of ANXA2 with the addition of P4 and roIFNT (COPI). This gene is considered as a positive regulator of invasive processes during the peri-implantation period( Reference Bauersachs, Mitko and Ulbrich 23 ). However, embryo implantation in cows is non-invasive( Reference Bauersachs, Mitko and Ulbrich 23 ), which may explain the modest up-regulation of ANXA2 mRNA in SC treated with the two hormones. Interestingly, we report for the first time the induction of the PARM1 transcript when both P4 and roIFNT are added to endometrial cells. This gene encodes for a mucin-like type I transmembrane protein that increases cell proliferation and whose expression is androgen-regulated( Reference Fladeby, Gupta and Barois 52 ). More recently, PARM1 was identified in the rat ovary where it acts as a regulator of the catabolic conversion of P4 to 5α-pregnanediol( Reference Yeon, Jang and Curry 53 ). The observed up-regulation of the PARM1 transcript with the addition of P4 and roIFNT suggests a putative role for PARM1 in the establishment of early pregnancy in cattle.
In the present primary endometrial cell-culture system, we observed that the different LA:ALA ratios and/or ENL concentrations affected the transcript abundance of selected genes and series 2 PG concentrations in culture media. Moreover, we reported that the SC response to the treatments differed from the EC response, thus suggesting that mechanisms influencing gene expression and PG secretion are regulated differently in those two cell types. In accordance with the present results, Xiao et al. ( Reference Xiao, Liu and Sirois 54 ) also observed a different regulation of PG synthesis in bovine EC and SC when steroid hormones were added to the cell-culture medium. In the bovine endometrium, SC and EC have a different morphology and present different functions( Reference Fortier, Guilbault and Grasso 32 ), which may explain the observed differences in mRNA abundance and series 2 PG concentrations. For example, it has been demonstrated earlier that PGE2 is mainly produced by SC, while EC secrete higher levels of PGF2α ( Reference Fortier, Guilbault and Grasso 32 ). Similar findings are reported in the present study. Herein we used SC and EC monocultures in order to investigate how these distinct endometrial cell types are affected by different LA:ALA ratios and/or ENL concentrations. This cell-culture system does not allow interactions between cell types, and we recognise that different cellular responses to the treatments may be observed in co-cultures of SC and EC. Ulbrich et al. ( Reference Ulbrich, Meyer and Zitta 55 ) previously reported that bovine endometrial SC are needed for the formation of tight junctions in endometrial EC. Moreover, modifications in cell proliferation, transepithelial resistance and cytokine secretion were observed when SC were co-cultured with human or mouse endometrial EC( Reference Arnold, Kaufman and Seppälä 37 , Reference Grant-Tschudy and Wira 56 ). In contrast, there was no difference between bovine endometrial epithelial monocultures and co-cultures with SC for the accumulation of PGE or PGF in response to oxytocin and AA( Reference Mackintosh, Schubert and Healy 39 ). Additional studies are required to determine whether co-cultures of SC and EC would respond differently to the treatment with LA and ALA at different ratios and/or ENL concentrations.
The in vitro culture approach used in the present study was based on previous experiments where FA were given for 24 h in BEND cells( Reference Mattos, Guzeloglu and Badinga 11 , Reference Caldari-Torres, Rodriguez-Sallaberry and Greene 40 ) or fed to cows for 4 weeks( Reference Cheng, Robinson and Pushpakumara 57 ), followed by in vitro tissue or cell culture with FA free culture media for up to 42 (tissue culture) or 6 h (BEND cells). Other studies have used alternative approaches where FA was included in culture media until measurements were performed( Reference Cheng, Elmes and Kirkup 58 , Reference Cheng, Abayasekara and Ward 59 ). Therefore, it would be of interest to investigate whether keeping FA in culture media throughout the whole in vitro experiments would result in different outcomes.
To the best of our knowledge, the present study is the first to evaluate the direct effects of different LA:ALA ratios on series 2 PG concentrations and gene expression in bovine endometrial primary cell cultures. The results show that transcript levels of selected genes were affected by the different LA:ALA ratios. Moreover, when considering only the three physiologically observed ratios (FA4, FA15 and FA25), many of these genes showed decreased mRNA abundance with increasing n-6:n-3 ratios in both cell types. FA4 corresponds to the observed circulating FA ratio when dairy cows are fed a diet containing 10 % flaxseed (rich in ALA). Interestingly, this ratio showed higher mRNA abundance of CXCL10, CYP39A1 and IFIT3 in SC and of AKR1B1, CYP39A1 and PTGES in EC when compared with FA25, which mimics cows fed 10 % sunflower seeds (rich in LA). The AKR1B1 and PTGES genes are coding for enzymes involved in the synthesis of PGF2α and PGE2, respectively. An up-regulation of the AKR1B1 gene may also lead to higher levels of series 3 PG at the expense of series 2 PG when n-3 FA levels increase( Reference Wathes, Abayasekara and Aitken 60 ), which may favour embryo survival. Moreover, an up-regulation of the mRNA levels of PTGES is desirable for embryo survival since PGE2 ( Reference Arosh, Banu and Kimmins 5 – Reference Pratt, Butcher and Inskeep 7 ) has known positive effects on uterine receptivity during the peri-implantation period( Reference Arosh, Banu and Kimmins 5 – Reference Pratt, Butcher and Inskeep 7 ). CXCL10 ( Reference Gray, Abbey and Beremand 48 ) and IFIT3 ( Reference Mansouri-Attia, Aubert and Reinaud 49 ) are up-regulated during early pregnancy in response to embryo IFNT signal, and in the ovine endometrium, CXCL10 is involved in the redistribution of immune cells that favours conceptus implantation( Reference Imakawa, Nagaoka and Nojima 61 ). Therefore, the up-regulation of CXCL10 and IFIT3 mRNA may be beneficial for embryo implantation and survival. The CYP39A1 gene encodes for a steroid hydroxylase, which prevents prolonged and inappropriate exposure to oestrogen in mice( Reference Omoto, Lathe and Warner 62 ). Because oestrogen levels must stay low during early pregnancy( Reference Henricks, Dickey and Hill 63 ), an up-regulation of CYP39A1 would favour embryo survival. Collectively, the above-mentioned results suggest that lower n-6:n-3 FA ratios contribute to an increase in the transcript abundance of genes that have positive effects on uterine receptivity and implantation.
High LA:ALA ratios increased PGE2 and PGF2α concentrations both in SC and EC culture media compared with control cells that were not exposed to LA and/or ALA (COPI). Moreover, the all-pairwise multiple comparisons revealed that PGF2α and PGE2 concentrations increased with greater LA:ALA ratios in EC, which is consistent with a previous study with BEND cells, where increasing LA:EPA ratios increases PGF2α secretion( Reference Caldari-Torres, Rodriguez-Sallaberry and Greene 40 ). In SC, PG concentrations were affected by the different LA:ALA ratios, with PGE2 and PGF2α concentrations being higher at FA4 (rich in n-3) than at FA25 (rich in n-6). An increase in the synthesis and secretion of PGF2α in SC is not desirable for embryo survival because of its luteolytic properties( Reference Abayasekara and Wathes 64 ). However, the contribution of PGF2α secretion in SC to the total endometrial PGF2α secretion is expected to be limited since PGF2α secretion is much lower in SC than in EC( Reference Fortier, Guilbault and Grasso 32 ). In contrast, increased PGE2 secretion in SC would favour embryo implantation based on its positive effect on uterine receptivity( Reference Arosh, Banu and Kimmins 5 ).
Flaxseed is rich in plant lignans that are mainly converted into the mammalian lignans ENL and enterodiol under the action of the cow's ruminal and intestinal microbiota( Reference Gagnon, Cortes and da Silva 21 , Reference Adlercreutz and Mazur 65 ). Mammalian lignans are absorbed from the intestine, and most of them are conjugated to glucuronides by specific enzymes in the intestinal wall and liver( Reference Lampe, Atkinson and Hullar 66 ). In dairy cows, most of the metabolism of flax lignans occurs in the rumen( Reference Gagnon, Cortes and da Silva 21 ) and, ENL is the main lignan metabolite found in goat serum( Reference Zhou, Wang and Han 67 ). Plant and mammalian lignans possess antioxidant activities( Reference Prasad 68 ) and can act as phyto-oestrogens( Reference Landete 22 ), which may explain, at least in part, the reproductive benefits observed when flaxseed is fed to cows( Reference Petit, Small and Palin 69 ). However, the effects of lignans on reproductive functions have been poorly explored so far. In the present study, we show for the first time that the presence of ENL modulates series 2 PG secretion in EC and SC as well as the mRNA abundance of genes known or suspected to play a role in uterine receptivity and conceptus implantation. Indeed, in both cell types, the addition of ENL decreased PGE2 and PGF2α concentrations in culture media. Moreover, the greatest reductions in PGF2α concentrations were observed in EC when FA (FA4) was combined with ENL. However, the present results demonstrate that the addition of ENL has no effect on the PGE2:PGF2α ratio, and that the presence of FA4 alone seems to be sufficient to increase the PGE2:PGF2α ratio in EC. Petit et al. ( Reference Petit, Small and Palin 69 ) observed a higher PGE metabolite:13,14-dihydro-15-keto-PGF2α ratio in the uterine flushes of non-pregnant cows fed with 9·1 % flaxseed in the diet (which corresponds to the FA4 treatment used in the present study) when compared with those fed the control diet (no added fat), thus showing that ALA can increase the PGE2:PGF2α ratio in vivo. The increase in the PGE2:PGF2α ratio in EC might protect the CL against luteolysis and help in preparing the endometrium for implantation. However, further work is required to determine which of the following factors contribute the most in maintaining the CL and in uterine receptivity: PGE2 and PGF2α secretion or the PGE2:PGF2α ratio.
Among the selected genes, IFIT3, DKK1 and IRF6 ( Reference Bauersachs, Ulbrich and Gross 24 , Reference Fleming, Song and Choi 70 ) are known interferon-stimulated genes whose expression increase in the ruminant endometrium during early pregnancy. The decreases observed in the mRNA levels of IFIT3, DKK1 and IRF6 in SC and in the transcript levels of IFIT3 in EC when ENL was added to the culture medium may, therefore, negatively affect uterine receptivity. ANXA2 is a gene involved in the positive regulation of invasive growth( Reference Bauersachs, Mitko and Ulbrich 23 ), and in the present study, the transcript levels of ANXA2 were up-regulated in EC by the addition of ENL. However, because implantation in the cow is a non-invasive process, an up-regulation of the mRNA abundance of ANXA2 would not necessarily improve embryo survival. It has earlier been reported that the gene expression of ZFP36L1 is essential to embryo survival( Reference Stumpo, Byrd and Phillips 31 , Reference Bell, Sanchez and Spasic-Boskovic 71 ). This gene is implicated in the post-transcriptional control of gene expression and is a negative regulator of the gene activity of vascular endothelial growth factor-A (VEGFA)( Reference Bell, Sanchez and Spasic-Boskovic 71 ). Here, we observed a down-regulation of the transcript levels of ZFP36L1 in SC with the addition of ENL. Further work is needed before being able to determine the physiological impact of the decreased expression of ZFP36L1.
Among the genes that were modulated by ENL, three code for enzymes involved in the synthesis of PG( Reference Arosh, Banu and Kimmins 5 ), which are as follows: AKR1B1, known as the primary enzyme responsible for PGF2α production( Reference Zakrzewska, Madore and Chapdelaine 72 ); PTGES previously identified as the main enzyme responsible for the synthesis of PGE2 ( Reference Parent and Fortier 73 ); PTGS2 that convert AA to PGH2, a common precursor of PGE2 and PGF2α ( Reference Arosh, Parent and Chapdelaine 34 , Reference Parent and Fortier 73 ). Interestingly, we reported that mRNA levels of AKR1B1 and PTGES, but not PTGS2, were decreased in SC with the addition of ENL, which was associated with lower PGF2α and PGE2 concentrations. This suggests that ENL may reduce the secretion of PGE2 and PGF2α by modifying the transcription levels of PTGES and AKR1B1 genes in endometrial SC. Further work is needed to determine the reasons why mRNA levels of AKR1B1 and PTGES were not affected by ENL in EC despite the fact that ENL decreased the secretion of PGE2 and PGF2α. The mRNA abundance of PTGS2 was higher in SC treated with FA4, which was associated with increases in PGF2α and PGE2 concentrations. Similar increases in the mRNA abundance of PTGS2 were observed with the addition of ALA and AA, and in PGF2α and PGE2 concentrations with the addition of ALA and stearidonic acid to ovine endometrial cells( Reference Cheng, Abayasekara and Ward 59 ). Interestingly, Caldari-Torres et al. ( Reference Caldari-Torres, Rodriguez-Sallaberry and Greene 40 ) previously reported an increase in the mRNA abundance of PTGS2 in BEND cells treated with increasing LA:EPA ratios, and this was accompanied by similar increases in PGF2α secretion. Moreover, in primary bovine endometrium EC, AA-induced PGF2α and PGE2 secretion was preceded by increases in the protein levels of PTGS2( Reference Parent, Villeneuve and Fortier 74 ). However, in BEND cells, EPA reduced and AA increased the synthesis of PGF2α ( Reference Caldari-Torres, Rodriguez-Sallaberry and Greene 40 , Reference Thatcher, Guzeloglu and Mattos 75 ), but neither PUFA affected the mRNA abundance of PTGS2 ( Reference Mattos, Guzeloglu and Badinga 11 ). In the absence of FA, the addition of ENL to EC affected the mRNA abundance of PTGS2, which followed the concentration profiles of PGF2α and PGE2. It remains to be determined why the observed association between the mRNA levels of PTGS2 and series 2 PG concentrations is lost with the addition of FA to EC. The absence of a concordance between the mRNA levels of AKR1B1, PTGES and PTGS2 and series 2 PG concentrations does not rule out the possibility of post-translational alteration of PG synthesis enzymes by ENL and/or FA that may affect the secretion of PG.
Among the different mechanisms that have been suggested for explaining how n-3 and n-6 FA modulate series 2 PG secretion in endometrial cells, one indicates the possible inhibition of PTGS2 enzyme activity by EPA, ALA and LA, previously identified as PTGS2 inhibitors( Reference Ringbom, Huss and Stenholm 76 ). However, this mechanism is unlikely because in the present study, PGE2 and PGF2α secretion was found to increase in EC treated with increasing LA:ALA ratios. In the present study, the lowest PGE2 and PGF2α concentrations were observed with the lowest LA:ALA ratios (i.e. high in n-3), whereas the highest PGE2 and PGF2α concentrations were obtained with high LA:ALA ratios (i.e. high in n-6). A possible competition between n-3 and n-6 FA for enzymes involved in the desaturation and elongation of long-chain FA or those involved in the synthesis of PG may explain how n-3 and n-6 FA modulate PGE2 and PGF2α concentrations( Reference Wathes, Abayasekara and Aitken 60 , Reference Abayasekara and Wathes 64 ). According to this last proposed mechanism, increased n-3 FA would favour the production of series 3 PG at the expense of series 2 PG. Although we found lower PGE2 and PGF2α secretion with higher n-3 FA, it remains to be determined whether this is accompanied with increases in series 3 PG.
In conclusion, the present results demonstrate that the addition of LA and ALA at different ratios and ENL can affect the mRNA abundance of the genes studied and PGE2 and PGF2α concentrations in primary bovine endometrial cells, with different effects being observed in EC and SC. The FA4 treatment, which corresponded to the observed circulating ratio when cows were fed a diet containing 10 % flaxseed, increased the mRNA abundance of genes that have positive effects on uterine receptivity and implantation, when compared with the FA25 treatment (rich in LA). This suggests that flax oil (rich in ALA) alone can modulate the transcript abundance of genes known to have beneficial effects on the fertility of dairy cows. The combination of ENL with FA (FA4) resulted in the greatest reduction in PGF2α concentrations in both cell types, suggesting that feeding flaxseed to dairy cows would have a greater impact on the reduction in PGF2α secretion than giving flax oil (rich in ALA) or flax meal (rich in lignans) alone. A decrease in endometrial PGF2α secretion is desirable for embryo survival because of its known luteolytic properties. Although the transcript levels of AKR1B1 and PTGES were modulated in SC with the addition of ENL, these genes were not affected by ENL in EC. This would suggest that different molecular mechanisms are involved in SC and EC, with respect to the synthesis of PGE2 and PGF2α. The results presented herein focused on mRNA abundance and PG secretion that were used as a functional endpoint. Since changes in mRNA abundance do not always reflect differences in protein expression, caution needs to be exercised when extrapolating these results to protein expression or activities. Future studies are required to assess whether the effects of the treatments observed on mRNA abundance and PG secretion correlate with protein expression and activities.
Acknowledgements
The authors thank Danièle Beaudry, Marian Mayhue and Karine L'Ériger for technical assistance, and Steve Methot for his help in the statistical analysis.
The present study was financially supported by grant IPDGR 114 from Agriculture and Agri-Food Canada and through a Natural Sciences and Engineering Research Council of Canada (NSERC) studentship granted to C. Hallé.
The authors’ contributions are as follows: C. H. and M. F. P. drafted the manuscript; M. F. P. and H. V. P. conceived and directed the study; C. H. coordinated the study and performed all the experiments; A. K. G. and R. B. provided advice on the experimental design. All authors critically revised the paper and approved the final version of the manuscript.
None of the authors has a personal or professional conflict of interest.










