The weaning transition is often associated with undesirable morphological and physiological changes in the piglet's gastrointestinal environment related to a reduced voluntary feed intake, which subsequently increase susceptibility to intestinal dysfunction(Reference Pluske, Hampson and Williams1, Reference Lallès, Boudry and Favier2). Traditional measures to alleviate weaning-associated intestinal dysfunction have centred on dietary manipulations post-weaning(Reference Pierce, Sweeney and Brophy3, Reference Boudry, Buldgen and Portetelle4). However, recent research has indicated that maternal dietary supplementation may influence growth performance, immune status and gastrointestinal health of weanling pigs(Reference Corino, Pastorelli and Rosi5–Reference Leonard, Sweeney and Bahar8).
Dietary inclusion of seaweed extracts (SWE) containing bioactive compounds to animal diets has received increasing attention in recent years(Reference Gardiner, Campbell and O'Doherty9, Reference O'Doherty, Dillon and Figat10). Evidence indicates that dietary provision of a Laminaria spp.-derived SWE containing laminarin and fucoidan is beneficial to weanling pigs, by enhancing growth performance(Reference Gahan, Lynch and Callan11) and reducing enteric Enterobacteriaceae numbers(Reference Reilly, O'Doherty and Pierce12). Furthermore, other studies have demonstrated that SWE supplementation may alter inflammatory responses through regulating the expression of cytokines, chemokines and mucins(Reference Reilly, O'Doherty and Pierce12, Reference Smith, Ryan and O'Doherty13). Laminarin represents a group of low-molecular-weight, water-soluble polysaccharides composed of β-(1 → 3)-linked glucans with β-(1 → 6)-linked side chains of varying distribution and length(Reference Read, Currie and Bacic14, Reference Brown and Gordon15). β-Glucans have the capacity to modulate immune function by stimulating the release of cytokines and chemokines, thus activating leucocytes including monocytes, macrophages and lymphocytes(Reference Yun, Estrada and Van Kessel16, Reference Volman, Ramakers and Plat17). In addition, fucoidans are highly sulphated polysaccharides, containing l-fucose as one of the major monosaccharides extracted from the extracellular matrix of various algae(Reference Berteau and Mulloy18).
Recently, Leonard et al. (Reference Leonard, Sweeney and Bahar8) reported that maternal SWE supplementation from day 109 of gestation until weaning (day 26) enhanced the growth performance of weaned pigs and decreased Escherichia coli numbers in the caecum and colon on day 9 post-weaning. These observations demonstrated that maternal dietary treatment exerted an influence on growth performance and aspects of gastrointestinal health in weaned pigs. This is of particular interest as the current European legislation prohibits antibiotic growth promoter inclusion in pig diets. Thus, maternal dietary treatment may potentially help reduce or alleviate weaning-associated intestinal dysfunction and growth depression immediately after weaning. Furthermore, another study has reported that piglets suckling SWE-supplemented sows had greater circulatory IgG concentrations on day 5 and 12 of lactation and a greater percentage of E. coli-phagocytising leucocytes at weaning, indicating an enhancement of cellular immune function(Reference Leonard, Sweeney and Bahar19).
The primary objective of the present study was to evaluate the effect of the supplementation period (lactation v. post-weaning) and dietary SWE inclusion on the growth performance of weaned pigs. Furthermore, the present study investigated the effect of dietary treatment on intestinal morphology, selected intestinal microflora, volatile fatty acid (VFA) concentrations and immune status of pigs at days 11 and 117 post-weaning. It was hypothesised that maternal SWE supplementation from day 107 of gestation until weaning (day 26) would enhance the growth performance of weaned pigs and improve aspects of gastrointestinal health. This could potentially replace the need for dietary SWE supplementation of pigs during the starter and grower–finisher (GF) periods.
Materials and methods
All experimental procedures described in the present study were conducted under experimental license from the Irish Department of Health in accordance with the Cruelty to Animals Act 1876 and the European Communities (Amendments of the Cruelty to Animals Act, 1876) Regulations (1994).
Experimental design and animal management
A total of twenty crossbred pregnant sows (Large White × Landrace genetic lines) were randomly assigned, accounting for parity (mean parity 2·4 (sd 1·1) and anticipated farrowing date, to one of the two dietary treatments (ten sows per treatment): basal lactation diet (LT) and basal lactation and 10·0 g SWE/d from day 107 of gestation until weaning (day 26). The SWE supplement (10·0 g) contained laminarin (1·0 g), fucoidan (0·8 g) and ash (8·2 g), and was extracted from a Laminaria sp. according to the procedure described by Lynch et al. (Reference Lynch, Sweeney and Callan20). The SWE was provided by a commercial company (Bioatlantis Limited, Tralee, County Kerry, Republic of Ireland).
At weaning, a total of eighty mixed-sex pigs (four pigs per litter; two males and two females) with an average body weight of 8·6 (sd 1·21) kg were selected. All pigs remained in the same treatment group defined by their dams; they were subdivided into two groups of two pigs (one male and one female), resulting in four experimental groups. The two factors, LT and post-weaning diet (PW), were arranged in a 2 × 2 factorial to provide the four treatment groups that were randomly assigned to replicate pens (n 10) as follows: (1) BB (basal sows–basal pigs); (2) BS (basal sows–treated pigs); (3) SB (treated sows–basal pigs); (4) SS (treated sows–treated pigs). Pigs from the BS and SS treatment groups were offered SWE-supplemented (2·8 g/kg feed) PW.
Sow and piglet management
The ingredient composition of the LT is presented in Table 1. Diets were formulated to contain similar concentrations of crude protein (CP) (192 g/kg), digestible energy (14 MJ/kg) and total lysine (10·1 g/kg). The amino acid requirements were met relative to lysine(21). Sows received specific amounts of feed in the following quantities: 2 kg/d of diet until the day of farrowing (day 0), and then the feed supply was increased by 1 kg/d until day 3 and then by 0·5 kg/d until day 6. Afterwards, they were allowed ad libitum consumption of the standard LT that was adjusted for each sow depending on daily intake. The sows were fed in two equal meals provided at 09.00 and 15.00 hours. The standard LT was top-dressed each morning (09.00 hours) with experimental supplements to ensure consumption.
Table 1 Diet and chemical composition of the experimental diets (as-fed basis)

CP, crude protein.
* Weaner diet provided (mg/kg completed diet): Cu, 175; Fe, 140; Mn, 47; Zn, 120; I, 0·6; Se, 0·3; retinol, 1·8; cholecalciferol, 0·025; α-tocopherol, 67; phytylmenaquinone, 4; cyanocobalamin, 0·01; riboflavin, 2; nicotinic, acid, 12; pantothenic, acid, 10; choline chloride, 250; thiamin, 2; pyridoxine, 0·015. Sow diet provided (mg/kg completed diet): Cu, 25; Fe, 140; Mn, 47; Zn, 120; I, 0·6; Se, 0·3; retinol, 1·8; cholecalciferol, 0·025; α-tocopherol, 67; phytylmenaquinone, 4; cyanocobalamin, 0·01; riboflavin, 2; nicotinic, acid, 12; pantothenic, acid, 10; choline chloride, 250; thiamin, 2; pyridoxine, 0·015. Grower diet provided (mg/kg completed diet): Cu, 25; Zn, 100; Se, 0·3; Mn, 25; I, 0·2; retinol, 3; cholecalciferol, 0·05; α-tocopherol, 40., Finisher diet provided (mg/kg completed diet): Cu, 25; Zn, 100; Se, 0·3; Fe, 100; Mn, 25; I, 0·2; retinol, 4·2; cholecalciferol, 0·07; α-tocopherol, 80.
† Calculated from tabulated nutritional composition(Reference Sauvant, Perez and Tran54).
The experiment was initiated on day 107 of gestation when sows were moved to the farrowing house, and sows were offered experimental supplements until weaning at day 26. The sows and piglets were individually housed in farrowing pens (2·2 × 2·4 m) with crates, slated floors and heat pads for piglets. The farrowing room temperature was maintained at 20°C. The sows were individually fed and had ad libitum access to drinking-water throughout the experimental period. Farrowings were not induced and were supervised. Litter size was adjusted shortly after birth by cross-fostering piglets within dietary treatments to ensure that sows nursed a similar number of piglets (twelve piglets per sow), and this was maintained throughout the suckling period.
Starter pig performance
The starter pig performance study measured performance between days 0 and 21 post-weaning, where pigs were supplemented with or without a SWE depending on the treatment group (ten replicates per treatment) as described earlier. The pigs were housed in groups of two (from original sow litter) on fully slatted pens (1·68 m × 1·22 m). The ingredient composition and chemical composition of the starter diet are presented in Table 1. Diets were formulated to contain similar concentrations of CP (210 g/kg), digestible energy (16 MJ/kg) and true ileal digestible lysine (14·5 g/kg). All amino acid requirements were met relative to true ileal digestible lysine(21). No medication, zinc oxide or other growth-promoting agents were included in the starter diet. Feed and water were available ad libitum throughout the experimental period. The ambient environmental temperature within the houses was thermostatically controlled. The temperature was maintained at 30°C for the first week and was reduced by 2°C/week thereafter. The pigs were individually weighed on days 0, 7, 14 and 21, and feed intake was recorded per pen on a daily basis.
In addition, the effect of dietary treatment on aspects of gastrointestinal health and immune status of pigs on day 11 post-weaning was examined. At weaning, a total of forty pigs (two female pigs per litter) with an average body weight of 8·6 (sd 0·44) kg were selected. All pigs remained in the same treatment group defined by their dams; they were subdivided into two groups of one pig each, resulting in four experimental groups (as described earlier): (1) BB; (2) BS; (3) SB; (4) SS. Pigs from the BS and SS treatment groups were offered SWE-supplemented (2·8 g/kg feed) diets. Pigs were offered a similar starter diet as in the performance study (Table 1). The pigs were housed individually. At day 11 post-weaning, the pigs were killed following a lethal injection of euthanal (pentobarbitone sodium) at a rate of 1 ml/1·4 kg live body weight. Intestinal tissues and digesta samples were recovered to facilitate analysis of small-intestinal morphology, selected intestinal microflora, VFA concentrations and immune status of the ileal and colonic tissues.
Grower–finisher performance study
At day 21 post-weaning, the performance pigs (twenty pigs per treatment: same pigs as in the starter pig performance study) were moved into the GF house. The pigs were supplemented with or without a SWE depending on the treatment group as described earlier. The pigs were penned in four mixed-sex groups of twenty with a space allowance of 0·75 m2/pig. The ingredient composition and chemical composition of the GF diet are presented in Table 1. The pigs were offered a grower diet from days 21 to 77 and a finisher diet from days 77 to 117. The grower diets were formulated to contain similar concentrations of CP (196 g/kg), digestible energy (14·4 MJ/kg) and standardised ileal digestible lysine (11·3 g/kg). The finisher diets were formulated to contain similar concentrations of CP (174 g/kg), digestible energy (13·8 MJ/kg) and total lysine (9·5 g/kg). The house was mechanically ventilated to provide an ambient temperature of 20°C. Each pen had a solid floor lying area with access to concrete slats at rear. The four group pens were equipped with single-space computerised feeders (Mastleistungsprufung MLP-RAP; Schauer Agrotronic AG, Sursee, Switzerland), as described previously by Pauly et al. (Reference Pauly, Spring and O'Doherty22). The pigs were weighed at the start of the experiment (day 21) and subsequently on days 77 and 117 (on the morning of slaughter), and feed intake was recorded daily. At slaughter (day 117), intestinal tissues and digesta samples were recovered to facilitate analysis of selected intestinal microflora, VFA concentrations and immune status of the colonic tissue. The Pigs were slaughtered in two batches of ten pigs per treatment (five males and five females) on days 117 and 118.
Carcass analysis
After overnight fasting (approximately 15 h), pigs were transported to a commercial slaughter plant, rested for 2 h, stunned by CO2 and killed by exsanguination. Carcass measurements of back fat and muscle thickness were measured at a point 6 cm from the edge of the split back at the level of the third and fourth last ribs using the Hennessy Grading Probe (Hennessy and Chong, Auckland, New Zealand). Lean meat content was estimated according to the following formula(23):
where x is the fat depth (mm) and y is the muscle depth (mm).
Further carcass data were determined by application of the following equation(Reference Varley, Sweeney and Ryan24):
where BW is the body weight.
Small-intestinal morphology of pigs at day 11 post-weaning
Intestinal tissues from middle sections of the duodenum, jejunum and ileum were aseptically isolated, flushed with 0·9 % salt solution and fixed in 10 % phosphate-buffered formalin. The preserved intestinal segments were prepared using standard paraffin-embedding techniques. Cross-sections at 5 μm thickness of each intestinal segment were stained with haemotoxylin and eosin. Villous height and crypt depth were measured on the stained sections (10 × objective) using a light microscope fitted with an image analyser (Image Pro Plus; Media Cybernetics, Bethesda, MD, USA). Lengths of fifteen well-orientated intact villi and their associated crypt were measured in duplicate for each segment. The villous height was measured from the crypt–villous junction to the tip of the villous, and the crypt depth was measured from the crypt–villous junction to the base. The results are expressed as the mean villous height or crypt depth in μm. The villous height:crypt depth ratio was calculated.
Intestinal microflora of pigs at day 11 post-weaning
Digesta samples (approximately 10 (sd 1) g) were aseptically recovered from the caecum and colon of each pig immediately post-slaughter, stored in sterile containers (Sarstedt, Wexford, Republic of Ireland), placed on ice and transported to the laboratory within 2 h. Populations of E. coli and Lactobacillus spp. were selectively isolated and enumerated as described previously(Reference Pierce, Sweeney and Brophy3). A 1·0 g sample was removed from each digesta sample, serially diluted (1:10) in 9·0 ml aliquots of maximum recovery diluent (Oxoid, Basingstoke, UK) and spread-plated (0·1 ml aliquots) onto selective agars as follows: Lactobacillus spp. were isolated on de Man, Rogosa and Sharpe agar (Oxoid) with overnight (18–24 h) incubation at 37°C in a 5 % CO2 environment, as recommended by the manufacturer's instructions (Oxoid). The API 50 CHL kit (BioMérieux, Marcy l'Etoile, France) was used to confirm suspect Lactobacillus spp. The E. coli species were isolated on MacConkey agar (Oxoid), following aerobic incubation at 37°C for 18–24 h. Suspect colonies were confirmed with API 20E (BioMérieux). This API system identifies the suspect colonies by measuring their ability to produce cytochrome oxidase. Typical colonies of each bacterium were counted, log transformed and presented per g digesta.
Intestinal microflora of pigs at day 117 post-weaning
Immediately after slaughter, the gastrointestinal tract was collected from individual animals, and digesta samples (approximately 10 (sd 1) g) were immediately aseptically removed from the ileum and the second loop of the proximal colon of each animal, stored in sterile containers (Sarstedt, Wexford, Republic of Ireland) on dry ice and transported to the laboratory within 2 h. Populations of Lactobacillus spp. and Enterobacteriaceae were isolated and enumerated according to the method described by O'Connell et al. (Reference O'Connell, Sweeney and Callan25). In brief, a 1·0 g sample was serially diluted (1:10) in 9·0 ml aliquots of maximum recovery diluent (Oxoid) and spread-plated (0·1 ml aliquots) onto selective agars as follows: Lactobacillus spp. as described earlier and Enterobacteriaceae were isolated on MacConkey agar (Oxoid), following aerobic incubation at 37°C for 18–24 h. Positive Enterobacteriaceae colonies were confirmed with API 20E (BioMérieux). Typical colonies of each bacterium were counted, log transformed and presented per g of digesta.
Volatile fatty acid analysis
Samples of digesta from the caecum and colon of individual pigs were recovered at day 11 post-weaning, and samples of digesta from the ileum and colon of individual pigs were recovered at day 117 for VFA analysis. Concentrations of VFA in the digesta were determined by a gas chromatographic method following the procedures of Pierce et al. (Reference Pierce, Sweeney and Brophy26).
RNA extraction and complementary DNA synthesis
Tissue samples were collected from the mesenteric side of the ileum and colon of pigs on day 11 and the colon of pigs on day 117 post-weaning, rinsed with ice-cold sterile PBS and stripped of the overlying smooth muscle. The tissue samples were cut into small pieces using a sterile scalpel blade and stored in 15 ml of RNAlater (Ambion, Inc., Austin, TX, USA) followed by storing at − 20°C until used for RNA extraction. Total RNA was extracted from 25 mg tissue samples using a GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer's instructions. To eliminate possible genomic DNA contamination, total RNA samples were subjected to DNase I (Sigma-Aldrich) treatment according to the protocol of the manufacturer. Then, RNA purification was preformed by a phenol–chloroform extraction method. The total RNA was quantified using a NanoDrop-ND1000 Spectrophotometer (Thermo Fisher Scientific, Inc., Boston, MA, USA), and purity was assessed by determining the ratio of the absorbance at 260 and 280 nm. All total RNA samples had 260/280 nm ratios above 1·8. In addition, RNA integrity was verified by visualisation of the 18S and 28S ribosomal RNA bands stained with ethidium bromide after gel electrophoresis on 1·2 % agarose gels (Egel; Invitrogen, Carlsbad, CA, USA). Total RNA (1 μg) was reverse transcribed using a First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, MD, USA) using oligodeoxythymidylic acid primers in a final reaction volume of 20 μl according to the manufacturer's instructions. The final reverse-transcribed product was adjusted to a volume of 120 μl using nuclease-free water.
Quantitative real-time PCR
Quantitative real-time PCR assays were performed on complementary DNA samples in ninety-six-well optical plates on a 7900HT ABI Prism Sequence Detection System (PE Applied Biosystems, Foster City, CA, USA) using SYBR Green PCR Master Mix (PE Applied Biosystems). The primers used for real-time PCR (IL-1α, IL-6, IL-10, TNF-α, MUC2, TFF3, glyceraldehyde-3-phosphate dehydrogenase, B2M, ACTB and PPIA) were designed using Primer Express™ software (PE Applied Biosystems) and were synthesised by MWG Biotech (Milton Keynes, Buckinghamshire, UK). Primer sequence data are presented in Table 2. Amplification was carried out in a total volume of 20 μl containing a 10 μl 2X SYBR PCR Master Mix (PE Applied Biosystems), forward and reverse primer mix (1 μl), 8 μl nuclease-free water and 1 μl template complementary DNA. The two-step PCR programme was as follows: 95°C for 10 min for one cycle, followed by 95°C for 15 s and 60°C for 1 min for forty cycles. Dissociation analyses of the PCR product were performed to confirm the specificity of the resulting PCR products. All samples were prepared in triplicate. The mean threshold cycle (C t) values of triplicates of each sample were used for calculations.
Table 2 Porcine-specific primers used for real-time PCR

F, forward; R, reverse; MUC2, mucin 2; TFF3, trefoil factor 3; GADPH, glyceraldehyde-3-phosphate dehydrogenase; B2M, β-2 microglobulin; ACTB, β-actin; PPIA, peptidylprolyl isomerase A.
Normalisation of real-time PCR data
Normalisation of the C t values obtained from real-time PCR was performed by (1) transforming the raw C t values to relative quantities using the formula, relative quantities = (PCR efficiency)ΔC t, where ΔC t is the change in the C t values of the sample relative to the highest expression (minimum C t value), (2) using geNorm, a normalisation factor was obtained from the relative quantities of four most stable housekeeping genes (glyceraldehyde-3-phosphate dehydrogenase, B2M, ACTB and PPIA), (3) and the normalised fold change or the relative abundance of each of the target genes was calculated by dividing its relative quantity by the normalisation factor.
Laboratory analysis
The total laminarin content of the SWE and feed was determined using a commercial assay kit (K-YBGL 10/2005; Megazyme International Ireland Limited, Bray, County Wicklow, Republic of Ireland). Laminarin was solubilised in 60 % H2SO4 and then hydrolysed to near completion in 2 m-HCl. Any remaining laminarin fragments were quantitatively hydrolysed to glucose using a mixture of highly purified exo-1,3-β-glucanase and β-glucosidase. An aliquot of the filtered extract was mixed with 3 ml of glucose oxidase/peroxidase, incubated at 40°C for 20 min. The absorbance of all solutions (extract and glucose standard) was measured at 510 nm against a reagent blank using a UV/VIS Spectrophotometer (model UV-mini-1240; Shimadzu, Duisburg, Germany). Fucoidan levels were determined using the method of Usov et al. (Reference Usov, Smirnova and Klochkova27). A weighted sample (150 mg) of the laminarin fucoidan extract was placed into a centrifuge tube (50 ml), treated with 25 ml of 0·2 m-HCl, magnetically stirred for 1 h at 70°C and centrifuged. The supernatant was separated, and the extract precipitate was extracted once more under the same conditions. The acidic extracts were combined, treated with sodium chlorite (75 mg), stirred until the salt dissolution, kept for 1 h at room temperature and dialysed for 3 d against distilled water. The solution was adjusted to a volume of 10 ml with distilled water in a volumetric flask and filtered through a paper filter, and fucoidan content was determined in 0·5 ml aliquots by the fucose colour reaction with l-cysteine hydrochloride and concentrated H2SO4. A calibration curve using a solution of fucose within the range of 0–80 μg was measured at 396 and 420 nm against a reagent blank using a UV/VIS spectrophotometer. The result of the determination was multiplied by 2, assuming the average fucose content in fucoidans to be 50 %. The feed samples were milled through a 1 mm screen (Christy and Norris hammer mill, Ipswich, UK). The DM of the feed was determined after drying at 103°C for a minimum of 16 h. Ash was determined after ignition of a known weight of concentrate in a muffle furnace (Nabertherm, Bremen, Germany) at 500°C for 4 h. CP content was determined as Kjeldahl N × 6·25 using the LECO FP 528 instrument. Neutral-detergent fibre content was determined according to Van Soest et al. (Reference Van Soest, Robertson and Lewis28). The gross energy of the feed was determined using a Parr 1201 oxygen bomb calorimeter (Parr, Moline, IL, USA).
Statistical analysis
The experimental data were analysed as a 2 × 2 factorial using the general linear model procedure of the SAS (SAS Institute, Inc., Carry, NC, USA)(29). Contrast statements were used to compare (1) T1+T2 v. T3+T4 – non-SWE-supplemented v. SWE-supplemented sows (lactation effect), (2) T1+T3 v. T2+T4 – non-SWE-supplemented v. SWE-supplemented PW (post-weaning effect) and (3) the interaction between the lactation effect and the post-weaning effect. The individual sow and the pen containing the weaned pigs served as the experimental unit for all variables measured. Piglet body weight at weaning was included as a covariate for all variables measured. The data were checked for normality using the Proc Univariate function of SAS. All data are expressed as least-squares means with their standard errors. The probability value that denotes statistical significance is P < 0·05.
Results
Starter pig performance
The number of live born piglets, litter weight, average piglet birth weight and weaning weight were not influenced by sow dietary treatment (data not shown).
Pigs weaned from SWE-supplemented sows had a higher average daily gain (ADG) between days 14 and 21 (0·525 v. 0·471; sem 0·016 kg/d; P < 0·05) and between days 0 and 21 (0·340 v. 0·306; sem 0·010 kg/d; P < 0·05) post-weaning compared with pigs weaned from non-SWE-supplemented sows (Table 3).
Table 3 Post-weaning growth performance for pigs born to basal (B) or seaweed extract (SWE)-supplemented (S) sows fed basal (B) or SWE-supplemented (S) starter diets between days 0 and 21 post-weaning
(Least squares mean values with their standard errors, ten sows per treatment)

LT, lactation diet; PW, post-weaning diet; ADG, average daily gain; ADFI, average daily feed intake; G, gain; F, feed.
a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05).
There was a LT × PW interaction on the gain:feed ratio between days 14 and 21 (P < 0·001) and between days 0 and 21 (P < 0·05) post-weaning. Pigs weaned from SWE-supplemented sows and offered non-SWE-supplemented diets post-weaning had a higher gain:feed ratio between days 14 and 21 (P < 0·05) compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented PW. However, maternal SWE supplementation had no effect on the gain:feed ratio when pigs were offered SWE diets post-weaning.
Pigs weaned from basal-fed sows and offered SWE-supplemented diets post-weaning had a higher gain:feed ratio between days 0 and 21 (P < 0·05) compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented PW. However, post-weaning inclusion of SWE had no effect on the gain:feed ratio in pigs weaned from SWE-supplemented sows.
Furthermore, dietary treatment had no effect on the growth performance of pigs that were killed between days 0 and 11 post-weaning. The ADG, average daily feed intake and gain:feed ratio were 0·175 (sd 0·030), 0·277 (sd 0·120) kg/d and 0·625 (sd 0·095) kg/kg, respectively, for pigs that were killed.
Grower–finisher pig performance
The effect of dietary treatment on GF pig performance is presented in Table 4. There was a LT × PW interaction on ADG between days 21 and 77 (P < 0·001), between days 77 and 117 (P < 0·05) and between days 21 and 117 (P < 0·001). Pigs weaned from basal-fed sows and offered SWE-supplemented GF diets had a higher ADG between days 21 and 77 (P < 0·05) and between days 21 and 117 (P < 0·05) post-weaning compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented GF diets. However, the inclusion of SWE in GF diets had no effect on ADG between days 21 and 77 and between days 21 and 117 in pigs weaned from SWE-supplemented sows.
Table 4 Grower–finisher growth performance for pigs born to basal (B) or seaweed extract (SWE)-supplemented (S) sows fed basal (B) or SWE (S)-supplemented diets
(Least squares mean values with their standard errors, ten sows per treatment)

LT, lactation diet; PW, post-weaning diet; ADG, average daily gain; ADFI, average daily feed intake.
a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
Pigs weaned from SWE-supplemented sows and offered non-SWE-supplemented diets had a lower ADG between days 77 and 117 compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented GF diets (P < 0·05). However, maternal SWE supplementation had no effect on ADG when pigs were offered SWE-supplemented GF diets.
There was a LT × PW interaction on the gain:feed ratio between days 21 and 77 (P < 0·001) and between days 21 and 117 (P < 0·01). Pigs weaned from basal-fed sows and offered SWE-supplemented GF diets had a higher gain:feed ratio between days 21 and 77 (P < 0·05) and between days 21 and 117 (P < 0·05) compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented GF diets. However, the inclusion of SWE in GF diets had no effect on the gain:feed ratio between days 21 and 77 and between days 21 and 117 in pigs weaned from SWE-supplemented sows.
Dietary treatment had no effect on carcass characteristics (data not shown). Kill-out proportion was 754 (sd 6·41) g/kg, fat depth was 10·7 (sd 0·16) mm, muscle depth was 49·0 (sd 1·8) mm and lean meat content was 580·0 (sd 5·1) g/kg.
Intestinal microflora and volatile fatty acid concentrations of pigs
Pigs offered PW containing SWE had decreased populations of E. coli in the colon on day 11 post-weaning (P < 0·01; Table 5) compared with non-SWE-supplemented diets. Furthermore, pigs offered SWE-supplemented PW had a greater Lactobacillus:E. coli ratio on day 11 post-weaning compared with pigs offered unsupplemented diets (P < 0·05). However, there was no effect of dietary treatment on caecal populations of E. coli and Lactobacillus spp.
Table 5 Selected intestinal microflora at days 11 and 117 post-weaning of pigs born to basal (B) or seaweed extract (SWE)-supplemented (S) sows fed basal (B) or SWE-supplemented (S) diets (log10 cfu/g digesta)
(Least squares mean values with their standard errors, ten sows per treatment)

cfu, Colony-forming units; LT, lactation diet; PW, post-weaning diet.
Dietary treatment had no effect on total VFA concentrations and molar proportions of VFA in the caecum and colon on day 11 post-weaning (P < 0·05; data not shown).
Pigs offered diets containing SWE had decreased Enterobacteriaceae populations in the colon at day 117 compared with pigs offered unsupplemented diets (P < 0·05). However, there was no effect of dietary treatment on ileal populations of Enterobacteriaceae and Lactobacillus spp.
At day 117, pigs offered diets containing SWE had a reduced molar proportions of valeric acid in the ileum (0·006 v. 0·013; sem 0·002; P < 0·05) and a tendency for reduced molar proportions of branched-chain fatty acids in the colon (0·036 v. 0·042; sem 0·002; P = 0·06) compared with pigs offered unsupplemented diets.
Intestinal morphology
Histological evaluation of the duodenum and ileum indicated no effect of dietary treatment on villous height, crypt depth and ratio of villous height:crypt depth (Table 6). However, there was a LT × PW interaction on villous height:crypt depth ratio (P < 0·05) in the jejunum. Pigs weaned from basal-fed sows and offered PW containing SWE had a greater villous height:crypt depth ratio on day 11 post-weaning compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented PW (P < 0·05). However, the inclusion of SWE in PW had no effect on the villous height:crypt depth ratio in pigs weaned from SWE-supplemented sows.
Table 6 Small-intestinal morphology on day 11 post-weaning of pigs born to basal (B) or seaweed extract (SWE)-supplemented (S) sows fed basal (B) or SWE-supplemented (S) diets
(Least squares mean values with their standard errors, ten sows per treatment)

LT, lactation diet; PW, post-weaning diet.
a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
Gene expression study
The present study evaluated inflammatory cytokine (IL-1α, IL-6, IL-8, IL-10 and TNF-α) and mucin (MUC2) mRNA expression in the ileal and colonic tissues on day 11 post-weaning. Pigs offered diets containing SWE post-weaning had a greater colonic MUC2 mRNA abundance (0·72 v. 0·51; sem 0·066 normalised fold change; P < 0·05) at day 11 post-weaning. Furthermore, pigs weaned from SWE-supplemented sows tended to have a lower mRNA abundance of IL-1α in the ileal tissue (0·22 v. 0·33; sem 0·044 normalised fold change; P = 0·08) at day 11 post-weaning.
In addition, there was no effect of dietary treatment on the mRNA expression profile of IL-1α, IL-6 and TNF-α in the colonic tissue at day 117 (Table 7).
Table 7 Normalised relative abundance of pro-inflammatory gene expression on day 117 post-weaning of pigs born to basal (B) or seaweed extract (SWE)-supplemented (S) sows fed basal (B) or SWE-supplemented (S) diets
(Least squares mean values with their standard errors, ten sows per treatment)
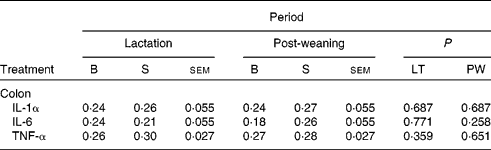
LT, lactation diet; PW, post-weaning diet.
Discussion
The major objectives of the present study were to investigate the influence of the supplementation period (lactation v. post-weaning) and dietary SWE inclusion on the growth performance and aspects of gastrointestinal health in pigs at days 11 and 117 post-weaning. The positive response observed in pigs weaned from SWE-supplemented sows on ADG between days 0 and 21 post-weaning compared with pigs weaned from non-SWE-supplemented sows and the absence of a response to SWE supplementation on ADG in GF pigs weaned from SWE-supplemented sows support the hypothesis.
Pig performance study
The present results indicate that pigs weaned from SWE-supplemented sows had a greater ADG during the starter period. Consistent with this, Leonard et al. (Reference Leonard, Sweeney and Bahar8) reported that supplementation of a similar Laminaria spp.-derived SWE to sows from day 109 of gestation until weaning bestowed a positive influence on the growth performance of weaned pigs, characterised by a greater ADG between days 0 and 21 post-weaning. Furthermore, pigs weaned from basal-fed sows and offered SWE-supplemented starter diets had a higher gain:feed ratio between days 0 and 21 post-weaning compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented starter diets. However, post-weaning inclusion of SWE had no effect on the gain:feed ratio in pigs weaned from SWE-supplemented sows. Similar results were recorded during the GF period, where pigs weaned from basal-fed sows and offered SWE-supplemented GF diets had a higher ADG and gain:feed ratio between days 21 and 117 post-weaning compared with pigs weaned from basal-fed sows and offered non-SWE-supplemented GF diets. However, SWE inclusion had no effect on the ADG and gain:feed ratio in pigs weaned from SWE-supplemented sows during the GF period. To our knowledge, this is the first investigation examining long-term beneficial effects of maternal SWE supplementation on the growth performance of pigs from weaning to slaughter (day 117). The improvements observed in growth performance of pigs weaned from SWE-supplemented sows may be attributed to an enhancement of cellular immune function at weaning and suppressed colonic E. coli numbers. Leonard et al. (Reference Leonard, Sweeney and Bahar19) have recently demonstrated that piglets suckling SWE-supplemented sows had a greater percentage of E. coli-phagocytising leucocytes at weaning. In addition, another study from our laboratory has demonstrated that piglets suckling SWE-supplemented sows had reduced colonic E. coli numbers and enhanced TNF-α mRNA expression in the ileal tissue following an ex vivo lipopolysaccharide challenge at weaning(Reference Leonard, Sweeney and Bahar30).
Consistent with this, recent studies have demonstrated that dietary provision of a similar SWE containing laminarin and fucoidan, enhanced the growth performance of weanling pigs(Reference Gahan, Lynch and Callan11, Reference McDonnell, Figat and O'Doherty31), and similar observations have been reported with yeast-derived β-glucans(Reference Dritz, Shi and Kielian32). Further investigations are warranted to elucidate the mechanism by which maternal SWE supplementation bestowed long-term positive effects on the growth performance of weaned pigs, as the majority of studies in the literature have failed to examine the influence of maternal supplementation beyond the suckling period.
Gastrointestinal health of pigs on days 11 and 117 post-weaning
The present study further examined the influence of dietary treatment on aspects of gastrointestinal health in pigs at days 11 and 117 post-weaning. Recently, O'Shea et al. (Reference O'Shea, Sweeney and Lynch33) demonstrated that pigs are susceptible to dietary manipulations that alter community dynamics of the resident gastrointestinal microflora. The present data indicate that dietary SWE inclusion post-weaning reduced colonic E. coli and Enterobacteriaceae populations at days 11 and 117, respectively, indicating an antimicrobial property of the SWE. These observations are in agreement with previous studies demonstrating that dietary supplementation of a similar SWE decreased enteric Enterobacteriaceae numbers(Reference Reilly, O'Doherty and Pierce12, Reference Lynch, Sweeney and Callan20) and faecal E. coli numbers in pigs(Reference O'Doherty, Dillon and Figat10, Reference McDonnell, Figat and O'Doherty31). The reduction in colonic E. coli and Enterobacteriaceae populations may be attributed to the agglutination properties of the extract. Yeast-derived β-glucans have the capacity to agglutinate certain bacterial species, thus preventing attachment to epithelial cell surfaces and subsequent colonisation of mucosal surfaces(Reference Mirelman, Altmann and Eshdat34, Reference Kogan and Kocher35). In addition, Dierick et al. (Reference Dierick, Ovyn and De Smet36) reported that supplementation of intact brown seaweed Ascophyllum nodosum containing bioactive polysaccharides (including laminarin and fucoidans) reduced E. coli numbers in the stomach and small intestine of weanling pigs. Furthermore, the present study indicates that SWE supplementation post-weaning induced an increase in the Lactobacillus: E. coli ratio in the colon of pigs at day 11 post-weaning. The ratio of Lactobacillus: E. coli is routinely regarded as a reliable indicator of gut health, with an increase commonly considered beneficial(Reference Pierce, Sweeney and Brophy3).
The quantity and composition of resident microbiota and fermentable substrate strongly influence the quantity and composition of VFA production in the large intestine(Reference Macfarlane and Macfarlane37). Changes in bacterial populations in the colon at day 11 were not reflected in fermentation patterns, as no differences were observed in VFA concentrations. However, pigs offered GF diets containing SWE had reduced molar proportions of valeric acid in the ileum and a trend for reduced molar proportions of branched-chain fatty acid in the colon at day 117. Branched-chain fatty acids mainly originate from protein fermentation(Reference Macfarlane and Macfarlane37, Reference Mortensen and Clausen38). An increase in proteolytic fermentation can result in the formation of potentially toxic metabolites such as NH3, amines and volatile phenols and indoles(Reference Macfarlane, Gibson and Beatty39, Reference Williams, Verstegen and Tamminga40). Therefore, SWE supplementation post-weaning provides a dietary means to improve gut health. It must be noted that maternal SWE supplementation exerted no influence on intestinal microflora and VFA concentrations in pigs on days 11 and 117.
Pigs weaned from basal-fed sows and offered SWE-supplemented PW had a greater villous height:crypt depth ratio in the jejunum on day 11 post-weaning; however, no effect was observed in pigs weaned from SWE-supplemented sows. However, there was no difference in the feed intake data of pigs that were killed. Numerous authors have reported significant post-weaning alterations in small-intestinal morphology, including villous atrophy and crypt hyperplasia; however, this result is often associated with differences in feed intake(Reference Pluske, Hampson and Williams1, Reference Cera, Mahan and Cross41, Reference McCracken, Spurlock and Roos42).
The effect of dietary treatment on mucin gene expression was evaluated in the ileum and colon of pigs on day 11 post-weaning. Mucin, the primary component of mucus, functions as biophysical barriers against enzymatic, mechanical insult and pathogenic invasion within the gastrointestinal tract(Reference Linden, Sutton and Karlsson43). Seaweed-derived polysaccharides are considered to be an important source of dietary fibre as they are resistant to hydrolysis by digestive enzymes in the upper gastrointestinal tract(Reference Devillé, Damas and Forget44). These results indicate that dietary SWE inclusion post-weaning induced an up-regulation of colonic MUC2 mRNA expression. Consistent with this, an increase in MUC2 mRNA expression has been reported in the ileum(Reference Ryan, Smith and O'Doherty45) and colon(Reference Smith, Ryan and O'Doherty13) of pigs supplemented with laminarin. The induced gene up-regulation of MUC2 in the colon may be attributed to the solubility of laminarin(Reference Read, Currie and Bacic14), allowing a direct effect at the cellular level, since laminarin delivered orally can bind directly and be internalised by intestinal epithelial cells and gut-associated lymphoid tissue cells in the murine model(Reference Rice, Adams and Ozment-Skelton46). Furthermore, elevated MUC2 gene expression may result from direct stimulation of colonic mucosa by laminarin, thus maintaining the protective gut barrier function of mucins after mucosal stimulation by dietary fibres(Reference Enss, Schmidt-Wittig and Höner47). Additionally, alterations in colonic microflora composition may directly influence mucin synthesis and secretion from goblet cells, as adherence of beneficial bacteria to mucosal epithelia stimulates the up-regulation of colonic MUC2 (Reference Moncada, Kammanadiminti and Chadee48).
In addition, the present study investigated whether dietary SWE could modulate mRNA expression of pro- and anti-inflammatory cytokines in ileal and colonic tissues of pigs at day 11 post-weaning. Pie et al. (Reference Pie, Lalles and Blazy49) demonstrated that weaning is associated with up-regulation of intestinal inflammatory cytokine gene expression. Interestingly, dietary treatment of sows did not significantly alter inflammatory cytokine expression in ileal and colonic tissues. However, the mRNA expression of IL-1α in the ileal tissue tended to be lower in pigs weaned from SWE-supplemented sows. It is widely accepted that pro-inflammatory mediators, including IL-1α, not only mediate immunity but can also directly regulate nutrient metabolism(Reference Spurlock50, Reference Webel, Finck and Baker51). Overproduction of pro-inflammatory cytokines can adversely affect growth and feed efficiency, as nutrients are partitioned away from normal growth to support components of the immune response(Reference Johnson52, Reference Klasing and Barnes53). Therefore, enhancing the immune function of weaned pigs and modulating the production of pro- and anti-inflammatory cytokines is beneficial to improving animal health status and minimising disease incidence(Reference Spurlock50). It must be noted, however, that no differences were observed on the growth performance between days 0 and 11 post-weaning in the present study. Dietary treatment had no effect on inflammatory gene expression profiles in pigs at day 117 post-weaning.
Conclusion
In summary, the present results demonstrate that SWE supplementation during lactation and post-weaning influences the growth performance of pigs. Pigs weaned from SWE-supplemented sows had a greater ADG between days 0 and 21 post-weaning compared with pigs weaned from non-SWE-supplemented sows. Dietary SWE supplementation post-weaning decreased E. coli and Enterobacteriaceae numbers in the colon on days 11 and 117, respectively, post-weaning, indicating an antimicrobial property of the extract. Dietary SWE supplementation during lactation tended to suppress pro-inflammatory IL-1α mRNA expression in the ileum of pigs 11 d after weaning. Furthermore, dietary SWE supplementation post-weaning induced an up-regulation in colonic MUC2 mRNA expression in pigs 11 d post-weaning. Collectively, these results demonstrate that SWE supplementation post-weaning, provides nutritionists a dietary means to improve gut health and growth performance in starter pigs. However, there was no growth performance response to SWE inclusion in the GF diet when pigs were weaned from SWE-supplemented sows.
Acknowledgements
Funding for the present study was provided under the National Development Plan, through the Research Stimulus Fund, administered by the Department of Agriculture, Fisheries and Food, Republic of Ireland. S. G. L. wrote the manuscript. J. V. O.’ D. was the principal investigator responsible for the design of the study, supervision of the data collection and statistical analysis. T. S., S. G. L., B. P. L. and B. B. contributed towards the data collection and laboratory collection. S. G. L. and B. B. contributed equally to the laboratory work. All authors contributed to the interpretation of the results, reviewed the manuscript content and approved the final version submitted for publication. None of the authors had a financial or personal conflict of interest in relation to the present study.









