The rapidly developing aquaculture industry is accompanied by a growing demand for fish oil (FO)(Reference Pickova and Mørkøre1). Due to the high price and limited supply of FO, terrestrial oils (TO), which mainly consist of vegetable oil and animal fat, are commonly used to replace FO in aquafeed industry(Reference Turchini, Torstensen and Ng2). Many studies have demonstrated that TO can partially replace FO without significantly decreasing growth performance or feed utilisation of European sea bass (Dicentrarchus labrax L.)(Reference Mourente, Good and Thompson3), gilt-head sea bream (Sparus aurata)(Reference Montero, Mathlouthi and Tort4), Atlantic salmon (Salmo salar)(Reference Bell, McEvoy and Tocher5), turbot (Scophthalmus maximus L.)(Reference Peng, Xu and Mai6) and silvery-black porgy (Sparidentex hasta)(Reference Mozanzadeh, Agh and Yavari7). However, the decrease in growth and non-specific immunity has also been repeatedly reported in fish when dietary FO is excessively replaced by TO, probably due to the unbalanced dietary fatty acid profile(Reference Emre, Kurtoğlu and Emre8,Reference Torrecillas, Mompel and Caballero9) .
All fish species have an essential demand of both n-3 and n-6 PUFA(Reference Sargent, McEvoy and Bell10). Generally, freshwater and diadromous fish species require linoleic acid (LNA) and α-linolenic acid (ALA), whereas marine fish species strictly require long-chain PUFA (LC-PUFA), such as EPA, DHA and arachidonic acid(Reference Sargent, Bell and McEvoy11,Reference Tocher12) . Furthermore, an appropriate ratio of n-3:n-6 PUFA is important for the growth and health performance of Malabar grouper (Epinephelus malabaricus)(Reference Wu and Chen13), salmon(Reference Menoyo, Lopez-Bote and Diez14,Reference Berge, Witten and Baeverfjord15) and yellow catfish (Pelteobagrus fulvidraco)(Reference Tan, Luo and Xie16). In addition to the need for PUFA, fish also need an appropriate amount of SFA and MUFA, which are good dietary energy sources for fish(Reference Turchini, Torstensen and Ng2,Reference Henderson and Sargent17) . Animal fat contains a high percentage of SFA and MUFA, and it is a cheap alternative lipid resource for aquafeed industry(Reference Monteiro, Matos and Ramos18). However, few studies have investigated the effects of a relatively appropriate dietary fatty acid profile on growth performance, metabolism and health of fish when dietary FO is replaced by a high level of TO.
Large yellow croaker (Larimichthys crocea) is an economically important fish in China. When dietary FO is excessively replaced by TO, inflammatory response and abnormal lipid deposition in large yellow croaker often cause huge economic losses(Reference Yan, Liao and Wang19,Reference Ai, Zhao and Mai20) . Progress is urgently needed to find effective additives to reduce inflammation and abnormal lipid deposition in fish fed the diet with a high level of TO. l-Carnitine plays a crucial role in shuttling long-chain fatty acids into mitochondria(Reference Harpaz21) and reduces abnormal lipid deposition(Reference Li, Li and Zhang22,Reference Chen, Sun and Liang23) . l-Carnitine can also increase the immunocompetence of several fish species to better cope with biotic and abiotic stresses(Reference Schreiber, Becker and Bresler24–Reference Schlechtriem, Bresler and Fishelson26). However, few studies have elucidated the effects of a high level of dietary TO supplemented with l-carnitine on fish. Therefore, the purpose of this study was to determine the effects of a high level of dietary TO supplemented with l-carnitine on growth, lipid deposition and inflammation of large yellow croaker for the wide usage of TO in aquafeed industry.
Methods
Animal ethics
The protocols for animal care and handling were conducted in strict accordance with the Management Rule of Laboratory Animals (Chinese order no. 676 of the State Council, revised 1 March 2017). The present study was approved by the Institutional Animal Care and Use Committee of the Ocean University of China.
Diets formulation
Three iso-nitrogenous (42 % crude protein) and iso-lipidic (12 % crude lipid) experimental diets were formulated, which included FO (the control group), 75 % TO (75 % FO was substituted by TO, dietary lipid source consisted of equal amounts of FO, soyabean oil, linseed oil and pork lard) and 75 % TOC (75 % TO supplemented with 800 mg/kg l-carnitine). 75 % FO was substituted by TO to preserve an appropriate fatty acid profile in the 75 % TO diet. The addition of l-carnitine was based on Sang’s(Reference Sang, Deng and Shentu27) study. Ingredients were purchased from Qingdao Great Seven Biotechnology Co. Ltd. Each diet was supplemented with vitamin and mineral premixes, an attractant mixture and a mold inhibitor. Procedures for making diets and storage of experimental diets were in accordance with the protocol described by Ai et al.(Reference Ai, Mai and Tan28). Ingredients of the three experimental diets (Table 1) and fatty acid profile of oil sources and diets (Table 2) were given in detail.
Table 1. Formulation and chemical proximate analysis of the experimental diets (% dry weight)
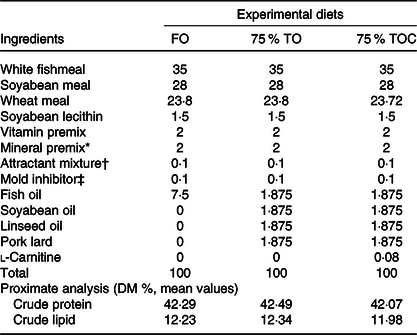
FO, fish oil; 75 % TO, 75 % fish oil substituted by terrestrial oils; 75 % TOC, 75 % TO supplemented with 800 mg/kg l-carnitine.
* The mixture of mineral mixture and vitamin mixture was purchased from Qingdao Master Biotechnology Co. Ltd.
† Attractant: mixture of 50 % glycine acid and 50 % betaine by weight.
‡ Mold inhibitor: mixture of 50 % calcium propionic acid and 50 % fumaric acid by weight.
Table 2. Fatty acid profile of oil sources and the experimental diets (% total fatty acids)*
(Mean values)
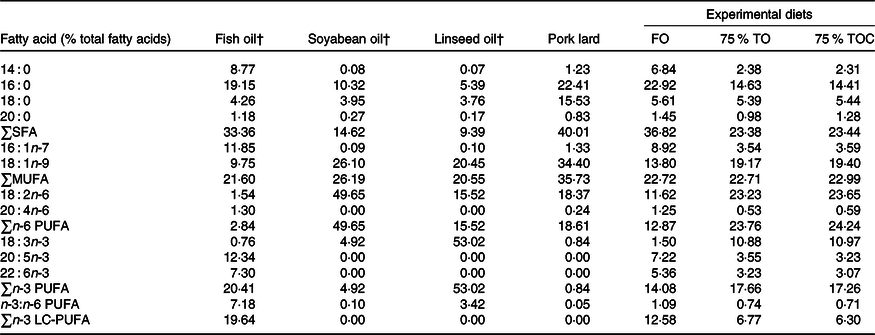
FO, fish oil; 75 % TO, 75 % fish oil substituted by terrestrial oils; 75 % TOC, 75 % TO supplemented with 800 mg/kg l-carnitine; LC-PUFA, long-chain PUFA.
* The contents of some fatty acids are minor and not detected, such as C20 : 0, C22 : 0, C24 : 0, C14 : 1, C20 : 1n-9, C22 : 1n-11, C20 : 2n-6, C18 : 3n-6, C20 : 3n-6, C18 : 4n-3 and C22 : 5n-3.
† Fatty acid profile of fish oil, soyabean oil and linseed oil is according to Li et al. (Reference Li, Pang and Xiang29) supplemental data.
Fish and experimental procedures
Large yellow croaker juveniles were obtained from Ningde Fufa Fishery Co. Ltd. Before the formal experiment, experimental fish were hand-fed twice daily (05.30 and 17.30 hours) to acclimatise the fish to the diet and environment for 2 weeks. Fish of disease-free and homogenous sizes (initial body weight 15·84 (sem 0·12) g) were fasted for 24 h. Then, 720 fish were randomly allocated to twelve floating net cages (1·0 m × 1·0 m × 2·0 m). Fish were hand-fed to apparent satiation, twice daily for 70 d. Each diet was randomly allocated to four replicate cages (sixty fish per cage). During the rearing period, the dissolved oxygen content was approximately 7·0 mg/l. Salinity ranged from 25·0 to 32·0 g/l and water temperature from 21·5 to 31·0°C.
Sample collection and analysis of growth data
At the termination of the experiment, fish were starved for 24 h and anaesthetised with MS222 (1:10 000; Sigma). Then, the number and weight of fish were determined for analyses of survival rate and final body weight. The wet weight of the liver, visceral and body and the length of six fish per cage were determined for analyses of morphometric parameters. The muscle and liver samples of the above six fish per cage were collected into 5 ml centrifuge tubes and stored at –80°C for compositional and fatty acid profile analyses. Blood samples of six fish per cage were collected from the caudal vein with a 1 ml syringe and clotted at room temperature to obtain serum samples for biochemical analyses. Liver samples were collected from twelve fish per cage, which contained six fish after serum collection. Samples were immediately frozen in liquid N2 and stored at –80°C for analyses of antioxidant capacity, enzyme activity and gene expression.
Proximate composition and fatty acid profile analyses
DM was analysed by drying whole fish samples to a constant weight at 105°C. Crude protein and crude lipid contents of ingredients, diets and fish bodies were measured in accordance with the standard methods of the Association of Official Analytical Chemists (AOAC, 1995)(30). Fish tissue and diet samples were freeze-dried in a lyophilised chamber (Alpha 1-4 LDplus; Christ) to analyse the moisture content. Lipid contents of freeze-dried liver and muscle samples were determined by chloroform/methanol (v/v, 2:1) following the procedures described by Folch et al. (Reference Folch, Lees and Sloane Stanley31). Fatty acid profile of oils and freeze-dried samples was measured according to procedures described by Metcalfe et al.(Reference Metcalfe, Schmitz and Pelka32) with minor modifications(Reference Zuo, Ai and Mai33). Fatty acid profile was separated and determined by a HP6890 gas chromatograph (Agilent Technologies Inc.). Results were shown as the percentage of each fatty acid to total fatty acid content.
Serum parameters and antioxidant capacity analysis
Contents of serum total cholesterol, total TAG, LDL-cholesterol and HDL-cholesterol and activities of serum alanine transaminase and aspartate aminotransferase were analysed with matching commercial reagent kits (Mindray Bio Medical Co. Ltd) by an automatic biochemical analyzer (BS180; Mindray). Activities of catalase, superoxide dismutase, total antioxidant capacity (T-AOC) and the content of malondialdehyde in the liver of fish were determined by commercial kits (Nanjing Jiancheng Bioengineering Institute).
Activities of carnitine palmitoyltransferase-I and acyl-CoA oxidase
Mitochondria was extracted, and its integrity was assessed according to procedures described by Suarez & Hochachka(Reference Suarez and Hochachka34) and Aprille & Asimakis(Reference Aprille and Asimakis35), respectively. CPT-I activity was determined according to the method of Bieber & Fiol(Reference Bieber and Fiol36) with some modifications(Reference Chen, Luo and Chen37,Reference Zheng, Luo and Liu38) . Briefly, CPT-I activity was determined by the CoA-SH formation in the 5’,5’-dithio-bis-(2-nitrobenzoic acid) (Sigma) reaction from palmitoyl-CoA (Sigma) at 412 nm. Results were expressed as 1 μmol of product formed per min per mg of mitochondrial protein at 25°C. Protein concentration of extracted samples was determined according to procedures of Bradford Protein Assay Kit (Beyotime Biotechnology).
Peroxisomal ACO activity was determined by the H2O2-dependent oxidation of 2’,7’-dichlorofluorescine (Sigma) according to the method of Small et al. (Reference Small, Burdett and Connock39). The reaction medium consisted of 11 mm potassium phosphate buffer (pH 7·4) (Sangon biotech), 50 μm horseradish peroxidase type II (Sigma), 0·05 mm 2’,7’-dichlorofluorescine (Sigma), 40 mm 3-Amino-1,2,4-triazole (Sigma) and 0·02 % Triton-X 100 (Sangon biotech), and the reaction was initiated by the addition of 30 μm palmitoyl-CoA (Sigma). The assay medium contained 10–40 mg of protein in a total volume of 1 ml.
Total RNA extraction, cDNA synthesis and real-time quantitative PCR
Total RNA was extracted from the liver of experimental fish using TRIzol reagent (Takara), and the quality and quantity of RNA were detected by a 1·2 % denaturing agarose gel and a NanoDrop spectrophotometer (Thermo Scientific), respectively. The extracted RNA was reversely transcribed to cDNA by PrimeScript™ RT reagent Kit (Takara). β-Actin and glyceraldehyde-3-phosphate dehydrogenase (gapdh) were used as reference genes to normalise the expression levels of genes(Reference Zheng, Zeng and Shen40). The real-time quantitative PCR primers (Table 3) of candidate genes were designed based on nucleotide sequences of large yellow croaker. The real-time quantitative PCR was performed in a quantitative thermal cycler (Mastercycler ep realplex; Eppendorf). The volume and procedure of real-time quantitative PCR were carried out as described previously(Reference Li, Ji and Cui41). Only one PCR product in these reactions was confirmed by melting curve analysis at the end of each reaction. Standard curves were generated with 4-fold serial dilutions (in triplicate) of cDNA sample, and the amplification efficiency was analysed by the equation: E = 10(–1/slope) − 1(Reference Nolan, Hands and Bustin42). The amplification efficiencies of all detected genes ranged between 0·95 and 1·04. The expression levels of all genes were calculated using the comparative Ct method (2–ΔΔCt method) as described by Livak & Schmittgen(Reference Livak and Schmittgen43).
Table 3. Sequences of the PCR primers used in this study

acc1, acetyl-CoA carboxylase 1; aco, acyl-CoA oxidase; arg-1, arginase-1; cd36, cluster of differentiation 36; cox-2, cyclo-oxygenase-2; cpt-I, carnitine palmitoyltransferase 1; dgat2, diacylglycerol acyltransferase 2; elovl4, long-chain fatty acid protein 4; elovl5, elongation of very long-chain fatty acid protein 5; fad6, fatty acyl desaturase 6; fas, fatty acid synthase; fatp1, fatty acid transport protein 1; gapdh, glyceraldehyde-3-phosphate dehydrogenase; hl, hepatic lipase; ifnγ, interferon γ; mtp, microsomal TAG transfer protein; scd1, stearoyl-CoA desaturase 1; srebp1, sterol-regulatory element binding protein 1.
Statistical analyses
All data analyses were processed by SPSS 19.0 (IBM). Results were shown as mean values with their standard errors. Statistics were performed to a one-way ANOVA and followed by Tukey’s test. Statistics with P < 0·05 were considered to be significant.
Results
Survival and growth performance
The survival rate of fish ranged from 87·92 to 90·83 %, but no significant differences were found among dietary treatments (P > 0·05). The dietary oil source markedly affected specific growth rate and feed efficiency ratio of fish in the present study, with specific growth rate and feed efficiency ratio significantly increased in fish fed diets with 75 % TO and 75 % TOC compared with the control group (P < 0·05). Hepatosomatic index was dramatically increased in fish fed the diet with 75 % TO compared with the control group (P < 0·05). When the 75 % TO diet was supplemented with 800 mg/kg l-carnitine, a decreasing trend (from 2·14 to 2·07) was observed in hepatosomatic index of fish compared with fish fed the diet with 75 % TO. There was no significant difference in feed intake, viscerosomatic index or condition factor of fish among dietary treatments (P > 0·05) (Table 4).
Table 4. Growth and somatic parameters of large yellow croakers
(Mean values with their standard errors; n 4)
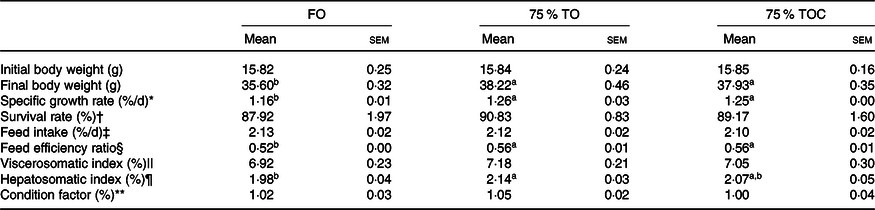
FO, fish oil; 75 % TO, 75 % fish oil substituted by terrestrial oils; 75 % TOC, 75 % TO supplemented with 800 mg/kg l-carnitine.
a,b Mean values in a row sharing the same superscript letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
* Specific growth rate (%/d) = (Ln (final body weight) – Ln (initial body weight)) × 100/duration of experimental days.
† Survival rate (%) = 100 × final fish number/initial fish number.
‡ Feed intake (%/d) = 100 × dry feed fed in g/((final body weight + initial body weight)/2)/duration of experimental days.
§ Feed efficiency ratio = wet weight gain in g/dry feed fed in g.
‖ Viscerosomatic index (%) = 100 × visceral wet weight/final body weight.
¶ Hepatosomatic index (%) = 100 × liver wet weight/final body weight.
** Condition factor (%) = 100 × final body weight/(final body length3).
Body composition analysis
There was no significant difference in the moisture, lipid or protein contents of whole fish body among dietary treatments (P > 0·05). Fish fed the diet with 75 % TO had markedly higher lipid content of the liver than the control group (P < 0·05). However, the lipid content of the liver was decreased in fish fed the diet with 75 % TOC compared with 75 % TO (P < 0·05). No significant difference was found in lipid content of the liver in fish fed the diet with 75 % TOC and the control group. The moisture was markedly decreased in the liver of fish fed the diet with 75 % TO compared with the control group (P < 0·05). However, the moisture was dramatically increased in the muscle of fish fed the diet with 75 % TOC compared with 75 % TO and the control group (P < 0·05) (Table 5).
Table 5. Body composition analysis of large yellow croakers
(Mean values with their standard errors; n 4)
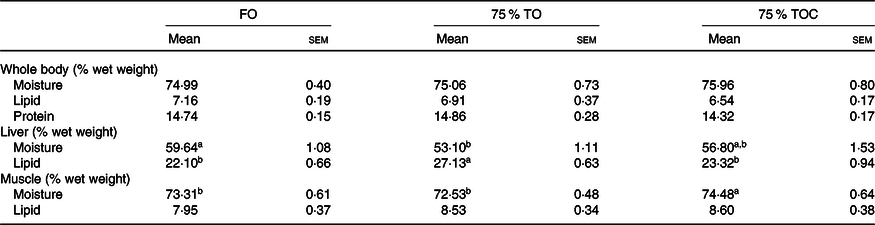
FO, fish oil; 75 % TO, 75 % fish oil substituted by terrestrial oils; 75 % TOC, 75 % TO supplemented with 800 mg/kg l-carnitine.
a,b Mean values in a row sharing the same superscript letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
Fatty acid profile in the liver
The contents of C14 : 0, C16 : 0, C16 : 1n-7, C20 : 4n-6 (arachidonic acid), C20 : 5n-3 (EPA), C22 : 6n-3 (DHA), SFA, n-3 LC-PUFA and the ratio of n-3:n-6 PUFA were dramatically decreased in the liver of fish fed diets with 75 % TO and 75 % TOC compared with the control group (P < 0·05). Furthermore, the contents of C18 : 1n-9, C18 : 2n-6, C18 : 3n-3 and n-6 PUFA were significantly increased in the liver of fish fed diets with 75 % TO and 75 % TOC compared with the control group (P < 0·05). The supplementation of 800 mg/kg l-carnitine in the 75 % TO diet significantly decreased the content of C18 : 3n-3, DHA and n-3 LC-PUFA in the liver of fish compared with fish fed the diet with 75 % TO (Table 6).
Table 6. Fatty acid profile (% total fatty acids) in the liver of large yellow croakers
(Mean values with their standard errors; n 4)*

FO, fish oil; 75 % TO, 75 % fish oil substituted by terrestrial oils; 75 % TOC, 75 % TO supplemented with 800 mg/kg l-carnitine; LC-PUFA, long-chain PUFA.
a,b,c Mean values in a row sharing the same superscript letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
* Some fatty acids, of which the contents are minor, trace amount or not detected, such as C20 : 0, C22 : 0, C24 : 0, C14 : 1, C20 : 1n-9, C22 : 1n-11, C20 : 2n-6, C18 : 3n-6, C20 : 3n-6, C18 : 4n-3, C22 : 5n-3, are not listed in the table.
Serum biochemical indexes
The content of TAG was dramatically increased in fish fed the diet with 75 % TO compared with the control group (P < 0·05). The content of HDL-cholesterol was significantly decreased in fish fed the diet with 75 % TO compared with the control group (P < 0·05). The content of LDL-cholesterol was significantly decreased, but the content of HDL-cholesterol was significantly increased in fish fed the diet with 75 % TOC compared with 75 % TO (P < 0·05). No significant difference was found in activities of serum aspartate aminotransferase or alanine transaminase in fish among dietary treatments (P > 0·05) (Table 7).
Table 7. Serum biochemical indexes and enzyme activities of large yellow croakers
(Mean values with their standard errors; n 4)
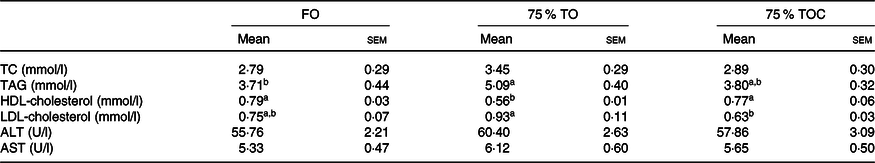
FO, fish oil; 75 % TO, 75 % fish oil substituted by terrestrial oils; 75 % TOC, 75 % TO supplemented with 800 mg/kg l-carnitine; TC, total cholesterol; ALT, alanine transaminase; AST, aspartate aminotransferase.
a,b Mean values in a row sharing the same superscript letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
Antioxidant capacity
A decreasing trend (from 1·16 to 1·03) was found in the activity of T-AOC (Fig. 1(C)) in fish fed the diet with 75 % TO compared with the control group, but no significant difference was found between the two treatments (P > 0·05). The supplementation of 800 mg/kg l-carnitine in the 75 % TO diet significantly increased the activity of superoxide dismutase (Fig. 1(B)) and T-AOC (Fig. 1(C)) in fish compared with fish fed the diet with 75 % TO (P < 0·05). No significant differences were found in the content of malondialdehyde (Fig. 1(A)) or the activity of catalase (Fig. 1(D)) in fish among dietary treatments (P > 0·05).
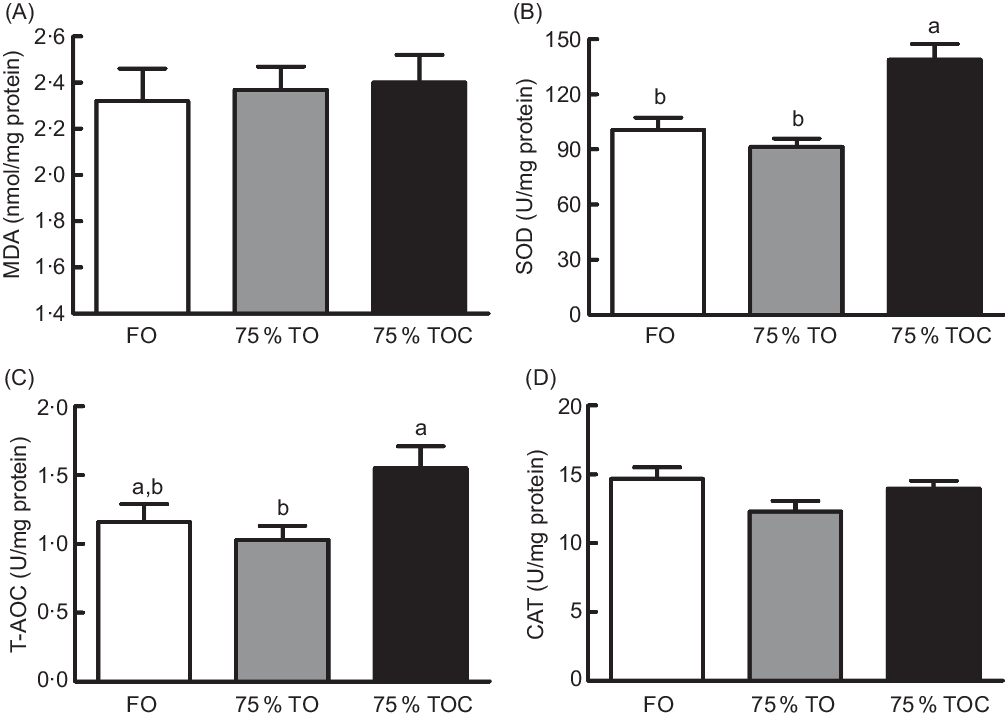
Fig. 1. Concentration of (A) malondialdehyde (MDA) and activities of (B) superoxide dismutase (SOD), (C) total antioxidant capacity (T-AOC) and (D) catalase (CAT) in the liver of large yellow croakers. FO, fish oil; 75 % TO, 75 % fish oil substituted by terrestrial oils; 75 % TOC, 75 % TO supplemented with 800 mg/kg l-carnitine. Values are means (n 4), with their standard errors represented by vertical bars. a,b Columns sharing the same letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
Enzyme activities of CPT-I and ACO
The activity of CPT-I was dramatically increased in fish fed the diet with 75 % TOC compared with fish fed the diet with 75 % TO and the control group (P < 0·05). However, no significant difference was found in the activity of ACO among dietary treatments (P > 0·05) (Fig. 2).
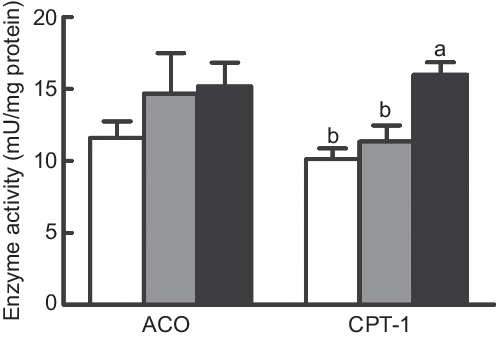
Fig. 2. Enzyme activities of carnitine palmitoyltransferase 1 (CPT-I) and acyl-CoA oxidase (ACO) in the liver of large yellow croakers. (![]() ), Fish oil (FO); (
), Fish oil (FO); (![]() ), 75 % FO substituted by terrestrial oils (75 % TO); (
), 75 % FO substituted by terrestrial oils (75 % TO); (![]() ), 75 % TO supplemented with 800 mg/kg l-carnitine. Values are means (n 4), with their standard errors represented by vertical bars. a,b Columns sharing the same letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
), 75 % TO supplemented with 800 mg/kg l-carnitine. Values are means (n 4), with their standard errors represented by vertical bars. a,b Columns sharing the same letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
Expression of genes related to lipid metabolism
The mRNA expression of diacylglycerol acyltransferase 2 (dgat2) and acetyl-CoA carboxylase 1 (acc1) was dramatically increased in fish fed the diet with 75 % TO compared with fish fed the diet with 75 % TOC and the control group (P < 0·05) (Fig. 3(C)). Expression of genes related to fatty acid synthesis (elongation of very long-chain fatty acid protein 4 (elovl4), elongation of very long-chain fatty acid protein 5 (elovl5) and fatty acyl desaturase 6 (fad6)) was dramatically increased in fish fed diets with 75 % TO and 75 % TOC compared with the control group (P < 0·05) (Fig. 3(D)). The mRNA expression of cpt-I and cluster of differentiation 36 (cd36) was significantly increased in fish fed the diet with 75 % TOC compared with fish fed the diet with 75 % TO and the control group (P < 0·05) (Fig. 3(A) and (B)). The mRNA expression of stearoyl-CoA desaturase 1 (scd1) and hepatic lipase (hl) was significantly increased in fish fed the diet with 75 % TOC compared with the control group (P < 0·05) (Fig. 3(A) and (D)). There was no significant difference in the mRNA expression of sterol-regulatory element binding protein 1 (srebp1), fatty acid synthase (fas), aco, fatty acid transport protein 1 (fatp1), microsomal TAG transfer protein (mtp) or apob100 among dietary treatments (P > 0·05) (Fig. 3(A)–(C)).
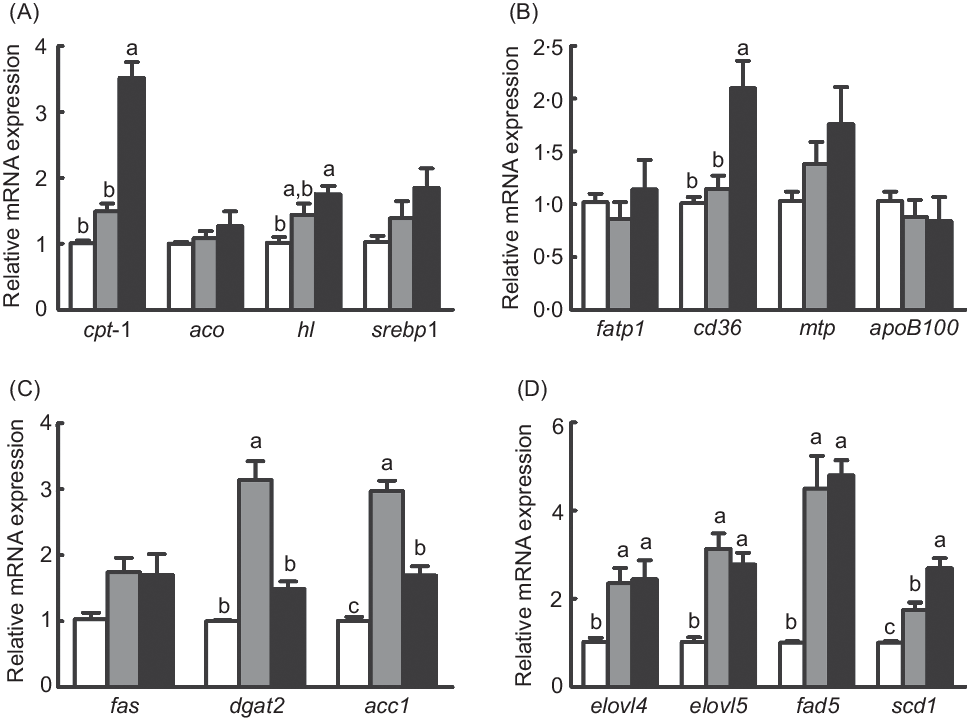
Fig. 3. Expression of genes related to lipid metabolism in the liver of large yellow croakers. cpt-I, Carnitine palmitoyltransferase I; aco, acyl-CoA oxidase; hl, hepatic lipase; srebp1, sterol-regulatory element binding protein 1; fas, fatty acid synthase; fatp1, fatty acid transport protein 1; cd36, cluster of differentiation 36; mtp, microsomal TAG transfer protein; dgat2, diacylglycerol acyltransferase 2; acc1, acetyl-CoA carboxylase 1; elovl4, long-chain fatty acid protein 4; elovl5, elongation of very long-chain fatty acid protein 5; fad6, fatty acyl desaturase 6; scd1, stearoyl-CoA desaturase 1. (![]() ), Fish oil (FO); (
), Fish oil (FO); (![]() ), 75 % FO substituted by terrestrial oils (75 % TO); (
), 75 % FO substituted by terrestrial oils (75 % TO); (![]() ), 75 % TO supplemented with 800 mg/kg l-carnitine. Values are means (n 4), with their standard errors represented by vertical bars. a,b,c Columns sharing the same letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
), 75 % TO supplemented with 800 mg/kg l-carnitine. Values are means (n 4), with their standard errors represented by vertical bars. a,b,c Columns sharing the same letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
Expression of genes related to inflammation
The mRNA expression of pro-inflammatory genes (tnfα and interferon γ (ifnγ)) was significantly increased in fish fed the diet with 75 % TO compared with the control group (P < 0·05), while they both were repressed in fish fed the diet with 75 % TOC compared with 75 % TO (P < 0·05) (Fig. 4(B)). The mRNA expression of anti-inflammatory genes (il-10 and arginase-1 (arg1)) was significantly increased in fish fed the diet with 75 % TOC compared with fish fed the diet with 75 % TO and the control group (P < 0·05) (Fig. 4(A) and (B)). There was no significant difference in the mRNA expression of il-1β, il-6 or cyclo-oxygenase-2 (cox-2) of fish among dietary treatments (P > 0·05) (Fig. 4(A) and (B)).
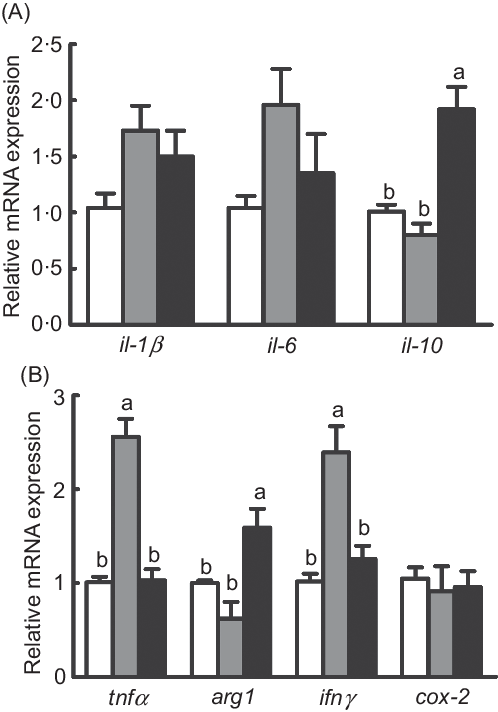
Fig. 4. Expression of genes related to inflammation in the liver of large yellow croakers. arg-1, Arginase-1; ifnγ, interferon γ; cox-2, cyclo-oxygenase-2. (![]() ), Fish oil (FO); (
), Fish oil (FO); (![]() ), 75 % FO substituted by terrestrial oils (75 % TO); (
), 75 % FO substituted by terrestrial oils (75 % TO); (![]() ), 75 % TO supplemented with 800 mg/kg l-carnitine. Values are means (n 4), with their standard errors represented by vertical bars. a,b Columns sharing the same letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
), 75 % TO supplemented with 800 mg/kg l-carnitine. Values are means (n 4), with their standard errors represented by vertical bars. a,b Columns sharing the same letter or absence of letters are not significantly different determined by Tukey’s test (P > 0·05).
Discussion
In the present study, specific growth rate significantly increased in fish fed diets with 75 % TO and 75 % TOC compared with the control group. The growth-promoting effect was possibly due to the balance of fatty acids with the supplementation of various TO. In the present study, the content of n-3 LC-PUFA in 75 % TO and 75 % TOC diets was approximately 0·83 %, which was supplied in an appropriate level for large yellow croaker. A previous study has reported that large yellow croaker fed 0·60–0·98 % dietary n-3 LC-PUFA have better growth performance, nonspecific immune responses and disease resistance than the control group (0·15 % n-3 LC-PUFA)(Reference Zuo, Ai and Mai33). Also, the ratio of ALA:LNA in 75 % TO and 75 % TOC diets was kept in an appropriate value (0·47) for large yellow croaker, while the corresponding ratio in the FO diet (0·13) was lower than the aforementioned ratio in TO diet. A previous study has shown that the ratio of dietary ALA:LNA in 0·5 is beneficial for the growth of large yellow croaker(Reference Zuo, Mai and Xu44).
Previously, Turchini et al. (Reference Turchini, Torstensen and Ng2) found that 60–75 % of dietary FO could be replaced by alternative TO in almost all finfish species when the requirement of essential fatty acid was met. However, a high level of TO might increase abnormal lipid deposition of fish(Reference Menoyo, Izquierdo and Robaina45). In this experiment, significantly higher contents of LDL-cholesterol and TAG as well as hepatic lipid content were found in fish fed the diet with 75 % TO than the control group. Similar results were also observed in Malaysian mahseer (Tor tambroides)(Reference Kamarudin, Ramezani-Fard and Saad46), rainbow trout (Oncorhynchus mykiss)(Reference Yıldız, Eroldoğan and Ofori-Mensah47) and turbot(Reference Peng, Xu and Mai6). Previous studies have demonstrated that oleic acid is more likely to increase steatosis and plasma lipid concentrations than palmitic acid(Reference Moravcová, Ervinková and Kuera48,Reference Ricchi, Odoardi and Carulli49) and PUFA(Reference Juárez-Hernández, Chávez-Tapia and Uribe50,Reference Fernandez and West51) . Accordingly, the increased lipid deposition of experimental fish could be due to the increase of dietary oleic acid in the 75 % TO diet compared with the FO diet. However, hepatic lipid deposition and the content of LDL-cholesterol were decreased in fish fed the diet with 75 % TOC compared with 75 % TO. Sang et al. (Reference Sang, Deng and Shentu27) reported that the supplementation of 800 mg/kg l-carnitine significantly improved growth performance and reduced serum TAG and total cholesterol levels in large yellow croaker compared with the control group (P < 0·05). The hypolipidemic effect was probably due to the role of l-carnitine in facilitating the transport of long-chain fatty acids into mitochondria for β-oxidation(Reference Li, Li and Qin52,Reference Ozorio, Van Ginneken and Bessa53) , as contents of C18 : 3n-3, DHA and total n-3 LC-PUFA were dramatically decreased, and the content of n-6 PUFA was also numerically lower in the liver of fish fed the diet with 75 % TOC compared with 75 % TO.
The decreased content of PUFA could be attributed to changes of fatty acid oxidation and synthesis in fish fed the diet with 75 % TOC compared with 75 % TO. As for fatty acid utilisation and oxidation, CPT-I is a rate-limiting enzyme that controls the mitochondrial uptake of long-chain acyl-CoA and facilitates long-chain fatty acids for β-oxidation(Reference Bonnefont, Djouadi and Prip-Buus54). CD36 is a key NEFA uptake transporter that involves transferring fatty acids into cells(Reference Zhou, Febbraio and Wada55) or mitochondria(Reference Bonen, Campbell and Benton56,Reference Campbell57) for utilisation. HL is a pivotal TAG lipase that contributes to vascular lipoprotein degradation, TAG hydrolysis and the uptake of lipoprotein into the liver(Reference Holmes, VandeBerg and Cox58). In the present study, the mRNA expression of cpt-I, cd36 and hl as well as the activity of CPT-I was significantly increased in fish fed the diet with 75 % TOC compared with 75 % TO. These results showed that l-carnitine facilitated the utilisation of lipid in the diet and promoted fatty acid oxidation. As for fatty acid synthesis, elovl4, elovl5 and fad6 were key genes for the biosynthesis of arachidonic acid, EPA and DHA from LNA and ALA in fish(Reference Tocher12). No significant difference was observed in the mRNA expression of elovl4, elovl5 or fad6 in fish fed diets with 75 % TOC and 75 % TO, which meant that the supplementation of l-carnitine had no significant effect on the capacity of LC-PUFA biosynthesis. Further, vertebrates cannot synthesise C18 : 2n-6 and C18 : 3n-3 from C16 : 1n-7 and C18 : 1 n-9(Reference Tocher12), and the capacity of biosynthesis of C20 and C22 LC-PUFA from C18 PUFA is very limited in most marine fish species(Reference Castro, Tocher and Monroig59). Above all, the unchanged PUFA synthesis and increased PUFA oxidation could be attributed to the decreased content of PUFA in fish fed the diet with 75 % TOC compared with 75 % TO. Similarly, dietary l-carnitine promoted the utilisation of fatty acid and decreased the content of PUFA in red porgy (Pagrus pagrus, L.)(Reference Nogueira, Cordeiro and Canada60), cobia (Rachycentron canadum)(Reference Wang, Han and Tian61) and beluga sturgeon (Huso huso)(Reference Mohseni, Ozorio and Pourkazemi62).
Antioxidant capacity is one of the most commonly used indexes to evaluate the physical condition of fish(Reference Tan, Sun and Liu63). In this study, the activity of T-AOC showed a decreasing trend (from 1·16 to 1·03) in fish fed the diet with 75 % TO compared with the control group. When the 75 % TO diet was supplemented with 800 mg/kg l-carnitine, the activity of superoxide dismutase and T-AOC was dramatically increased in fish compared with fish fed the diet with 75 % TO. A previous study has reported that l-carnitine induces Nrf2/Keap1 pathway activation in vivo and in vitro and increases the antioxidant enzyme activities (catalase, T-superoxide dismutase, GSH-PX) in Rhynchocypris lagowski (Reference Zhang, Guo and Zhao64). Further, l-carnitine played some crucial physiological roles in the inhibition of superoxide radical formation(Reference Gülçin65). The increased antioxidant capacity could be attributed to the modulatory effects of l-carnitine on critical antioxidant enzymes in fish fed the diet with 75 % TOC compared with 75 % TO.
In addition to antioxidant capacity, the inflammation in fish was also an important indicator to evaluate the health status. In the present study, the mRNA expression of pro-inflammatory genes (tnfα and ifnγ) was significantly higher in fish fed the diet with 75 % TO than the control group. However, the mRNA expression of the pro-inflammatory genes was decreased in fish fed the diet with the 75 % TOC compared with 75 % TO. The mRNA expression of anti-inflammatory genes (il-10 and arg-1) was increased in fish fed the diet with the 75 % TOC compared with 75 % TO. Previous studies have demonstrated that dietary l-carnitine can improve the immunity and disease resistance of juvenile narrow clawed crayfish (Astacus leptodactylus leptodactylus)(Reference Safari, Atash and Paolucci66), black carp (Mylopharyngodon piceus)(Reference Ming, Ye and Zhang67) and common carp (Cyprinus carpio L.)(Reference Chen, Guo and Sun68). l-Carnitine exerted protective effects by inhibiting the generation of ammonia and xenobiotics in rat hepatocytes(Reference Nakamura, Iida and Nakatake69). Future research is required to explore the specific mechanism how l-carnitine decreased the inflammation of fish fed the diet with 75 % TOC compared with 75 % TO.
In conclusion, dietary TO mixture (75 % TO and 75 % TOC diets) increased the growth of large yellow croaker compared with the control group, which was probably due to the appropriate level of n-3 LC-PUFA and the appropriate ratio of ALA:LNA. Although the growth was not significantly different, the supplementation of 800 mg/kg l-carnitine in the 75 % TO diet (75 % TOC) could increase antioxidant capacity, fatty acid oxidation and decrease lipid abnormal deposition and the expression of inflammatory genes compared with 75 % TO.
Acknowledgements
This work was supported by the Scientific and Technological Innovation of Blue Granary (grant number: 2018YFD0900402), National Science Fund for Distinguished Young Scholars of China (grant number: 31525024), the China Agriculture Research System (grant number: CARS47-11), Key Program of National Natural Science Foundation of China (grant number: 31830103), Leading Talent of Technological Innovation of Ten-Thousands Talents Program (grant number: CS31117200001) and Aoshan Talents Program Supported by Qingdao National Laboratory for Marine Science and Technology (grant number: 2015ASTP).
The authors’ contributions were as follows: Q. A. contributed to funding acquisition, project administration and supervision; Q. A. and K. M. designed the research; X. L., Q. C. and Q. C. conducted the research; X. L. analysed the data and wrote the paper. We appreciate Yunqiang Zhang, Jiamin Li, Weiwei Dai, Xiufei Cao, Ye Gong, Tao Ding and Md Golam Sajed Riar for their help in revising the article.
The authors declare that there are no conflicts of interests associated with the manuscript.














