Dietary SFA are associated with higher blood concentrations of LDL-cholesterol(Reference Sacks, Lichtenstein and Wu1), a well-established risk factor for CVD(Reference Carr, Hooper and Sullivan2), and may also have metabolic effects such as decreased insulin sensitivity when compared with unsaturated fatty acids(Reference Lopez, Bermudez and Pacheco3,Reference Lopez, Bermudez and Ortega4) . Although the evidence indicates the substitution of dietary saturated fats with unsaturated fats is beneficial for CVD risk, and a number of health and advisory committees have made recommendations for reducing the intake of SFA in the diet(Reference Sacks, Lichtenstein and Wu1,Reference DeSalvo, Olson and Casavale5) , there are still conflicting reports about the effect of dietary SFA on CVD(Reference Hooper, Martin and Jimoh6,Reference Hamley7) . This heterogeneity in findings may partly be related to different dietary sources of SFA(Reference Khaw, Sharp and Finikarides8).
Ghee is produced from butter by traditional methods and is rich in SFA and also contains moderate amounts (about 30 %) of MUFA. Palmitic acid, which is associated with an increase in LDL-cholesterol(Reference van Rooijen and Mensink9), accounts for a relatively high percentage of the ghee’s SFA (about 62 %), but it also contains stearic acid (about 10 %), which has been shown to have a neutral effect on LDL-cholesterol(Reference Meng, Matthan and Wu10). In addition to SFA and MUFA, ghee also contains some conjugated linoleic acid (CLA), a fatty acid that has anti-atherosclerotic effects and may improve insulin sensitivity(Reference den Hartigh11,Reference Chinnadurai, Kanwal and Tyagi12) in animal studies, with inconsistent effects on cardiovascular risk factors in human studies(Reference den Hartigh11,Reference Raff, Tholstrup and Basu13–Reference Riserus, Vessby and Arnlov15) . Previous studies have shown inconsistent results regarding the effect of ghee on plasma lipoprotein cholesterols levels. Results from animal studies indicated that ghee increased serum total cholesterol (TC), LDL-cholesterol and HDL-cholesterol in rabbits(Reference Hosseini and Asgary16) or had no significant effect on serum TC, but increased serum TAG concentration in rats when it included at a level of 10 % energy in the diet(Reference Sharma, Zhang and Dwivedi17,Reference Kumar, Sambaiah and Lokesh18) . However, in human studies, the inclusion of ghee in the diet of healthy subjects had no adverse effect on serum LDL-cholesterol levels when compared with mustard oil(Reference Shankar, Yadav and Ray19) or hydrogenated vegetable oil(Reference Mohammadifard, Nazem and Naderi20).
The effects of ghee consumption on plasma lipids and lipoprotein might be better interpreted by examining its effects on other risk factors for CVD, including insulin sensitivity, as well as hemostatic markers such as plasminogen activator inhibitor 1 (PAI-1), which is also associated with individual fatty acids intake(Reference Pacheco, Bermudez and Lopez21,Reference Masquio, de Piano and Campos22) and insulin sensitivity(Reference Masquio, de Piano and Campos22,Reference Potter van Loon, Kluft and Radder23) . Few randomised trials have assessed the effects of consuming ghee on plasma lipids and lipoproteins. Furthermore, all trials have used a parallel design, making it difficult to distinguish between person from variation within person. Moreover, information about the postprandial metabolic effect after consumption of a test meal containing ghee is sparse. Therefore, we conducted a randomised crossover trial to determine the effect of the inclusion of ghee compared with olive oil in the diet on fasting and postprandial blood cardiometabolic risk markers in healthy men and women. Olive oil was considered as the reference oil for comparison in this study since it has been promoted as a healthy oil, and previous researches have shown its potential cardioprotective benefits(Reference Guasch-Ferre, Liu and Li24–Reference Khandouzi, Zahedmehr and Nasrollahzadeh26).
Experimental methods
Study design and subjects
The present study was a randomised, not blinded, crossover, clinical trial. The study was conducted between December 2020 and March 2021 at the Shahid Beheshti University of Medical Sciences. Participants were recruited via public advertisements. Adult male and non-menopausal females aged 20–60 years were included in the study. Participants were excluded if they were on lipid-lowering medication, had a history of type 2 diabetes, history of coronary heart disease, abnormal liver function test (alanine aminotransferase and aspartate aminotransferase > 45 U/L), abnormal kidney function test (createnine > 1·4 mg/dl), were at pregnancy and breastfeeding periods or were on oral contraceptive unless they had no intention to change during the study. A simple randomisation method was performed using computer-generated random numbers with a 1:1 randomisation between the two treatments. Each number was placed in a sealed opaque envelope and was consecutively opened by the research assistant. The research assistant involved in the procedure of randomisation did not have access to any information about the laboratory characteristics of the participants. In this study, the effect of the inclusion of ghee in the diet was compared with a reference oil (olive oil). The study included a 2-week run-in period in which reference oil (olive oil) was used and at the end of which baseline measurements were performed. Subjects were then randomised to one of the two treatments for 4 weeks in which participants were asked to consume ghee or olive oil in their diet. The intervention was crossed within each group after two weeks wash-out during which reference oil (olive oil) was consumed. Anthropometric and biochemical parameters were assessed at baseline and the end of each intervention period.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethical Committee of National Nutrition and Food Technology Research Institute, Tehran, Iran (the Ethical No: IR.SBMU.nnftri. Rec.1399·053), and written informed consent was obtained from all patients. This clinical trial was registered at the Iranian Registry of Clinical Trials (IRCT20160702028742N9).
Diets
Volunteers were instructed to have a diet at their estimated total energy expenditure. Energy levels were determined using equations to determine energy requirements (by Mifflin St Jeor’s equation(Reference Mifflin, St Jeor and Hill27) multiplied by self-reported physical activity). The estimated weight maintenance energy content were distributed as ∼55 % from carbohydrates, ∼15 % from protein and ∼30 % from fat. Then, the number of servings of each food group was determined for each level of energy requirement. The daily intake of olive oil or ghee was about 15 % of the total daily energy content, which varied between 20 and 48 g per day (mean and standard deviation of 30·6 ± 6·7), depending on the daily energy requirement. Olive oil and ghee were delivered to the subjects every 2 weeks. Participants received written dietary information booklets with portion advice. Except for studied oil/fat, all foods were self-selected by participants. During the study period, participants prepared two daily meals (lunch and dinner) using the studied oil/fat so that more than 80 % of the daily oil/fat used in the meal preparation were from study oil/fat. For each person, the amount of oil/fat allowed to prepare food in each of the two daily meals was determined, and usually consumed oils were replaced with ghee or olive oil. Participants were instructed to use each of the studied oil/fat to prepare meals using household utensils (such as spoons and cups). During each of the two intervention periods, dietary intake was assessed through six telephone-administered 24-h dietary recalls (four weekdays and two weekends) obtained from each participant. The mean of the 24-h dietary recalls was calculated for each individual over a 4-week diet period, and the means were used for data analysis. Diet composition was analysed by use of the Nutritionist software (version IV, N-Squared Computing) to which was added local food data. In addition, to assess their overall compliance with consuming the oil/fat, participants were asked to self-rate between 0 % and 100 % of their overall experience of consuming the assigned oil/fat in the study.
Participants attended the clinic at baseline and the end of each dietary intervention. In the post-intervention period (week 4), participants were exposed to a meal challenge that included studied oil/fat in accordance with the intervention diets. The patients attended the clinic in the morning after 12 h fast, and a fasting blood sample was collected before ingestion of the meal, in <15 min. The subsequent blood sample was taken at 2 h. For each person, the meal was considered to be at 20 % of his/her daily energy content. This meal consisted of 48 % energy content from carbohydrates, 7 % energy content from protein, 45 % energy content from fat so that 40 % of the total energy content, equivalent to 15–29 g, depending on the daily energy content, were from the tested fat (olive oil or ghee). The subjects did not consume any other food for 2 h after the meal challenge but were allowed to drink water during the postprandial assessment.
Ghee was sourced from a commercial manufacturer (Nikmanesh® Animal Oil) that meets nationally accredited manufacturing standards. In this study, olive oil was used as a reference oil because it has long been recognised to be favourable for cardiovascular health(Reference Foscolou, Critselis and Panagiotakos28). Refined olive oil was selected since it was preferred by some consumers, probably because of its milder flavour. Refined olive oil was produced by the company (Etka) from harvested olive in Rudbar County (Gilan Province). The composition of fatty acids in studied oil/fat (Table 1) was determined by GC of the corresponding methyl esters using standard methods (ISO 5509:2000 and ISO 5508:1990).
Table 1. Fatty acid composition of ghee and olive oil

ND, not detected. Expressed as % of total fatty acids.
The level of physical activity over each treatment period was determined two times by using the short form of a valid International physical activity questionnaire(Reference Craig, Marshall and Sjostrom29), and the mean of them was regarded in data analysis.
Anthropometric and blood pressure measurement
Height and weight were measured during the baseline and post-intervention visits. Weight was measured in light street clothes. Height was measured without shoes. Waist circumference was measured midway between the iliac crest and the lower rib margin by an anthropometric flexible tape. During each visit, while the participant was seated after 5 min of rest, two blood pressure measurements were obtained using a digital arm sphygmomanometer (Omron digital automatic blood pressure monitor HEM-907).
Biochemical measurement
Blood samples were collected in the heparin tubes in the fasting (at least 12 h) and 2-h postprandial state and plasma samples were separated using centrifugation at 2400 rpm for 10 min at 20°C and were stored at −80°C until they were analysed. Plasma glucose, TAG, TC, HDL-cholesterol, LDL-cholesterol and apolipoprotein B (apo B) were measured in batches using commercial kits (Pars-Azmoon by an automated analyser (Selectra ProXL, Vital Scientific). The intra-assay coefficient of variations were 1·49 %, 1·82 %, 1·62 %, 0·82 %, 0·67 % and 2·18 % for glucose, TAG, TC, HDL-cholesterol, LDL-cholesterol and apo B, respectively. Plasma insulin (Monobind, Inc., Lake Forest) and PAI-1 (R and D system) were measured in batches in frozen plasma samples via enzyme-linked immunosorbent assays kit according to the manufacturer’s protocol. The intra-assay coefficient of variations were 5·1 % and 6·8 % for insulin and PAI-1, respectively. All measurements of each of the studied parameters were analysed in batch and together (baseline samples along with end-of-study samples) to minimise inter-assay variation. Homoeostasis model assessment-insulin resistance was calculated according to the formula: fasting insulin (mIU/ml) × fasting glucose (mmol/l)/22·5.
Statistical analysis
The sample size was calculated considering a change in LDL-cholesterol as the primary outcome. A previous study that compared the effect of diets enriched with olive oil or butter for 4 weeks generated an approximately 0·38 mmol/l between-treatment difference in plasma LDL-cholesterol concentrations and standard deviations of 0·39 and 0·48 mmol/l(Reference Khaw, Sharp and Finikarides8). Accordingly, using the G × Power 3.1 software, it was determined that a sample size of n 28 would allow the power of 80 % at the level of significance of 5 % and a correlation between treatment values of 0·4. By considering a dropout, the required sample size was n 30.
The normality of continuous variables was assessed by using the Kolmogorov–Smirnov test. All studied variables had normal distribution (P > 0·05). A paired t test was performed to assess the effect of each intervention compared with its baseline. Differences in study outcomes between treatments were analysed using a two-factor repeated-measures ANOVA, with treatment and time (baseline and week 4 within each treatment period) as within-subject factors. The main effects of treatment and time, as well as the time × treatment interaction, were investigated. The possible carryover effect was determined by including allocation order as a fixed factor in the model and investigating its interaction with treatment × time. No statistically significant carryover was evident; therefore, data from both treatment sequences were pooled. For postprandial data, the baseline to postprandial response was analysed by a two-factor repeated-measures ANOVA, with treatment and time (baseline and 2-h postprandial in each treatment period) as within-subject factors. To analyse the dietary intake and physical activity level values, a paired t test was used. Data are presented as means ± standard deviations (sd) unless otherwise mentioned. SPSS Statistics software version 21.0 (IBM Corporation) was used for the statistical analyses and significance was defined as P ≤ 0·05.
Results
All thirty individuals randomised in the two interventions completed both 4 week intervention periods and were included in final analyses (Fig. 1 and Table 2). Table 3 shows energy and macronutrient intake during each of the two dietary interventions. The energy content, carbohydrates, protein and fibre intake did not differ between the two diet periods. The total amount of fat intake did not differ between the two diet periods, but the dietary intervention changed the composition of the fat intake. During the diet with olive oil, MUFA, as well as PUFA intake, were higher, whereas SFA and dietary cholesterol were higher during the diet with ghee intervention. Regarding self-reported compliance, participants reported 80 % or more compliance with the studied oil/fat over the 4 weeks. Evaluation of daily physical activity by physical activity questionnaire indicated no difference between the two diet periods.

Fig. 1. Flow diagram of participants.
Table 2. Baseline characteristics of participants by treatment sequence*
(Mean values and standard deviations)

* Data are means ± sd. BP, blood pressure.
Table 3. Dietary intake and physical activity level over the 4-week consumption of diets rich in olive oil or ghee*
(Mean values and standard deviations; mean values and 95 % confidence intervals)

* For dietary intake assessment, six 24-h dietary recalls were taken from each participant over each 4-week diet period, the mean of which was calculated for each individual, and the means were used for data analysis.
† Data were analysed using a paired t test.
In Table 4, values of anthropometric measures are shown. There was no statistical difference between the two diet periods in body weight, waist circumference and blood pressure.
Table 4. Anthropometry and blood pressure in healthy adults at baseline and following 4-week consumption of diets rich in olive oil or ghee
(Mean values and standard deviations)

* P for time × treatment, analysed using a two-factor repeated-measures ANOVA. Δ, change from baseline; DBP, diastolic blood pressure; SBP, systolic blood pressure; WC, waist circumference.
A decrease in the consumption of olive oil and an increase in the intake of ghee in the diet increased plasma apo B concentration (treatment × time, P = 0·018) and in non-HDL-cholesterol (treatment × time, P = 0·046) (Table 5). However, despite the difference in apo B concentration between the two diets, the plasma LDL-cholesterol concentrations were not different after consumption of the two diets (treatment × time, P = 0·092). Further, there were no significant differences in plasma HDL-cholesterol and TAG concentrations, or the ratio of TC: HDL-cholesterol between the two diets.
Table 5. Fasting plasma concentrations of glucose, insulin, lipids and plasminogen activator inhibitor-1 (PAI-1) in healthy adults at baseline and following 4-week consumption of diets rich in olive oil or ghee
(Mean values and standard deviations)
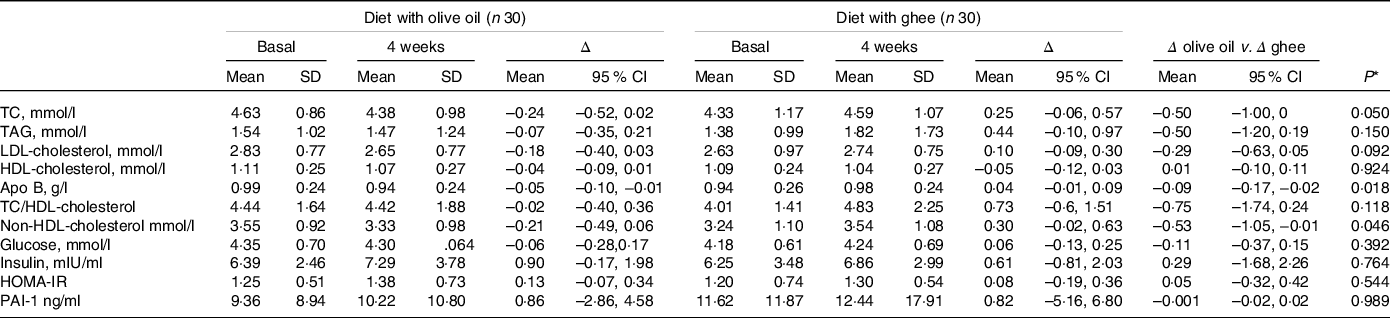
* P for time × treatment, analysed using a two-factor repeated-measures ANOVA. Δ, change from baseline; TC, total cholesterol.
Fasting plasma glucose had no significant changes, but fasting insulin tended to increase after both olive oil and ghee consumption (time effect, P = 0·059). However, neither of the dietary interventions had a significant time nor time × treatment effect on insulin resistance as assessed by homoeostasis model assessment-insulin resistance (Table 5).
Although fasting TAG concentration before ingestion of the olive oil was slightly and non-significantly (P = 0·18) lower than and ghee meals, the 2-h postprandial TAG response of meals was not different. TAG concentrations increased (P < 0·01) after both test meals, but no significant difference was observed in the 2-h postprandial TAG response of meals (Fig. 2(a)). Meals had no significant effect on postprandial plasma HDL-cholesterol concentration (Fig. 2(b)). Glucose concentration return to lower than basal values after 2 h and the response was similar after ingestion of both test meals (Fig. 2(b)). Both meals markedly increased (P < 0·001) plasma insulin concentrations at 2 h with no significant difference between meals (Fig. 2(c)). Likewise, PAI-1 concentrations markedly increased after both meals, but there was no difference between the two meals (Fig. 2(e)).

Fig. 2. Comparison of plasma 2-h postprandial responses of study participants to meals containing olive oil or ghee. Values are the means ± standard error of the mean. 2-h postprandial values are after ingestion of a meal containing olive oil or ghee in accordance with the background diets at week-4 of each dietary intervention. Data were analysed using a two-factor repeated-measures ANOVA. Δ, change from baselinePAI-1, plasminogen activator inhibitor-1.
Discussion
This study aimed to evaluate the cardiovascular health-related effects of consuming ghee in the usual diet. Compared with the diet containing olive oil, consumption of ghee instead of olive oil in the diet, significantly increased non-HDL and apo B concentrations, but it had a neutral effect on insulin resistance and other cardiovascular risk markers. In addition, similar postprandial TAG and glycaemic responses were observed after ingestion of meals containing ghee or olive oil.
There were no significant differences in blood pressure, body weight, central adiposity as measured by waist circumference and physical activity between the two treatments; therefore, the observed changes in plasma lipid and lipoprotein concentrations can be attributed to changes in the dietary intake of participants. The subjects reported increased SFA and cholesterol intake by the ghee intervention, whereas MUFA and PUFA intakes were increased by olive oil intervention. This could indicate good compliance with consuming the studied dietary fats.
The clinical relevance of the increase in apo B after consuming the diet containing ghee compared with the diet with olive oil remains unclear given that LDL-cholesterol increased marginally. However, the plasma apo B concentration has been considered to be a more accurate representation of atherogenic particles(Reference Carr, Hooper and Sullivan2,Reference Sniderman, Thanassoulis and Glavinovic30) . A meta-analysis reported that each 0·1 g/L decrease in apo B was associated with a 9 % decrease in coronary heart disease and a 6 % decrease in major CVD risk(Reference Robinson, Wang and Jacobson31). In addition to apo B, ghee consumption increased the non-HDL-cholesterol level. Non-HDL-cholesterol represents the cholesterol in all particles causing CVD(Reference Nordestgaard, Langlois and Langsted32). Non-HDL-cholesterol is an important target of therapy for the prevention of coronary heart disease and there is a consistent direct relationship between cardiovascular risk reduction and the magnitude of non-HDL-cholesterol lowering(Reference Carr, Hooper and Sullivan2,Reference Robinson, Wang and Smith33,Reference Zhang, Wu, Li and Zhu34) . Other studies examining the effect of ghee on plasma lipid and lipoproteins concentrations have yielded conflicting results. In an experimental study in which male rabbits were fed a normal chow diet or diets containing ghee or olive oil, ghee significantly increased TC, LDL-cholesterol and HDL-cholesterol as compared with chow or olive diet(Reference Hosseini and Asgary16). Replacing ghee in the diet of healthy young people on a vegetarian diet did not increase LDL-cholesterol concentration compared with their baseline, but in the control group who consumed mustard oil, atrend towards a decline in LDL-cholesterol was observed(Reference Shankar, Yadav and Ray19). In another parallel study in healthy subjects who used mostly hydrogenated vegetable oil in their usual diets before the study, replacing hydrogenated oil with ghee did not change serum LDL-cholesterol and apo B levels, but did increase HDL-cholesterol and lowered TAG levels. On the other hand, in the group that replaced hydrogenated oils with non-hydrogenated ones, the levels of total cholesterol, apo B and TG were decreased(Reference Mohammadifard, Nazem and Naderi20). The discrepancy between the results may be caused by differences in study design and subjects. These studies were not a crossover, thus between-person differences may have influenced the observed results. Moreover, in the first study(Reference Shankar, Yadav and Ray19), all analyses compared final values to their baselines, and between groups, analysis was not performed, and the second study(Reference Mohammadifard, Nazem and Naderi20) did not have a lead-in period to control participants’ baseline diets.
There was no difference between the effects of ghee or olive oil intake on fasting plasma concentrations of glucose and insulin or insulin resistance index. Fasting plasma insulin tended to increase after both dietary interventions. This tendency to increase was observed despite no change in body weight or waist circumference and also without significant change in energy, carbohydrate or fibre intake. Regarding postprandial glycaemic response, the two test meals showed similar postprandial glucose and insulin responses. These findings are somewhat consistent with the results of other studies in which the effects of diets or meals rich in SFA or MUFA have been compared. Chang et al. investigated the effect of MUFA or SFA-enriched diets in centrally obese subjects and found no significant difference between SFA or MUFA diets on fasting and postprandial insulin and glucose secretion(Reference Chang, Vethakkan and Nesaretnam35). Roche et al. evaluated the postprandial response during 9 h after the ingestion of meals containing different proportions of MUFA and SFA in healthy males and reported no significant difference in postprandial plasma glucose and insulin response(Reference Roche, Zampelas and Jackson36). Itoh et al. evaluated the glucose and insulin responses after the ingestion of an SFA-enriched high-fat meal or a reduced SFA high-fat meal in healthy women and found no difference in insulin response between SFA enriched and reduced SFAs high-fat meals(Reference Itoh, Moriguchi, Yamada and Fujita37). However, our finding may not be consistent with some previous studies in which the consumption of SFA instead of MUFA changed the insulin sensitivity index. Lopez et al. evaluated acute postprandial insulin sensitivity effect over the 8 h after ingestion of high-fat meals enriched in SFA (high-palmitic sunflower oil and butter) or MUFA (refined olive oil) in normotriglyceridaemic subjects and found that the early postprandial insulin response (0–2 h) increased as the ratio of dietary MUFA:SFA decreased(Reference Lopez, Bermudez and Pacheco3). Christiansen et al. studied the effects of diets (for 6 weeks) enriched in SFA or MUFA in obese patients with non-insulin-dependent diabetes mellitus and found that in the presence of unchanged fasting glycaemia, dietary SFA induced an increase in postprandial insulinaemia compared with a diet with MUFA(Reference Christiansen, Schnider and Palmvig38). However, the health status of participants (healthy v. diabetes mellitus), as well as the difference in the composition of fatty acids oil/fat, may have influenced the results. Ghee’s fatty acid composition is somewhat different from the SFA-rich fats used in the above studies. In addition to being rich in SFA, ghee is also a relatively good source of MUFA and CLA. Although the effects of CLA on glucose metabolism are controversial, the administration of CLA has improved insulin sensitivity in non-obese, regularly exercising women(Reference Lambert, Goedecke and Bluett39) or in young sedentary humans(Reference Eyjolfson, Spriet and Dyck14). However, CLA supplementation did not affect plasma glucose and insulin concentrations or insulin resistance index in healthy subjects(Reference Noone, Roche and Nugent40,Reference Tricon, Burdge and Kew41) or even impaired insulin sensitivity in non-diabetic abdominally obese men(Reference Riserus, Vessby and Arnlov15,Reference Riserus, Vessby and Arner42) .
In the present study, the fasting and postprandial TAG concentrations were not statistically significant between the two interventions. The relative effects of SFA and MUFA rich meals on postprandial TAG concentrations have been investigated in only a small number of studies, and none of these studies have evaluated the effect of ghee. However, the postprandial TAG response may be best measured at times longer than 2 h since similar previous studies have shown that peak TAG concentrations occur between 3 and 5 h after high-fat meals(Reference Lopez, Bermudez and Pacheco3,Reference Teng, Chang and Vethakkan43) .
With respect to PAI-1 concentration, the main regulator of fibrinolysis, the effect of ghee consumption was not significantly different from olive oil. This suggests that a diet rich in ghee does not increase PAI-1. No previous study has reported the effect of ghee consumption on haemostatic factors. It has been shown that enrichment of test meals with refined olive oil, butter and high-palmitic sunflower oil induces a peak in PAI-1 2-h postprandially(Reference Pacheco, Bermudez and Lopez21). Tholstrup et al. compared the effects of test meals rich in stearic acid, palmitic acid or oleic acid on haemostatic profile, including PAI-1, in 16 young men and consistent with our finding, they reported no significant difference in postprandial PAI-1 between test fats(Reference Tholstrup, Miller and Bysted44). Oakley et al. compared the postprandial effects of three high-fat (95 g) meals (butter, high oleate and oleate + medium-chain triacylglycerols) with an isoenergetic low-fat meal (18 g medium-chain triacylglycerols) in twelve men and found neither the amount nor type of fat meals influenced PAI-1 concentration(Reference Oakley, Sanders and Miller45). Similarly, Stonehouse et al. showed that consumption of meals containing palmolein or olive oil did not affect postprandial plasma PAI-1 in overweight and obese men(Reference Stonehouse, Brinkworth and Noakes46). However, Pacheco et al. reported that when the ratio of MUFA:SFA in dietary fats (from butter, refined olive oil and high-palmitic sunflower oil) was lower, postprandial concentrations of PAI-1 increased in healthy subjects(Reference Pacheco, Bermudez and Lopez21).
The main strengths of this study are the randomised crossover design, pragmatic trial in free-living individuals with a high completion rate over 4 weeks. The trial has some limitations. The sample size was rather small, and the power to detect subtle changes is therefore restricted. Furthermore, the generalisability of the data is limited to healthy men and women. In addition, it was a short-term trial of 4 weeks intervention, and the study would have benefited if continued for a longer duration to assess long-term effects. Moreover, the postprandial period was too short and limited by only two measurements (fasting and 2 h). Ghee as one of the treatment oils in this study was included at more than 80 % of the total cooking fat which may be higher than what is expected for the usual intake of this oil. Thus, some of the treatment effects under the experimental conditions of this study may have been larger than what would be expected when a more typical amount of ghee is consumed. Although self-reported compliance was good, it was subjective, and the study would have benefited if objective biomarkers of compliance such as the blood fatty acid profile were measured in participants.
Conclusion
Dietary recommendations to decrease intakes of SFA and, more recently, to replace SFA with unsaturated fat have been emphasised by some health and advisory committees(Reference Sacks, Lichtenstein and Wu1,Reference DeSalvo, Olson and Casavale5) and researchers(Reference Kris-Etherton and Krauss47). In addition, it has been proposed that different foods rich in SFA may have different effects on cardiovascular risk factors, and the dietary recommendation to reduce intake of SFA should distinguish between specific food sources of SFA(Reference Astrup, Magkos and Bier48). The results of this study indicate that a decrease in olive oil intake which is predominantly monounsaturated fat, and a concomitant increase with ghee intake, a predominantly saturated fat, increases blood apo B and non-HDL-cholesterol levels. Given that plasma apo B concentrations could be regarded as a representation of atherogenic particles(Reference Sniderman, Thanassoulis and Glavinovic30) and regarding a direct relationship between the magnitude of non-HDL-cholesterol levels and cardiovascular risk(Reference Zhang, Wu, Li and Zhu34), the current trial adds further evidence to emphasise the existing prudent recommendations to replace dietary fats high in SFA with dietary fats high in unsaturated fats to reduce CVD risk(Reference Sacks, Lichtenstein and Wu1,Reference DeSalvo, Olson and Casavale5) .
Acknowledgements
The authors would like to thank Elaheh Tajeddin for her assistance in laboratory measurements and the participants who completed the trial.
This research is funded by a project grant supporting by the National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Science, Tehran, Iran.
The authors’ contributions were as follows – J. N. and S. M. H. designed the research; S. M. H. conducted the research, including the recruitment of study volunteers and the collection of sample and data; J. N. and S. M. H. analysed and interpreted data; J. N. performed the statistical analysis and wrote the paper.
The authors declare that there is no conflict of interest.










