Zn is required for normal growth and development, being an essential nutrient for nearly all organisms, and is most notably involved as an important component of over 300 enzymes and 1000 transcription factors(1–Reference Kambe, Tsuji and Hashimoto4). Thus, Zn serves as a catalytic or structural cofactor in several enzyme systems as a component of metalloenzymes that are involved in many physiological and metabolic pathways including carbohydrate and protein metabolism(Reference Kambe, Tsuji and Hashimoto4,Reference Olechnowicz, Tinkov and Skalny5) , and especially lipid metabolism(Reference Dieck and Frank Döring6–Reference Tayeb, Nakbi and Cheraief9). As Zn has been demonstrated to be involved in lipid metabolism of mammals, the effect of dietary Zn supplementation on lipid metabolism has been recently studied in aquatic animals, such as goby Synechogobius hasta (Reference Zheng, Luo and Chen10) and yellow catfish Pelteobagrus fulvidraco (Reference Zheng, Luo and Hu11,Reference Wei, Luo and Hogstrand12) .
AMPK-activated protein kinase (AMPK) is a crucial cellular energy sensor and is composed of a catalytic α-subunit and two regulatory β- and γ-subunits(Reference Carling13). Upon energy deficiency, AMPK is phosphorylated at Thr172 in the catalytic α-subunit by calmodulin-dependent protein kinase kinase-β (CaMKKβ)(Reference Carling13). Activated AMPK leads to a concomitant activation of ATP-producing catabolic pathways such as fatty acid oxidation and glycolysis and inhibition of energy-consuming biosynthetic pathways like protein, glycogen and fatty acid synthesis(Reference Hardie, Ross and Hawley14). Thus, AMPK not only restrains lipid biosynthesis pathways but also activates fatty acid oxidation-related genes to maintain energy in vivo (Reference Angin, Beauloye and Horman15). A previous study demonstrated that dietary Zn had a potent effect in lowering levels of hepatic lipids by up-regulating the activity of AMPK(Reference Wei, Luo and Hogstrand12). In vivo and in vitro studies have demonstrated that Zn plays an essential protective role in the regulation of lipid accumulation and metabolism in fish(Reference Wei, Luo and Hogstrand12). However, in contrast, there are no studies on the relationship between Zn and lipid metabolism in shrimp.
Litopenaeus vannamei is an important cultured shrimp worldwide, accounting for 80 % of total penaeid shrimp production due to its great economic value and rapid growth rate(1). Nutrition and feeding of L. vannamei under semi-intensive or intensive conditions have received a great deal of attention. Studies on Zn nutrition in various crustaceans have mainly focused on requirement levels and/or immunity(1). L. vannamei required 33 mg Zn/kg diet to maintain normal tissue mineralisation and growth(Reference Davis, Lawrence and Gatlin16). A similar requirement value of 32–34 mg Zn/kg based on weight gain (WG) and whole-body Zn retention was reported for grass shrimp Penaeus monodon (Reference Shiau and Jiang17). However, until now, the regulation of lipid metabolism by dietary Zn has not been explored in L. vannamei. Therefore, the aim of the present study was to determine the relationship between Zn and lipid metabolism, including the Zn-induced Ca2+/CaMKKβ/AMPK pathway, to reveal the mechanism of Zn-induced lipolysis in L. vannamei.
Methods
Ethics statement
The study was performed in strict accordance with the Standard Operation Procedures of the Guide for Use of Experimental Animals of Ningbo University. The experimental protocol and procedures were approved by the Institutional Animal Care and Use Committee of Ningbo University.
Experimental diets
The formulation and proximate composition of the diets are presented in Table 1. Fishmeal, soyabean meal, poultry meal, soya protein concentrate, krill meal and peanut meal were used as protein sources; fish oil, soyabean oil and soya lecithin were used as lipid sources; and wheat flour was used as the carbohydrate source. Five experimental diets were formulated with ZnSO4.H2O (Zn content = 35·5 %; Sinopharm Chemical Reagent Co. Ltd) as Zn source, with the analysed values of Zn being 46·4 (basal diet), 77·2, 87·0, 117·1 and 136·8 mg/kg diet, respectively. All dry ingredients were ground through 80 mesh and weighed according to the formulation. The mineral and vitamin premixes were mixed thoroughly by the progressive enlargement method, and then lipid and distilled water (35 %) were added. The ingredients were mixed in a Hobart-type mixer, and cold-extruded pellets produced (F-26, Machine Factory of South China University of Technology) with pellet strands were cut into uniform sizes (1·5 and 2·5 mm diameter) (G-250, Machine Factory of South China University of Technology). Pellets were heated for 30 min at 90°C, then air-dried to approximately 10 % moisture, sealed in vacuum-packed bags and stored at −20°C until used for the feeding trial.
Table 1. Formulation and proximate compositions of the experimental diets

* Mineral premix (g/kg diet): NaCl, 0·74; K2SO4, 2·24; MgSO4.7H2O, 3·58; FeC6H5O7, 0·29; C6H10CaO6.5H2O, 0·51; MnSO4.H2O, 0·12; CuSO4.5H2O, 0·16; KIO3 (1 %), 0·02; Na2SeO3 (1 %), 0·07; CoSO4.7H2O, 0·02; zeolite, 2·25. The mineral premix does not supply Zn.
† Vitamin premix (mg/kg diet): d-calcium pantothenate, 120; inositol, 200; menadione, 60; nicotinic acid, 100; pyridoxine hydrochloride, 60; riboflavin, 50; thiamin nitrate, 60; all-rac-α-tocopherol, 100; cyanocobalamin, 0·1; biotin, 6·0; folic acid, 10; retinyl acetate, 5000 IU; cholecalciferol, 2000 IU.
Shrimp rearing and experimental conditions
Juvenile L. vannamei were obtained from Chia-Tai Ningbo Company. Prior to the start of the feeding trial, the shrimp were reared in cement pools and fed a commercial feed (40 % protein, 8 % lipid; Yue-Hai Aquafeed Corp.) for 2 weeks to acclimatise to the experimental conditions. Juveniles (initial weight 1·33 (se 0·01) g) were randomly distributed into 300-litre cylindrical fibre-glass tanks filled with 250 litres of seawater at a stocking density of thirty shrimp per tank, and each experimental diet was randomly assigned to five replicate tanks. Shrimp were fed three times/d (daily ration of 6–8 % of biomass) at 08.00, 12.00 and 17.00 hours, with the rations in the morning and evening being 70 % of the total given. Shrimp in each tank were weighed every 2 weeks, and the daily ration was adjusted accordingly. Dead shrimp were immediately removed, weighed and recorded. All tanks were cleaned daily by siphoning out the waste material and exuviae, and over 70 % of the tank seawater was exchanged daily prior to the morning feed. The seawater in the tanks was provided with continuous aeration through air stones, and dissolved O2 level was not <6·0 mg/l. During the experiment, photoperiod was maintained on a natural cycle, the temperature was 26–30°C, salinity was 23–27 g/l, pH was 7·6–7·8 and ammonia nitrogen concentration was lower than 0·05 mg/l. Salinity, pH, dissolved O2 and ammonia nitrogen were measured by YSI Proplus (YSI, Yellow Springs). The duration of the feeding trial was 8 weeks.
Sample collection
A total of 120 juvenile L. vannamei were randomly sampled at the beginning of the feeding trial and frozen at −20°C for analysis of Zn in whole body. At the termination of the experiment, shrimp were fasted for 24 h before sampling. All shrimp from each tank were counted and weighed to determine survival, WG, specific growth rate, feed conversion ratio and feed intake. Furthermore, body length, whole-body and hepatopancreas weight from four shrimp in each tank were taken to calculate condition factor and hepatosomatic index. Five shrimp from each tank were used to analyse the Zn concentration in tissues (whole body, hepatopancreas and shell). Haemolymph samples from five shrimp in each tank were taken from the pericardial cavity using a 1-ml syringe, placed in 1·5-ml microfuge tubes and centrifuged at 4°C, 850 g for 10 min (Eppendorf centrifuge 5810 R). The supernatant was collected and stored at −80°C until analysis of haematological characteristics. Hepatopancreas samples were also collected and stored at −80°C until analysis of lipid metabolism-related parameters and gene expressions.
Proximate composition and mineral concentration analysis
Crude protein, crude lipid, ash and moisture contents of the diets and shrimp tissues (whole body, hepatopancreas and muscle) were analysed by standard methods of the AOAC(18). Crude protein (N × 6·25) was determined using the Dumas combustion method with an auto-protein analyzer (FP-528). Crude lipid was determined by the ether extraction method using Soxtec (Soxtec System HT6, Tecator). Moisture content was determined by drying the samples to a constant weight at 105°C, and ash content was determined in a muffle furnace at 550°C for 8 h.
Zn concentrations in tissues (whole body, hepatopancreas and shell), experimental diets and water were measured using Inductively Coupled Plasma Optical Emission Spectrometer (PE 2100DV, Perkin Elmer) in Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences. The concentration of Zn in seawater ranged from 1·8 to 2·0 μg/l among the five groups fed diets containing different Zn levels.
Haematological and hepatopancreas characteristic analysis
TAG, total cholesterol (CHOL), LDL-cholesterol and HDL-cholesterol contents in haemolymph were determined using an automatic chemistry analyzer (Hitachi 7600–110).
Hepatopancreas samples were homogenised on ice in nine volumes (w/v) of ice-cold physiological saline 8·9 g/ml and then centrifuged at 850 g for 10 min at 4°C. The resultant supernatant was collected, and aliquots were stored at −80°C until analysis. TAG, CHOL, LDL-cholesterol, HDL-cholesterol, lipase (LPS), NEFA, adiponectin (ADP), malondialdehyde (MDA), catalase and Cu/Zn superoxide dismutase (Cu/Zn SOD) were determined using the relevant diagnostic reagent kits (Nanjing Jiancheng Co.) according to the manufacturer’s instructions.
Total RNA extraction, reverse transcription and real-time PCR
Total RNA was extracted from 10 to 20 mg hepatopancreas with Trizol reagent (TaKaRa) following the manufacturer’s protocol. RNA was quantified by a NanoDrop spectrophotometer (Thermo Scientific NanoDrop 2000) at 260 and 280 nm, and RNA ratio (A260:A280) between 1·9 and 2·0 was used for further experiments. The isolated RNA quality was electrophoresed on a 1·2 % denatured agarose gel through the Molecular Imager® Gel Doc™ XR System (Bio-Rad) to check integrity. Complementary DNA (cDNA) was generated from 1000 ng of DNAase-treated RNA and synthesised using a Prime Script™ RT Reagent Kit with gDNA Eraser (perfect real-time) (TaKaRa) according to the manufacturer’s protocol, using Mastercycler nexus GSX1 PCR (Eppendorf). cDNA was diluted four times using RNA-free water.
The core fragments of all genes were obtained from the National Center for Biotechnology Information database. β-Actin (GenBank accession no. AF300705.2), a housekeeping gene whose expression was found to be unaffected by dietary treatment in the present experiment, was used as an endogenous reference to normalise the template amount. The gene-specific primers used for mRNA quantification by real-time PCR were designed by Primer Premier 5.0 and are shown in Table 2. All primers were synthesised by BGI (The Beijing Genomics Institute). The quantitative PCR was carried out in a quantitative thermal cycler system (Roche, Light cyclern96) using SYBR Green I (Roche). The amplification was performed in a ninety-six-well plate in a 20 µl reaction volume containing 10 μl of 2 × SYBR Green I Master Mix (Roche), 1 μl (each) gene-specific forward and reverse primers (10 μm), 6 μl diethyl pyrocarbonate (DEPC) water and 2 μl of diluted cDNA. The real-time PCR programme was 95°C for 2 min, followed by forty-five cycles of 95°C for 10 s, 58°C for 10 s and 72°C for 20 s. Standard curves were made with six different dilutions (2-, 4-, 8-, 16- 32- and 64-fold dilutions in triplicate) of the cDNA samples, and amplification efficiency was analysed according to the following equation E = 10(−1/slope)−1. During analysis, each sample was run in triplicate and the E-values ranged from 95·1 to 103·6 %. The relative quantification method was used to analyse data with expression levels of target genes calculated using the 2–ΔΔCt method as described by Livak & Schmittgen(Reference Livak and Schmittgen19).
Table 2. Real-time quantitative PCR primers for genes related to lipid and energy metabolism and β-actin of Litopenaeus vannamei

Tm, melting temperature; F, forward primer; R, reverse primer; srebp, sterol regulatory element-binding protein; fas, fatty acid synthase; ampk, 5′-AMP-activated protein kinase; ob-rb, leptin receptor; adipor, adiponectin receptor; cpt1, carnitine palmitoyl transferase 1; camkkβ, Ca/calmodulin-dependent protein kinase kinase; acc1, acetyl-CoA carboxylase 1; scd1, stearoyl-CoA desaturase; mcd, malonyl-CoA decarboxylase; cd36, cluster of differentiation 36; zip3, solute carrier family 39 member 3; zip9, solute carrier family 39 member 9; zip11, solute carrier family 39 member 11; zip14, solute carrier family 39 member 14.
Calculations
Parameters were calculated as follows:
-
WG (%) = 100 × (final body weight (g) − initial body weight (g))/initial body weight (g).
-
Specific growth rate (%/d) =100 × (Ln (final body weight (g)) − Ln (initial body weight (g)))/days.
-
Survival (%) = 100 × (final number of shrimp)/(initial number of shrimp).
-
Feed conversion ratio = feed consumption (g, dry weight)/(final body weight (g) − initial body weight (g)).
-
Feed intake (%/body weight day) = 100 × feed consumption (g, dry weight)/((initial body weight (g) + final body weight (g))/2)/days.
-
Hepatosomatic index (%) = 100 × (hepatopancreas wet weight (g))/(body wet weight (g)).
-
Condition factor (g/cm3) = 100 × body weight (g)/body length3 (cm3).
-
Deposition rate of Zn (%) = 100 ×(final body weight (g) × final whole shrimp of Zn (mg/kg) − initial body weight (g) × initial whole shrimp of Zn (mg/kg))/(feed consumption (g) × feed Zn content (mg/kg)).
Statistical analysis
Results are presented as mean values with their standard errors. Data were checked for normality and homogeneity of variances and were normalised when appropriate. Proportional data were arcsine square root transformed before statistical analyses. Mean values were compared through one-way ANOVA followed by Duncan’s multiple-range test. The level of significance was set at P < 0·05. All statistical analyses were conducted using the SPSS 20.0 software package (IBM Crop.) for Windows.
Results
Growth performance and morphometric index
Survival ranged from 92 to 94·7 %, and there were no significant differences among treatments (Table 3). Shrimp fed the diet containing 117·1 mg/kg Zn had higher WG and specific growth rate than those fed the other diets (P < 0·05). The lowest feed intake and feed conversion ratio were also observed in shrimp fed the diet supplemented with 117·1 mg/kg Zn. Moreover, shrimp fed the diet containing 46·4 mg/kg Zn had lower condition factor than those fed the other diets (P < 0·05). The hepatosomatic index was not significantly influenced by dietary Zn level.
Table 3. Effects of different dietary zinc levels on growth performance, feed utilisation and morphologic index of juvenile Litopenaeus vannamei
(Mean values with their standard errors for five determinations)

IBW, initial mean body weight; WG, weight gain; SGR, specific growth rate; FI, feed intake; FCR, feed conversion ratio; HSI, hepatosomatic index; CF, condition factor.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Zinc concentration in tissues and zinc deposition rate
Zn concentration in tissues and Zn deposition rate of L. vannamei are presented in Table 4. Zn concentration in whole body was not significantly affected by dietary Zn level. Shrimp fed the diets containing 117·1 and 136·8 mg/kg Zn had the highest Zn concentrations in hepatopancreas and shell among the treatments, and the lowest Zn concentrations in hepatopancreas and shell occurred in shrimp fed the basal diet (46·4 mg/kg Zn). The deposition rate of Zn significantly decreased as dietary Zn level increased from 46·4 to 136·8 mg/kg.
Table 4. Effects of different dietary zinc levels on zinc concentration in tissues (mg/kg, wet weight) and zinc deposition rate of juvenile Litopenaeus vannamei (Mean values with their standard errors for five determinations)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Proximate compositions of tissues
Moisture, protein, lipid and ash contents of muscle were not significantly influenced by dietary Zn level (Table 5). Shrimp fed the basal diet (46·4 mg/kg Zn) had lower ash content in whole body than those fed the other diets (P < 0·05); however, there were no significant differences of moisture, protein and lipid contents in whole body among all treatments. Lipid content in hepatopancreas significantly decreased as dietary Zn level increased from 46·4 to 136·8 mg/kg, whereas moisture, protein and ash contents in hepatopancreas were not significantly affected by dietary Zn level.
Table 5. Effects of different dietary zinc levels on proximate composition of whole body, hepatopancreas and muscle (%, wet weight) in juvenile Litopenaeus vannamei
(Mean values with their standard errors for five determinations)

a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Haematological metabolites and enzyme activity
Haematological metabolites related to lipid metabolism and health indicators are shown in Table 6. Haemolymph CHOL and HDL-cholesterol concentrations were significantly influenced by dietary Zn level (P < 0·05). Shrimp fed the diet containing 87·0 mg/kg Zn had the lowest CHOL and the highest HDL-cholesterol in haemolymph among all treatments. However, LDL-cholesterol and TAG concentrations and LPS activity in haemolymph were not significantly affected by dietary Zn level. In addition, shrimp fed the basal diet (46·4 mg/kg Zn) had the lowest Cu/Zn SOD and ceruloplasmin in haemolymph.
Table 6. Effects of different dietary zinc levels on haematological metabolites related to lipid metabolism and health indicators of juvenile Litopenaeus vannamei
(Mean values with their standard errors for five determinations)

CHOL, total cholesterol; LPS, lipase; CP, ceruloplasmin; Cu/Zn SOD, Cu/Zn superoxide dismutase.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Hepatopancreas biochemical parameters
TAG, CHOL, HDL-cholesterol, MDA, NEFA, ADP concentrations and LPS, Cu/Zn SOD activities in hepatopancreas were significantly influenced by dietary Zn level (Table 7). Shrimp fed the basal diet (46·4 mg/kg Zn) had the highest CHOL, TAG, HDL-cholesterol and NEFA concentrations in hepatopancreas among all treatments. In addition, shrimp fed the diet containing 136·8 mg/kg Zn had lower HDL-cholesterol and NEFA concentrations in hepatopancreas than those fed the basal diet (P < 0·05). Shrimp fed the diet supplemented with 117·1 mg/kg Zn had the highest ADP concentration in hepatopancreas. LPS activity in hepatopancreas was significantly higher in Zn inclusion groups compared with the basal group (P < 0·05), but LDL-cholesterol concentration in hepatopancreas was not affected by dietary Zn level. Shrimp fed the basal diet had the highest MDA concentration and the lowest Cu/Zn SOD activity in hepatopancreas, but catalase activity in hepatopancreas was not affected by dietary Zn level.
Table 7. Effects of different dietary zinc levels on hepatopancreas parameters related to lipid metabolism and health indicators of juvenile Litopenaeus vannamei
(Mean values with their standard errors for five determinations)

ADP, adiponectin; MDA, malondialdehyde; CAT, catalase.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Gene expression
Transcript level of genes involved in lipid and energy metabolism in hepatopancreas is shown in Figs. 1 and 2. Transcript levels of adipor, ob-rb and camkkβ were significantly up-regulated with increasing dietary Zn level, and shrimp fed the basal diet (46·4 mg/kg Zn) had the lowest expression levels of adipor, or-rb and camkkβ in hepatopancreas. In contrast, the opposite was the case for hepatopancreas sterol regulatory element-binding protein (srebp) mRNA level, with expression level of srebp being significantly down-regulated in shrimp fed the diet containing 77·2, 87·0, 117·1 and 136·8 mg/kg Zn (Fig. 1).

Fig. 1. mRNA levels of genes involved in lipid metabolism in the hepatopancreas of juvenile Litopenaeus vannamei fed the experimental diets. ![]() , 46·4 mg/kg zinc diet;
, 46·4 mg/kg zinc diet; ![]() , 77·2 mg/kg zinc diet;
, 77·2 mg/kg zinc diet; ![]() , 87·0 mg/kg zinc diet;
, 87·0 mg/kg zinc diet; ![]() , 117·1 mg/kg zinc diet;
, 117·1 mg/kg zinc diet; ![]() , 136·8 mg/kg zinc diet. Expression values are normalised by β-actin-expressed transcripts. Relative fold difference among treatments are presented as means (n 8), with their standard errors. a,b,c Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA).
, 136·8 mg/kg zinc diet. Expression values are normalised by β-actin-expressed transcripts. Relative fold difference among treatments are presented as means (n 8), with their standard errors. a,b,c Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA).

Fig. 2. mRNA levels of genes involved in energy metabolism in the hepatopancreas of juvenile Litopenaeus vannamei fed the experimental diets. ![]() , 46·4 mg/kg zinc diet;
, 46·4 mg/kg zinc diet; ![]() , 77·2 mg/kg zinc diet;
, 77·2 mg/kg zinc diet; ![]() , 87·0 mg/kg zinc diet;
, 87·0 mg/kg zinc diet; ![]() , 117·1 mg/kg zinc diet;
, 117·1 mg/kg zinc diet; ![]() , 136·8 mg/kg zinc diet. Expression values are normalised by β-actin-expressed transcripts. Relative fold difference among treatments are presented as means (n 8), with their standard errors. a,b,c Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA).
, 136·8 mg/kg zinc diet. Expression values are normalised by β-actin-expressed transcripts. Relative fold difference among treatments are presented as means (n 8), with their standard errors. a,b,c Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA).
Hepatopancreas transcript level of key enzymes involved in lipogenesis (fas, scd1), β-oxidation (carnitine palmitoyl transferase 1, cpt1) and lipolysis (cd36) was significantly affected by dietary Zn level (Fig. 1). The expression levels of fas and scd1 were significantly down-regulated as dietary Zn level increased from 46·4 to 136·8 mg/kg, with no significant differences in the expression of fas and scd1 were observed in shrimp fed the diets containing 87·0, 117·1 and 136·8 mg/kg Zn. However, the expression levels of cd36 and cpt1 were significantly up-regulated with increasing dietary Zn level, and shrimp fed the basal diet had lower expression of cd36 and cpt1 in hepatopancreas.
Dietary Zn level also affected the expression of genes involved in energy metabolism (Fig. 2). The expression levels of ampk and mcd in hepatopancreas were significantly up-regulated as dietary Zn level increased from 46·4 to 77·2 mg/kg, whereas there were no significant differences in the expression of ampk and mcd when dietary Zn level exceeded 87·0 mg/kg. Shrimp fed the diets containing 46·4 and 77·2 mg/kg Zn had higher expression of acc1 than those fed the other diets, while no significant difference in the expression of acc1 was observed in shrimp fed the diets with Zn above 87·0 mg/kg.
The mRNA expression of genes involved in the SLC39 family is shown in Fig. 3. The mRNA expression levels of zip3, zip9, zip11 and zip14 in hepatopancreas were significantly up-regulated as dietary Zn level increased. Shrimp fed the basal diet had lowest expression levels of zip3, zip9, zip11 and zip14.

Fig. 3. mRNA levels of genes involved in SLC39 family genes (zip3, zip9, zip11, zip14) in the hepatopancreas of juvenile Litopenaeus vannamei fed the experimental diets. ![]() , 46·4 mg/kg zinc diet;
, 46·4 mg/kg zinc diet; ![]() , 77·2 mg/kg zinc diet;
, 77·2 mg/kg zinc diet; ![]() , 87·0 mg/kg zinc diet;
, 87·0 mg/kg zinc diet; ![]() , 117·1 mg/kg zinc diet;
, 117·1 mg/kg zinc diet; ![]() , 136·8 mg/kg zinc diet. Expression values are normalised by β-actin-expressed transcripts. Relative fold difference among treatments are presented as means (n 8), with their standard errors. a,b,c Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA).
, 136·8 mg/kg zinc diet. Expression values are normalised by β-actin-expressed transcripts. Relative fold difference among treatments are presented as means (n 8), with their standard errors. a,b,c Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA).
Discussion
The dietary requirement for Zn has been quantified for a variety of marine shrimp fed semi-purified or commercial diets. The requirement of Zn was demonstrated to be substantially higher when fed with practical diets compared with purified diets(Reference Delbert, Gatlin and Wilson20–Reference Satoh, Tabata and Izume22). In the present study, 117·1 mg/kg Zn improved growth performance and feed utilisation, and higher or lower dietary Zn reduced growth and feed efficiency of juvenile L. vannamei, similar to results reported previously for this shrimp species(Reference Davis, Lawrence and Gatlin16). Davis & Lawrence(Reference Davis, Lawrence and Gatlin16) reported that WG of Pacific white shrimp Penaeus vannamei was significantly affected by dietary Zn levels from 18 to 60 mg/kg in shrimp fed a casein/gelatin-based semi-purified diet. In that study, best growth was obtained in P. vannamei fed a diet supplemented with 33 mg/kg Zn, lower than the 117·1 mg/kg Zn found in the present study. This may be due to different feed ingredients. The fishmeal and plant protein sources used in diets in the present study may contain anti-nutritional factors (e.g. tri-calcium phosphate from hard tissues and phytic acid from plant meals), which would form complexes with Zn and inhibit Zn bioavailability(Reference Apines, Satoh and Kiron23–Reference Satoh, Tabata and Izume25). Thus, higher levels of supplemented Zn were required to overcome the depressed bioavailability caused by the presence of anti-nutritional factors in the commercial diet(Reference Davis, Lawrence and Gatlin16,Reference Gatlin and Phillips26) .
In addition to growth indicators, tissue Zn deposition is also used to evaluate the Zn nutritional status of animals(Reference Forbes, Parker and Erdman27). Jeng & Sun(Reference Jeng and Sun28) demonstrated that Zn firstly accumulated in the digestive tract, followed by skeletal tissue, and then skin and muscle in common carp cyprinus carpio, indicating that Zn in these tissues was useful indices for evaluating Zn status. In the present study, incremental dietary Zn significantly increased Zn concentrations in hepatopancreas and shell and did not reach a plateau implying that increasing dietary Zn level promoted Zn deposition in these tissues. These results were in accordance with previous studies on grass shrimp P. monodon (Reference Shiau and Jiang17) and juvenile grouper Epinephelus malabaricus (Reference Chen, Cheng and Hu29). Shiau & Jiang(Reference Shiau and Jiang17) reported that Zn concentration in hepatopancreas ranged from 0·6 to 21·8 μg/g when dietary Zn level increased from 7 to 127 mg/kg in juvenile grass shrimp. Chen et al. (Reference Chen, Cheng and Hu29) reported that Zn concentrations in vertebra and scale significantly increased with increasing dietary Zn level in juvenile grouper. However, no differences were found in whole-body Zn concentrations in this study, suggesting that this was not a sensitive indicator for evaluating Zn status in L. vannamei. While Zn involved in bone metabolism by stimulating collagen synthesis to increase Ca content in bone(Reference Oner, Bhaumick and Bala30), invertebrates mainly deposit minerals in exoskeleton(Reference Chen, Cheng and Hu29). Therefore, the higher the ash content in whole body of shrimp fed the diet supplemented with Zn might be due to the increased Ca and Zn in shell.
Zn plays an important role in enhancing antioxidant status and regulating lipid metabolism(Reference Olechnowicz, Tinkov and Skalny5). In the present study, clinical haematological parameters demonstrated that dietary Zn could improve lipolysis and antioxidant responses by decreasing haematological CHOL and increasing ceruloplasmin and Cu/Zn SOD. In addition, the lipid content of hepatopancreas was significantly reduced by increased dietary Zn level. Similar results were reported in previous studies, in which waterborne Zn exposure decreased anterior intestine TAG content in juvenile goby S. hasta, which was considered to be due to the down-regulation of lipogenesis and increased lipolysis(Reference Ling, Luo and Chen31). Similarly, dietary Zn of about 156 mg/kg reduced lipid content in liver and muscle and increased activities of CPT1 and lipoprotein LPS of liver in yellow catfish P. fulvidraco (Reference Zheng, Luo and Hu11). Furthermore, Wei et al. (Reference Wei, Luo and Hogstrand12) reported that Zn reduced hepatic lipid deposition and activated lipophagy via Zn2+/MTF-1/PPAα and Ca2+/CaMKKβ/AMPK signalling pathways. In contrast, 8-week chronic Zn exposure induced lipid accumulation in yellow catfish P. fulvidraco (Reference Zheng, Luo and Liu32). Zheng et al. (Reference Zheng, Luo and Wei33) also reported that dietary Zn at 155 mg/kg induced Zn accumulation in liver, muscle and whole body of yellow catfish P. fulvidraco. Thus, contradictory results on the influence of dietary Zn on lipid metabolism have been reported in different fish species, which may reflect differences due to species, trial duration or other experimental conditions. In the present study, some hepatic lipid metabolism-related parameters were determined to confirm the relationship between Zn and lipid metabolism. TAG are major constituents of body lipid in humans and other animals, and TAG content can reflect the state of lipid metabolism(Reference Ling, Luo and Chen31). Cholesterol is an essential structural component of animal cell membranes, and excessive accumulation of cholesterol in cells results in disorders of lipid metabolism(Reference Maxfield and Tabas34). Lipid can be broken down to produce energy by LPS which catalyses the hydrolysis of TAG into glycerol and NEFA(Reference Svendsen35,Reference Zechner, Strauss and Haemmerle36) , then ADP breaks down NEFA(Reference Diez and Iglesias37). A pharmacological study showed that administration of ADP to mice resulted in lower body weight associated with a reduction in NEFA and TAG(Reference Fruebis, Tsao and Javorschi38). The results of the present study showed that dietary Zn supplementation reduced hepatopancreas CHOL, HDL-cholesterol, TAG and NEFA and increased ADP content and LPS activity, suggesting that Zn supplementation could accelerate lipid breakdown by hydrolysing TAG through LPS activity to release NEFA, simultaneously increasing the content of ADP and promoting fatty acid oxidation, with the final manifestation being reduced lipid content of the hepatopancreas.
Many studies have demonstrated that the antioxidant defence system is associated with the physical health status of animals by inhibiting oxidation and removing excess reactive oxygen species (Reference Li, Ma and Liu39–Reference Ding, Zhang and Ye41). Two of the essential antioxidant enzymes are catalase and ceruloplasmin. Catalase catalyses the decomposition of hydrogen peroxide to water and oxygen, protecting the cell from oxidative damage(Reference Chelikani, Fita and Loewen42), and ceruloplasmin can serve as a scavenger of superoxide radicals(Reference Goldstein, Kaplan and Edelson43). Reactive oxygen species can degrade polyunsaturated lipids forming MDA, a marker for lipid oxidative stress(Reference Lushchak44). In addition, Zn is an active central ion for Cu/Zn SOD, which converts highly reactive superoxide anion radical (O2−) to less reactive hydrogen peroxide (H2O2)(Reference Li, Jiao and Chen45). In the present study, shrimp fed the basal diet had the lowest Cu/Zn SOD activity and the highest MDA content in hepatopancreas, indicating that dietary Zn supplementation could prevent the accumulation of free radicals and improve antioxidant activities in hepatopancreas of juvenile L. vannamei.
AMPK is one of the most well-recognised modulators of the guardians in lipid homoeostasis(46,47) . It can be activated by CaMKK(Reference Hurley, Anderson and Franzone48,Reference Woods, Dickerson and Heath49) in response to increased intracellular Ca2+. Activated Ca2+/CaMKKβ phosphorylates the subunit of AMPK and forms a multimeric protein complex comprising (Ca2+/CaMKKβ)/AMPK(Reference Green, Anderson and Means50,Reference Zhao, Huang and Zhang51) . In this process, Zn2+ is a significant factor in regulating cytosolic Ca2+ homoeostasis, which is mediated by triggering sensitive Ca2+ pumps in the endoplasmic reticulum(Reference Wei, Luo and Hogstrand12,Reference Sensi, Yin and Carriedo52) . While Zn2+ absorption and homoeostasis depend on ZIP transporters (known as SLC39 family), which promote uptake of extracellular Zn2+ and release of vesicular Zn2+ into the cytosol, eventually leading to increased free Zn2+ in the cytoplasm(Reference Jeong and Eide53,Reference Schweigel-Röntgen54) . At the whole-body level, AMPK is regulated by a diverse range of hormones, including leptin(Reference Minokoshi, Kim and Peroni55) and ADP(Reference Yamauchi, Kamon and Minokoshi56). Yamauchi et al. (Reference Yamauchi, Kamon and Ito57) reported that the ADP receptor (AdipoR) serves as the receptor for full-length ADP and activates AMPK. Iwabu et al. (Reference Iwabu, Yamauchi and Okadaiwabu58) showed that ADP induces extracellular Ca2+ influx by ADP receptor, which was necessary for subsequent activation of Ca2+/CaMKKβ and AMPK.
Under the condition of low energy, AMPK phosphorylates specific enzymes to increase ATP generation and decrease ATP consumption which by accelerating the decomposition of fatty acids and inhibiting the synthesis of fatty acids(Reference Sébastien and Shaw59). AMPK inhibits fatty acid synthesis by inducing the inhibitory phosphorylation of acetyl-CoA carboxylase (ACC) and SREBP(46). SREBP is a family of transcription factors that regulate the expression of enzymes required for endogenous fatty acid synthesis, affecting multiple genes such as ACC1, FAS and SCD1(Reference Zhang, Hao and Zhang60–Reference Xu, Wang and Chang63). AMPK is also associated with lipid catabolism. In vivo, AMPK activated CD36 (also known as fatty acid translocase), which increases cellular fatty acid uptake(Reference Habets, Coumans and Hasnaoui64). Once inside cells, fatty acids are transported into the mitochondria interior by CPT1 for β-oxidation and accompanied with the production of large amounts of ATP(46,Reference Townsend, An and Lynes65) .
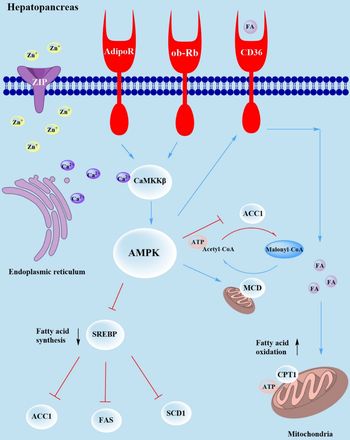
AMPK also directly involved in energy metabolism by increasing intracellular acetyl-CoA content. Bergeron et al. (Reference Bergeron, Ren and Cadman66) reported that decreased malonyl-CoA occurs as a result of the action of malonyl-CoA decarboxylase (MCD), which prevents acetyl-CoA conversion to malonyl-CoA, which may be regulated by AMPK. In the present study, mRNA expression levels of the SLC39 family such as zip3, zip9, zip11 and zip14 were significantly higher in shrimp fed dietary Zn supplementation compared with the basal group, indicating that more Zn2+ was transferred into the cell. However, intracellular Zn2+ promotes the release of Ca2+ from the endoplasmic reticulum, followed by Ca2+/CaMKKβ/AMPK signalling pathway induced lipophagy, which was confirmed by increased mRNA levels of camkkβ and ampk. Then, activated ampk inhibited the transcription factor srebp binding with fas, acc1 and scd1, which ultimately led to decreased expression of these genes. In the meantime, activated ampk promoted the expression of cd36 and cpt1, enhancing fatty acid oxidation to produce ATP. Finally, ampk inhibited the expression of acc1 and promoted the expression of mcd, increasing the content of intracellular acetyl-CoA that, in turn, might affect energy metabolism (Fig. 4).

Fig. 4. Working model of how Zn2+ regulates lipophagy via the Ca2+/CaMKKβ/AMPK axes. The blue lines indicate promotion, and the red lines indicate suppression. Extracellular Zn2+ activates SLC39 family genes (zip3, zip9, zip11, zip14) which increases intracellular Zn2+, which promotes the release of Ca2+ and activation ampk via camkkβ, reducing the mRNA expression of fas, acc1 and scd1, resulting in inhibition of fatty acid synthesis. Meanwhile, ampk activates cd36, which increase the mRNA expression of cpt1 and enhancement of fatty acid oxidation. Moreover, activated ampk promotes the mRNA expression of mcd, which might affect energy metabolism.
Conclusion
In the present study, dietary Zn level affected growth performance, Zn deposition in tissues (hepatopancreas and shell) and lipid metabolites, and we identified a novel mechanism of Zn-induced lipolysis. Dietary deficient or excessive Zn retarded growth and reduced feed utilisation. Furthermore, incremental dietary Zn levels reduced total lipid, NEFA, CHOL and TAG levels in hepatopancreas. Importantly, dietary Zn-induced lipolysis was dependent on the activation of Zn transporters (SLC39 family) and involved up-regulation of the Ca2+/CaMKKβ/AMPK pathway. Further studies on the relationship between dietary Zn and energy products, cytokines, adipokines and receptors are required to fully explain the role of Zn in energy metabolism. Modulation of Zn status may become a new target for the prevention and treatment of metabolic disorders. Deeper knowledge and understanding of the physiological functions of Zn transporters and the ability to control their activity may be an important factor in the mechanism of coordinated lipid metabolism in hepatopancreas or liver mediated by Zn-induced lipophagy.
Acknowledgements
The authors are grateful to Lihui Zhu for helping with chemical analysis.
This study was supported by National Key R & D Program of China (2018YFD0900400), China Agriculture Research System (CARS-48), Nature Science Foundation of Zhejiang Province (LY17C190002), Key Research Program of Zhejiang Province of China (2018C02037) and Zhejiang Aquaculture Nutrition & Feed Technology Service Team (ZJANFTST2017-2). This research was also sponsored by the K. C. Wong Magna Fund in Ningbo University.
B. S. formulated the research question, designed the study, carried out the study, analysed the data and wrote the article. M. J. designed the study, assisted in the correction and developed the questions. M. B. B. developed the questions and revised the manuscript. D. R. T. assisted in developing the research questions and revising the manuscript. Q. Z. formulated the research question, designed the study and revised the manuscript. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.