One of the biggest expenses in modern aquaculture is feed cost. Thus, maximised feed utilisation per unit cost is of paramount importance in maintaining an economically viable aquaculture enterprise. In commercial fish feed production, the main concern is the quality, quantity and ratio between nutrients supplied through the raw materials used, and the cost of combining these to fit the nutritional requirements of a specific farmed species at a specific life stage. These nutrient requirements, however, are not absolute. Rather, they should be present in the correct proportion to each other as pointed out by Wilson(Reference Wilson1) with respect to protein requirements. According to him, the protein requirement of an animal comprises a well-balanced mixture of essential and non-essential amino acids, where protein digestibility, amino acid profile and energy concentration of the diet are also considered. Consequently, two of the most commonly used diet optimisation ‘tools’ in aquaculture comprise amino acid optimisation (‘ideal protein concept’)(Reference Akiyama, Oohara and Yamamoto2–Reference Peres and Oliva-Teles4), and optimisation of the ratio between digestible protein (DP) and digestible energy (DE)(Reference Wilson, Halver and Hardy5–Reference Bowen9). The optimal DP:DE ratio refers to the minimum amount of DP required for optimising a certain production trait, such as growth, feed conversion or protein retention at a given DE density. Diets containing DP in excess of requirements will lead to excessive protein deamination, which in turn increases the discharge of nitrogenous compounds into the environment(Reference Cowey10, Reference Watanabe11). Additionally, protein is the most costly macronutrient in aquaculture diets. Thus, there is an economic incentive not to include this nutrient in excess of requirements.
Historically, gilthead sea bream (Sparus aurata) has been perceived to have a high dietary protein requirement, and relatively poor protein utilisation and feed conversion compared with other aquacultured species such as salmonids. This is also reflected in the reported optimal DP:DE ratios for this species(Reference Lupatsch, Kissil and Sklan12, Reference Lupatsch, Kissil and Sklan13), which are considerably higher than for farmed salmonids at the same life stage(Reference Beamish and Medland14–Reference Kaushik and Médale16). Irrespective of species, practically all DP/DE studies reported so far have focused on optimising protein retention. In practice, this is typically done by reducing the dietary DP/DE level, by substituting DE supplied from DP with DE supplied from non-protein DE sources such as fat(Reference Hillestad and Johnsen17–Reference Hemre and Sandnes20) or carbohydrates(Reference Grisdale-Helland and Helland18, Reference Kaushik and Oliva-Teles21–Reference Ekmann, Dalsgaard and Holm25). Only a few studies have commented on the metabolic fate of non-retained (deaminated) protein in this respect(26, Reference Figueiredo-Silva, Corraze and Rema27).
Recent studies have indicated that a substantial part of the deaminated amino acids in blackspot seabream (Pagellus bogaraveo) were converted into fatty acids de novo (Reference Figueiredo-Silva, Corraze and Rema27). This was expressed by increased hepatic lipogenic enzyme activities and the increased hepatic content of palmitic and stearic acids, which are generally recognised to be the main products of de novo lipogenesis(Reference Sargent, Henderson, Tocher and Halver28). Additionally, studies by Enes(Reference Enes, Panserat and Kaushik29, Reference Enes, Panserat and Kaushik30) have shown a positive correlation between dietary protein level and lipogenic enzyme activity both in gilthead sea bream and European seabass (Dicentrarchus labrax), indicating that protein may contribute to lipid biosynthesis in this species. However, since deaminated protein can precede both gluconeogenesis, lipogenesis and complete oxidation for energy purposes, it is hard to quantitatively conclude on the fate of deaminated protein.
The main purpose of the present study was to quantify the amount of dietary protein endogenously converted to body lipid de novo in gilthead sea bream using nine diets enriched with stable isotopes that differed in DP/DE levels and energy density. Additionally, macronutrient retention efficiencies, growth and feed performance parameters were determined. To achieve this, a study comprising two trial periods was conducted. First, an 89 d growth period was carried out feeding gilthead sea bream nine diets differing in DP and DE content. Based on this specific growth rate (SGR), feed conversion ratio (FCR), nutrient digestibility coefficients (ADC), DP retention (DPR), DE retention (DER) and apparent lipid retention (aDLR) were determined. Second, and immediately following the growth period, fish were fed their respective diets for three more days, only now diets were added trace amounts of 13C-labelled protein isolate. This was done to determine the extent to which dietary protein was converted into body lipid endogenously, and to determine how much this lipid biosynthesis contributed to the overall lipid deposition in the fish. As deposited lipid could originate from both dietary and endogenous sources (de novo lipogenesis), digestible lipid retention efficiencies are henceforth referred to as ‘apparent’.
Materials and methods
Culture conditions and fish
Gilthead sea bream with an average individual weight of approximately 120 g were obtained from a commercial fish farm (Ferme Marine de Douhet). They were subsequently stocked into a recirculated aquaculture system comprising twenty-seven fibreglass tanks with a volume of 800 litres, each at a stocking density of twenty fish/tank (BioMar research facility; The North Sea Research Centre). The tanks were fitted with a central bottom drain designed to quickly and efficiently remove faeces and uneaten feed pellets from the water by means of externally mounted swirl separators. The trial facility was supplied with filtered North Sea water with a salinity of 34 g/l, and the temperature was kept at 24°C throughout the trials. Water quality was monitored daily, maintaining O2 saturation between 80 and 100 %, NH4+ below 1·0 mg/l, NO2 − below 1·0 mg/l and NO3 − below 100 mg/l. pH was adjusted to 7·0 using sodium bicarbonate when necessary. The tanks were supplied with system water at a flow rate of 1200 litres/tank per h. A 14 h light–10 h dark photoperiod was maintained throughout the trials. All fish were acclimatised to the facility for 2 weeks during which they were fed a commercial diet (BioMar EFICO YM 664; DP/DE level 21·7 g/MJ) according to a commercial feeding table value (1·5 % of the biomass per d).
Experimental diets
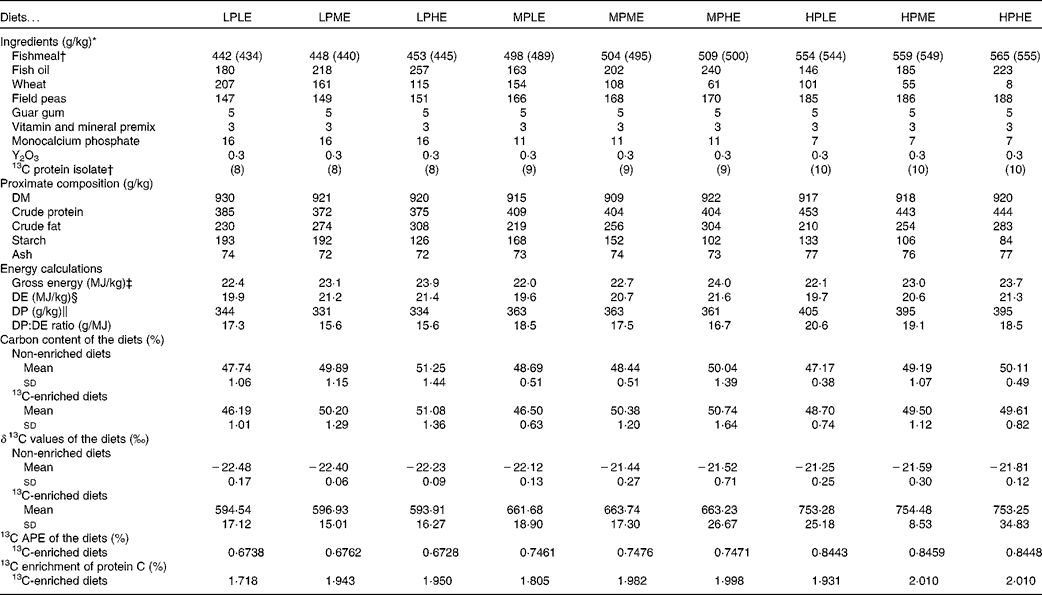
A total of nine experimental diets were prepared using Allix2 feed formulation software (A-systems S.A.; Table 1). The main dietary ingredients were fishmeal, fish oil, wheat and field peas, and the diets were formulated to contain three DP levels (330, 360 or 380 g/kg) and three DE levels (20, 21 or 22 MJ/kg) in a 3 × 3 factorial design. The diets were named according to their DP and DE content (LP, MP or HP for low, medium or high DP content, respectively, and LE, ME or HE for low, medium or high DE content, respectively). For example, the diet LPLE refers to the low DP:low DE diet (expected to contain 330 g/kg DP and 20 MJ/kg DE). The following two versions of each diet were made: one where approximately 1·8 % of the dietary fishmeal was substituted with a 13C-labelled (97–98 % 13C) Spirulina protein isolate (Cambridge Isotope Laboratories, Inc.), and one unlabelled version. The Spirulina protein isolate was chosen as an isotope marker due to its close resemblance to fishmeal regarding the amino acid profile and the lack of non-protein macronutrients that could potentially adversely affect the enrichment data. The diets were supplemented with a vitamin and mineral premix, as well as monocalcium phosphate. Guar gum was added (5 g/kg) to enhance pellet stability and accurately quantify feed waste, and yttrium oxide (Y2O3) was added (0·3 g/kg) as an inert marker enabling indirect measurements of nutrient and stable isotope digestibility. The diets were prepared at the BioMar TechCenter using a twin-screw extruder (Clextral BC-45; Firminy) to produce 4·5 mm pellets. Following extrusion, the diets were dried in a six-level Geelen counterflow continuous dryer (Geelen Counterflow), vacuum coated with fish oil and cooled.
Table 1 Diet formulation, chemical and isotope composition of the experimental diets (Mean values and standard deviations; percentages)

LPLE, low digestible protein (DP):low digestible energy (DE) diet; LPME, low DP:medium DE diet; LPHE, low DP:high DE diet; MPLE, medium DP:low DE diet; MPME, medium DP:medium DE diet; MPHE, medium DP:high DE diet; HPLE, high DP:low DE diet; HPME, high DP:medium DE diet; HPHE, high DP:high DE diet; APE, atom per cent excess.
* Fishmeal: TripleNine Fish Protein; Fish oil: South American (Peru); Guar gum: HV200; LCH A/S; 13C-labelled protein isolate: Cambridge Isotope Laboratories, Inc., algal crude protein fraction isolated from Spirulina algae (U-13C, 97–98 %), lot no. BP-733, catalogue no. CLM-3348-0; Cambridge Isotope Laboratories, Inc.; vitamin and mineral mix is estimated to meet the requirements according to the US National Research Council recommendations(49).
† Each of the nine diets were produced in a 13C-enriched version (where 13C-labelled protein isolate replaced approximately 1·8 % of the con-kix fishmeal; shown in brackets) and a non-enriched version in order to determine 13C APE of the experimental diets.
‡ Gross energy (MJ/kg) was calculated as the sum of the dietary content of protein, lipid and N-free extract (NFE), multiplied by their respective energetic values upon complete oxidation(Reference Blaxter50): Gross energy = (P diet× 23·66)+(L diet× 39·57)+(NFEdiet× 17·17), where P diet, L diet and NFEdiet refer to the dietary protein, lipid and NFE content (%), respectively. NFE was calculated as the sum of dietary protein, lipid, ash and water deducted from 100 % (by difference).
§ The DE (MJ/kg) content was calculated as the dietary gross energy, but with the apparent nutrient digestibility coefficients (ADC) of each nutrient multiplied into their respective terms: DE = (P diet× 23·66 × ADCprotein)+(L diet× 39·57 × ADClipid)+(S diet× 17·17 × ADCstarch), where S diet is the dietary starch content, and ADCprotein, ADClipid and ADCstarch are the ADC of protein, lipid and starch, respectively.
∥ DP (g/kg) = dietary crude protein content × ADCprotein.
Experimental procedures
The study comprised two trial periods: (1) an 89 d growth period (growing fish from approximately 140 to 340 g) feeding each of the nine unlabelled diets to three replicate tanks (i.e. twenty-seven tanks in all), and concluded by a faeces stripping procedure to determine the ADC of macronutrients, and subsequently (2) a 3 d enrichment period feeding the 13C-enriched versions of the experimental diets to determine the proportion of dietary protein converted into body lipid de novo, and to determine the ADC of the two stable carbon isotopes (12C and 13C). All procedures were carried out in accordance with the EC directive 86/609/EEC for animal experiments(31).
Growth period
At start-up, five randomly chosen fish were removed from each of the twenty-seven tanks and euthanised using 250 mg/l of tricaine methanesulfonate (MS-222). The 5 × 27 fish were subsequently pooled and stored at − 20°C until analysis, constituting the initial fish sample. The remaining fifteen fish in each tank were weighed, and the nine experimental diets were fed to triplicate tanks for 89 d. The fish were fed a ration recalculated from a commercial feeding table value for gilthead sea bream, allowing a restrictive iso-DE feeding regimen based on the expected DE content of the respective experimental diets. Any uneaten feed was collected daily and subtracted in the calculations of feed intake. Fish were fed continuously from 08.00 to 14.00 hours using automatic belt feeders. On day 89, the final meal was administered 18 h before faeces stripping, where the fish were anaesthetised using MS-222 (50 mg/l), and a gentle bilateral pressure was applied to the hindgut in order to provoke defecation. Faeces obtained from the fish within each tank were pooled and immediately frozen at 20°C. At 24 h after the stripping procedure, fish were bulk weighed, and seven fish from each tank were removed, euthanised using MS-222 (250 mg/l) and subsequently stored at 20°C for chemical and isotopic analysis.
13C enrichment period
Following the stripping procedure of the growth trial, the eight remaining fish in each tank were fed their respective nine experimental diets for three more days, only now in the 13C protein-enriched version at a feeding rate calculated as described in the ‘Growth period’ section. Uneaten feed was collected daily and subtracted in the calculations of feed intake. At 18 h after the final meal, fish fed the diet MPME (three tanks in all) were stripped for faecal matter according to the method described in the ‘Growth period’ section. This was done in order to determine the ADC of the two stable carbon isotopes (12C and 13C), assuming that they were representative of all the experimental diets. Fish were starved for 48 h after the final meal, and subsequently euthanised using MS-222 (250 mg/l), weighed and stored at 20°C for chemical and isotopic analysis.
Sample preparation and chemical and isotopic analysis
Feed samples
Feed samples were homogenised before analysis using a Krups Speedy Pro homogeniser. Crude protein was determined according to the ISO(32), crude fat according to Bligh & Dyer(Reference Bligh and Dyer33), and DM and ash according to Kolar(Reference Kolar34). Yttrium was determined according to the ISO(35) and Danish Standards(36). Starch analyses were carried out according to the method by Bach Knudsen(Reference Knudsen37), while amino acids were determined according to the EC(38) and ISO(39). Aliquots of the homogenised feed samples were lyophilised and finely ground using a mortar and pestle before the determination of 13C isotope enrichment and elemental carbon (see the ‘Isotopic analysis’ section).
Faecal samples
Faecal samples were freeze-dried before analysis using a Christ Beta 2-16 freeze dryer (Martin Christ Gefriertrocknungsanlagen GmbH). Faecal protein was determined by elemental analysis according to the method described in the ‘Isotopic analysis’ section, assuming that protein equals 6·25 × N. Faecal lipid was determined according to Bligh & Dyer(Reference Bligh and Dyer33). Faecal starch was determined using a BioVision Starch assay kit (catalogue no. K647–100, Tecan GENios microplate reader (Austria) fitted with a 570 nm colorimetric filter), and yttrium was determined according to the ISO(35) and Danish Standards(36). Aliquots of the faecal samples were lyophilised and finely ground using a mortar and pestle before the determination of 13C isotope enrichment, and elemental C and N (see the ‘Isotopic analysis’ section).
Fish samples
Fish sampled initially (one pooled sample), at the end of the growth trial (twenty-seven samples), and after the 13C enrichment trial (twenty-seven samples) were homogenised in a two-step procedure before chemical and/or isotopic analysis. Frozen fish samples were homogenised for 60 s using a Tecator 1094 homogeniser (Tecator AB), and an aliquot of each sample was further homogenised for 30 s using a Büchi Mixer B-400 (BÜCHI Labortechnik AG). All sample aliquots were subjected to crude protein, crude lipid, DM and ash analyses, using the same methodology as described for feed (see the ‘Feed samples’ section). Samples obtained at the end of the growth trial and at the end of the isotope enrichment trial were additionally subjected to stable carbon isotope analysis of their respective lipid fractions. Lipid samples for isotopic analysis were obtained during the lipid extraction process of the Bligh & Dyer(Reference Bligh and Dyer33) procedure.
Isotopic analysis
Feed (enriched and unenriched) and isolated whole-body lipid samples were all subjected to stable isotope (δ13C) and elemental carbon analysis, while faecal samples were additionally subjected to elemental N analysis. Before isotopic analysis, aliquots of all samples were packed and weighed into tin capsules (standard weight pressed tin capsules 5 × 3·5 mm, catalogue no. D1002; Elemental Microanalysis Limited) using an analytical microbalance (Mettler Toledo MT5; Mettler). All stable isotope enrichment, elemental C and N analyses were carried out using a Thermoquest EA1110 CHNS-O elemental analyser coupled to a Thermo Scientific Delta V advantage isotope ratio mass spectrometer via a Thermo Scientific ConFlo IV module.
Calculations
Stable 13C isotope enrichment (δ13C, ‰) of samples was calculated as:
where R sample is the 13C:12C ratio of the sample, and R standard is the 13C:12C ratio of the reference standard calibrated against the international standard V-PDB (Pee Dee Belemnite). The 13C atom per cent excess (APE, %) of the samples was determined as the difference between the atom percentage (13C atm%) of the enriched sample and the unenriched sample (‘blank’), according to:
Atomic percentages were calculated as:
where AR is the absolute 13C:12C ratio of V-PDB (0·0112372) as given by Craig(Reference Craig40).
Lipid deposition of protein origin (LDPO, %) expressed as a fraction of total lipid deposited was calculated according to:
where BMend is the end biomass (in g); BLend is the end body lipid content (in %); C wbl is the end whole-body lipid carbon content (in %); 13C APEwbl is the 13C APE in the whole-body lipid fraction of the fish (in %) at the end of the 3 d enrichment period; PEdiet is the 13C enrichment of dietary protein (in %); FIenr is the intake of 13C-enriched feed (in g); DL is the dietary lipid content (in %); ADClipid is the ADC of dietary lipid (in %); aDLR is the aDLR (in %) obtained from the growth trial.
The recovery of carbon derived from dietary protein in whole-fish lipid (RPCL, %) was calculated according to:
where C diet is the diet carbon content (in %); 13C APEdiet is the 13C APE of the enriched diets (in %); ADCprotein is the ADC of protein (in %).
The ADC of nutrient X (ADC(X)) was calculated according to:
where I diet and I faeces are yttrium concentrations recovered in the diet and faeces, respectively, and X faeces and X diet are the concentrations of X (protein, lipid, starch or carbon isotope) recovered in the faeces and diet, respectively(Reference Maynard and Loosli41).
Statistical analysis
Data on FCR, SGR, ADC, DPR, aDLR, DER, DE intake, LDPO, RPCL, and the proximate composition of whole fish were subjected to two-way ANOVA to test for the main effects of, and interactions between, dietary DE and DP, respectively. Significant differences caused by a main effect were subsequently assessed using the Holm–Sidak all-pairwise multiple comparison test. A probability of P< 0·05 was considered as significant in all analyses.
Results
Diets and dietary 13C enrichment
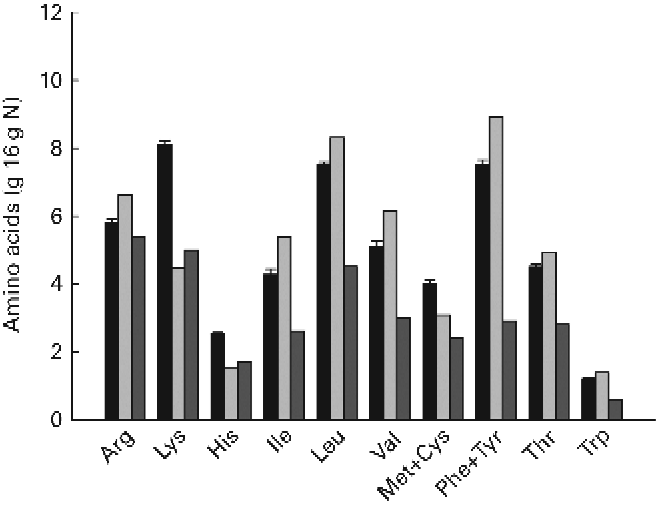
The ingredient composition and chemical and isotopic analyses of the experimental diets are shown in Table 1. The nine experimental diets were designed to comprise three different DP levels combined with three different DE levels in a 3 × 3 factorial design. The crude protein content of the LP, MP and HP diets ranged between 372–385, 404–409 and 443–453 g/kg feed, respectively. Similarly, the DE levels ranged between 19·6–19·9, 20·6–21·2 and 21·3–21·6 MJ/kg feed for the LE, ME and HE diets, respectively. Collectively, the nine experimental diets covered a DP/DE range from 15·6 to 20·6 g/MJ. The dietary carbon content ranged between 46·2 and 51·3 %. The δ13C values of the non-enriched diets ranged between − 22·5 and − 21·3 ‰, while the δ13C values of the enriched diets ranged between 593·9 and 754·5 ‰, corresponding to the 13C APE values from 0·673 to 0·846 %. The measured 13C enrichment of dietary protein carbon ranged between 1·718 and 2·010 %. The indispensable amino acid (IAA) profile of the nine experimental diets is presented in Fig. 2. Also, the IAA profile of the Spirulina protein isolate employed and the IAA requirements of gilthead sea beam approximated by Kaushik(3) are presented.
Digestibility of macronutrients, energy and carbon isotopes
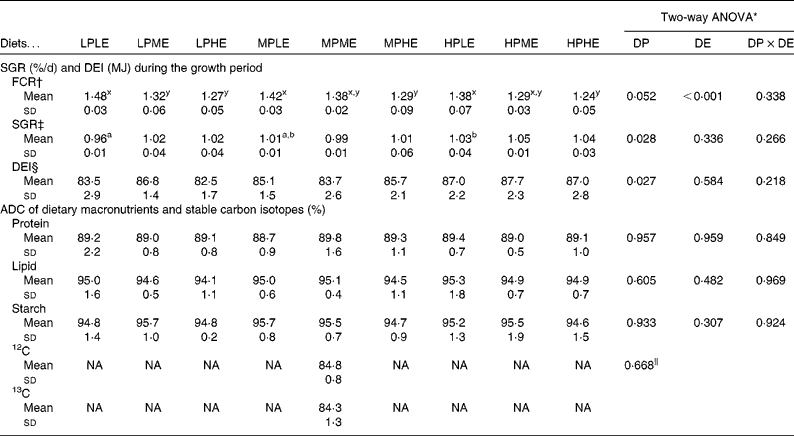
The ADC of macronutrients and stable carbon isotopes are shown in Table 2. The ADC of protein, lipid and starch ranged from 88·7 to 89·8, 94·1 to 95·3 % and 94·6 to 95·7 %, respectively, and were not significantly affected by the dietary treatment. The ADC of the two stable carbon isotopes, 12C and 13C, were 84·8 and 84·3 %, respectively. No significant differences between the ADC of the two carbon isotopes were observed.
Table 2 Feed conversion ratio (FCR), specific growth rate (SGR), digestible energy (DE) intake (DEI) and apparent nutrient digestibility coefficients (ADC) of macronutrients and stable carbon isotopes (Mean values and standard deviations, n 3)

LPLE, low digestible protein (DP):low DE diet; LPME, low DP:medium DE diet; LPHE, low DP:high DE diet; MPLE, medium DP:low DE diet; MPME, medium DP:medium DE diet; MPHE, medium DP:high DE diet; HPLE, high DP:low DE diet; HPME, high DP:medium DE diet; HPHE, high DP:high DE diet; NA, not available.
a,bMean values of DP within the DE groups were significantly different (P< 0·05; Holm–Sidak method).
x,yMean values of DE within the DP groups were significantly different (P< 0·05; Holm–Sidak method).
* Two-way ANOVA (df = 2, 26) on the effects of DP, DE and their interaction (DP × DE).
† FCR = feed consumed/biomass gain.
‡ SGR(Reference Hopkins51)= ln(biomass(final)/biomass(initial))/(days in the trial) × 100.
§ DEI = feed intake(growth trial)× DEdiet.
∥ P value is based on a t test comparing the ADC of the two carbon isotopes.
Feeding, growth, feed conversion ratio and mortality
The results on SGR, FCR and total DE intake from the 89 d feeding trial are presented in Table 2. FCR (ranging from 1·24 to 1·48) were significantly lowered by increasing DE in all the DP groups (LP, MP and HP), while no significant effects of DP were observed in FCR. SGR (ranging from 0·96 to 1·05 %/d) were slightly, but significantly, higher with increasing DP in fish fed the LE diets, while no significant effects of DE on SGR were observed for any of the dietary treatment groups. DE intake ranged between 82·5 and 87·7 MJ, and was significantly different among the DP groups. No mortality occurred throughout the trial.
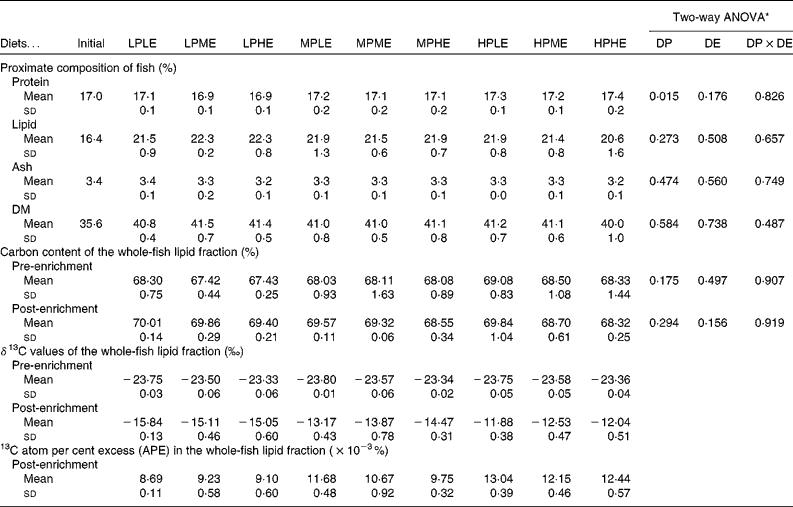
Chemical composition of fish
The chemical composition of whole fish at the beginning and at the end of the 89 d growth period is presented in Table 3. A two-way ANOVA showed no significant effects of dietary treatment on whole-body lipid, ash or DM, while the whole-body protein content was significantly higher in fish fed the HP diets than in fish fed the LP and MP diets. After 3 d of feeding using diets with a 13C-enriched protein content, 13C APE in the lipid fraction of whole fish ranged between 8·69 and 13·04 × 10− 3% (Table 3).
Table 3 Chemical and isotopic composition of whole fish (Mean values and standard deviations, n 3)*

LPLE, low digestible protein (DP):low digestible energy (DE) diet; LPME, low DP:medium DE diet; LPHE, low DP:high DE diet; MPLE, medium DP:low DE diet; MPME, medium DP:medium DE diet; MPHE, medium DP:high DE diet; HPLE, high DP:low DE diet; HPME, high DP:medium DE diet; HPHE, high DP:high DE diet.
* Two-way ANOVA (df = 2, 26) on the effects of DP, DE and their interaction (DP × DE).
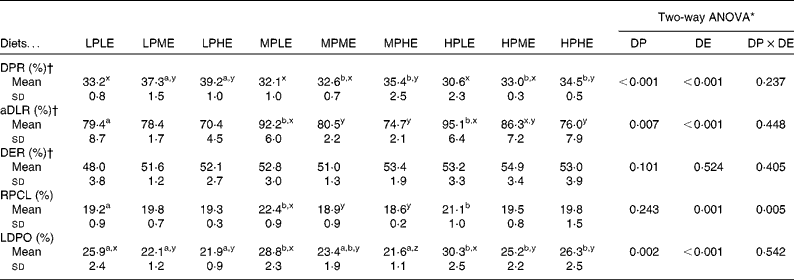
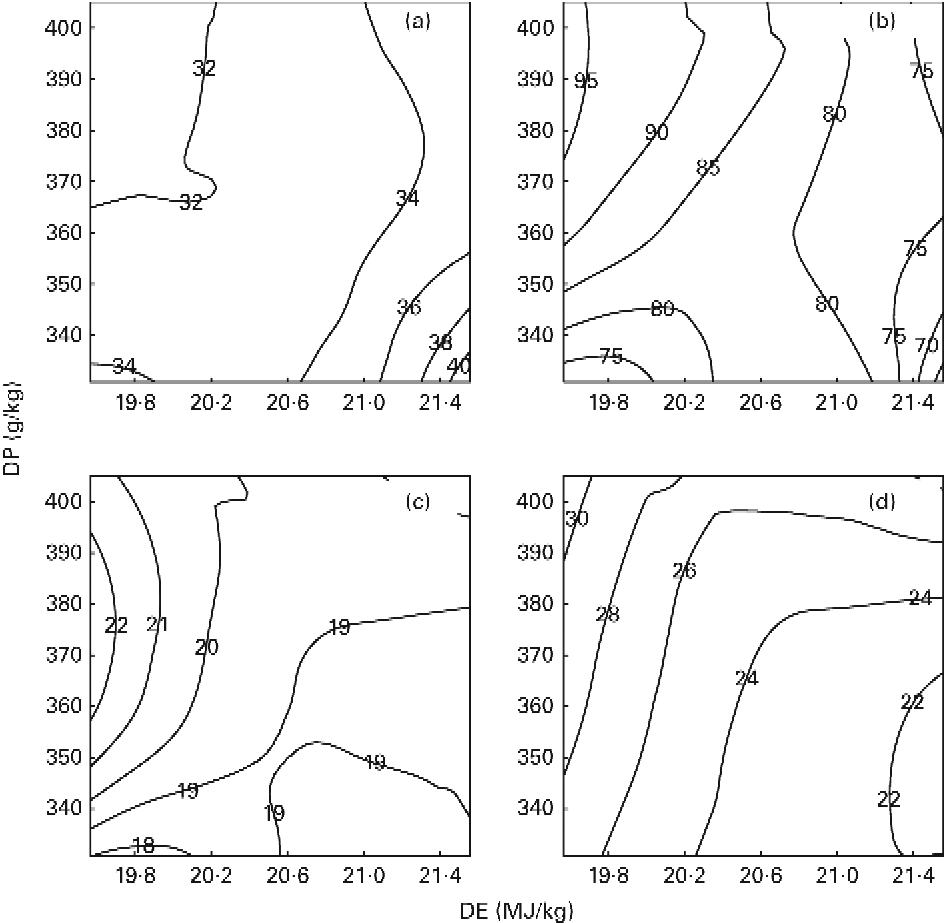
Nutrient retention efficiencies
The results on DPR and aDLR based on the 89 d feeding trial are presented in Table 4 and Fig. 1(a) and (b). DPR ranged between 30·6 and 39·2 %, and was significantly affected by both dietary DP and DE levels, showing increased retention efficiency with increasing DE and decreasing DP (Fig. 1(a)). aDLR ranged between 70·4 and 95·1 %, and was also significantly affected by both dietary DP and DE levels, showing increased retention efficiency with increasing DP within the LE groups and/or with decreasing DE within the MP and HP groups (Fig. 1(b)). The results on DER are also presented in Table 4 ranging between 48·0 and 54·9 %. DER was not significantly affected by the dietary treatment.
Table 4 Digestible macronutrient retention and recovery of protein-derived carbon in the whole-fish lipid fraction (Mean values and standard deviations, n 3)*

LPLE, low digestible protein (DP):low DE diet; LPME, low DP:medium DE diet; LPHE, low DP:high DE diet; MPLE, medium DP:low DE diet; MPME, medium DP:medium DE diet; MPHE, medium DP:high DE diet; HPLE, high DP:low DE diet; HPME, high DP:medium DE diet; HPHE, high DP:high DE diet; DPR, digestible protein retention; aDLR, apparent digestible lipid retention; DER, digestible energy retention; RPCL, recovery of protein-derived carbon in fish lipid; LDPO, lipid deposition of protein origin.
a,bMean values of DP within the DE groups were significantly different (P< 0·05; Holm–Sidak method).
x,yMean values of DE within the DP groups were significantly different (P< 0·05; Holm–Sidak method).
* Two-way ANOVA (df = 2, 26) on the effects of DP, DE and their interaction (DP × DE).
† DPR, aDLR and DER were calculated as the ratio between the amount of protein, lipid and energy retained by the fish and the amount of protein, lipid and energy digested by the fish during the growth trial, respectively.

Fig. 1 Contour plots of the effects on (a) digestible protein (DP) retention (%), (b) apparent digestible lipid retention (%), (c) recovery of protein carbon in the lipid fraction of fish (%) and (d) the percentage of total lipid deposit originating from dietary protein (%) in fish fed the nine diets differing in DP content and digestible energy (DE) content for a period of 89 feeding days. The response values of changes in DE (horizontal axis) and DP (vertical axis) are given directly on the contour curves seen in the four plots. All diets were fed to triplicate tanks.
Recovery of protein-derived carbon in fish lipid and contribution to the total lipid deposition of lipid synthesised from dietary protein de novo
RPCL as determined from the stable isotope analyses ranged between 18·6 and 22·4 %, and was significantly affected by DE and the interaction between DP and DE. Pairwise comparisons showed that RPCL was significantly increased by increasing DP in the LE group and by decreasing DE in the MP group (Table 4; Fig. 1(c)). The contribution to the total lipid deposition of lipid synthesised from dietary protein de novo (LDPO) ranged between 21·6 and 30·2 %, and was significantly affected by both dietary DP and DE levels, showing increased contribution with decreasing DE and/or increasing DP (Table 4; Fig. 1(d)).
Discussion
The main purpose of the present study was to determine: (1) the magnitude of de novo lipogenesis originating from dietary protein in gilthead sea bream fed nine diets with different DP (330, 360 or 380 g/kg) and DE (20, 21 or 22 MJ/kg) levels set up in a 3 × 3 factorial design, and (2) the overall contribution to lipid deposition from de novo lipogenesis originating from dietary protein in this species. Simple, high-quality raw material matrices were applied to assure the highest possible quality of dietary nutrients and to avoid possible anti-nutritional effects associated with certain plant raw materials(Reference Gatlin, Barrows and Brown42). DP/DE levels of the nine experimental diets were deliberately formulated to also cover a lower range (from 15·6 to 20·1 g/MJ) than previously recommended for gilthead sea bream by, for example, Lupatsch et al. (Reference Lupatsch, Kissil and Sklan12) (ranging from 19·0 to 22·6 g/MJ for present fish size). This was done in order to incite the possible effects of protein deficiency on de novo lipogenesis, performance and nutrient retention efficiencies. Also, recommendations on dietary DE densities from the same authors were slightly more conservative (ranging between 15 and 20 MJ/kg) than dietary DE densities of the present study (ranging between 19·6 and 21·6 MJ/kg).
The dietary IAA profile of all the experimental diets satisfied the requirements put forward by Kaushik(Reference Kaushik3). However, since these recommendations were expressed relatively to dietary N content, fish fed the low DP/DE diets might have experienced a general lack of DP.
The present study clearly demonstrated that DP, irrespective of the diet, did indeed contribute significantly to endogenous lipid biosynthesis in gilthead sea bream, as seen both from RPCL and from the contribution of lipid synthesised de novo to total lipid deposition (LDPO; Table 4; Fig. 1(c) and (d)). The results thereby corroborate the findings by Enes et al. (Reference Enes, Panserat and Kaushik30) and Figueiredo-Silva et al. (Reference Figueiredo-Silva, Corraze and Rema27) who both observed a significant correlation between hepatic lipogenic enzyme activity (glucose-6-phosphate dehydrogenase) and dietary protein level in diets for gilthead sea bream and blackspot seabream, respectively. These studies, including the present study, are thereby in contrast to the review by Tocher(Reference Tocher43), who claims that biosynthesis of fatty acids de novo is not likely to occur to any significant extent in marine predatory species. Also, using 13C-labelled dietary protein, Campbell(26) found that between 9·7 and 44·5 % of whole-body lipids in rainbow trout (Oncorhynchus mykiss) juveniles were derived from dietary protein, using diets with a protein:energy ratio ranging between 17·7 and 26·6 g/MJ, respectively. In the present study, approximately one-fifth (18·6–22·4 %) of the dietary DP supplied was converted into body lipid, irrespective of the dietary treatment. Fish fed the MPLE and HPLE diets displayed slightly higher RPCL values (22·4 and 21·1 %, respectively) than fish fed the remaining seven diets. This might have been due to these two diets having the highest DP/DE level in their respective DE groups, triggering excessive protein deamination and donation of extra carbon for lipid biosynthesis (Table 4; Fig. 1(c)). The contribution of lipid synthesised de novo from DP to total lipid deposition (LDPO) ranged between 21·6 and 30·2 %, confirming that de novo lipid synthesis from DP plays a major role in the overall lipid deposition in gilthead sea bream. LDPO was clearly elevated in the low-energy diets of each DP group, and by increasing DP generally (Table 4; Fig. 1(d)). Thus, LDPO values were directly related to dietary DP/DE levels. This was also reflected in the aDLR values that ranged between 70·4 and 95·1 % (Table 4; Fig. 1(b)). Hence, similar to the LDPO results, aDLR increased with increasing dietary DP and decreasing DE (i.e. increasing DP:DE ratio). Conversely, the DPR results showed increasing retention efficiencies with decreasing DP and/or increasing DE levels (i.e. decreasing DP:DE ratio). These results substantiate the so-called protein-sparing effect of substituting DE originating from DP with DE from non-protein sources, as already reported in a number of aquacultured species(Reference Venou, Alexis and Fountoulaki23, Reference Dias, Corraze and Arzel44, Reference Hemre, Mommsen and Krogdahl45), including gilthead sea bream(Reference Company, Calduch-Giner and Pérez-Sánchez19, Reference Fernández, Miquel and Córdoba24, Reference Vergara, Robainà and Izquierdo46) (Table 4; Fig. 1(a)). Thus, the aDLR and DPR results combined suggest that while protein was spared by a decreasing dietary DP/DE level, the opposite was true for lipid, substantiating that deaminated DP was indeed converted into body lipids. The results of these opposing nutrient retention dynamics have supposedly rendered differences in the overall DER insignificant, as shown in Table 4. In addition, the proximate composition of whole fish was largely unaffected by the dietary treatment. No significant effects were observed in whole-body lipid, ash and DM, while a very small, but significant, effect of dietary DP was observed on whole-body protein content. This is in accordance with the finding of Bonaldo et al. (Reference Bonaldo, Isani and Fontanillas47), who found no difference in the body composition of gilthead sea bream fed three diets differing in dietary DP:DE ratio for 81 d. Thus, considering lipogenesis, nutrient retention and body composition results of the present study collectively, fish appeared to endeavour to rigorously maintain a certain whole-body energy status under a wide variety of dietary DP:DE ratios, even if substantial amounts of dietary protein were sacrificed to achieve this.
It was expected that the SGR values obtained from the growth period would not differ significantly among the dietary treatment groups, as fish were fed iso-DE throughout the trial. However, a small, but significant, difference was observed between the LPLE and HPLE fish. This could be partly explained by the slightly lower DE intake observed in fish fed the LPLE diet (83·5 MJ) compared with fish fed the HPLE diet (87·0 MJ), or by a possible general lack of DP experienced by LPLE fish. However, since dietary DP levels did not have any significant effect on the FCR obtained from the growth trial, the latter point probably does not apply. In contrast, FCR were clearly improved by a dietary DE increase. This clear link between dietary DE and FCR responses has been reported earlier in a number of aquacultured fish species(Reference Hillestad and Johnsen17, Reference El-Mowafi, Ruohonen and Hevroy48), including gilthead sea bream(Reference Lupatsch, Kissil and Sklan12, Reference Lupatsch, Kissil and Sklan13) when growth was not limited by dietary protein content. The present results thereby indicate that gilthead sea bream have the ability to efficiently utilise diets with lower DP:DE ratios and higher energy densities (virtually resembling commercial diets for salmonid species) than previously recommended(Reference Lupatsch, Kissil and Sklan12) without showing adverse effects on the proximate composition or performance of the fish.
The measured ADC of protein, lipid and starch did not differ significantly among the dietary treatments, which was also expected from diet optimisation, considering that the same raw materials were used in all diets, and only inclusion levels differed.
An inherent problem by using a tracer to investigate metabolic pathways is the potential difference in functional behaviour between the tracer and the tracee. In the present study, a uniformly 13C-labelled Spirulina protein isolate was used to trace the fate of dietary protein, which mainly originated from con-kix fishmeal. However, possible differences in overall protein digestibility, amino acid profile and individual amino acid digestibility between the Spirulina protein isolate and dietary protein could potentially lead to differences in the way that the tracer and the tracee were metabolised, rendering the tracer unsuitable for the purpose. However, when comparing the IAA profile of the Spirulina protein isolate and experimental diets, only small differences were apparent (Fig. 2). The only clear difference was a considerably higher lysine content of the experimental diets. This difference, however, was unlikely to change the overall pattern in amino acid deamination since the remaining amino acids were basically in balance, and all experimental diets, as such, fulfilled the general IAA requirements of gilthead sea bream(Reference Kaushik3, Reference Peres and Oliva-Teles4). The ADC of individual amino acids were not determined in the present trial. However, the ADC of measured stable carbon isotopes displayed no significant differences between 12C and 13C, indicating that Spirulina whole protein was indeed digested similarly to the remaining dietary protein fraction. Thus, it was assumed that the Spirulina protein isolate could be considered a true tracer, not behaving functionally different from the tracee.

Fig. 2 Indispensable amino acid (IAA) profile including Cys and Tyr of the diets (■) and Spirulina protein isolate (![]() ). The IAA requirements of gilthead sea bream (Sparus aurata) as approximated by Kaushik(Reference Kaushik3) are shown as
). The IAA requirements of gilthead sea bream (Sparus aurata) as approximated by Kaushik(Reference Kaushik3) are shown as ![]() . Values are means (n 9), with standard deviations represented by vertical bars.
. Values are means (n 9), with standard deviations represented by vertical bars.
Conclusion
For the first time, orally administered 13C-labelled protein was applied to quantify de novo lipogenesis originating from dietary protein, and to determine the importance of this in the overall body lipid deposition in gilthead sea bream. Irrespective of the dietary treatment, the fish converted substantial amounts of carbon derived from dietary protein into body lipids, which in turn contributed significantly to total body lipid deposition. Despite dietary effects on protein and lipid retention efficiencies and de novo lipogenesis, the fish were able to maintain a constant retention of DE with no significant effects seen on the whole body composition. The results indicate that this species may have evolved to maximise energy storage (in the form of lipid) for seasonal, migratory or maturation purposes at the expense of increasing body size through more efficient use of protein for growth. Additionally, the improvement of FCR by increased DE combined with an improvement of DPR with decreasing DP/DE levels suggest that gilthead sea bream is able to efficiently utilise feeds within a wide range of dietary DP:DE ratios, which could be taken into consideration in the future production of commercial feeds for this species.
Acknowledgements
The authors would like to thank the technical and laboratory staff at DTU Aqua, Hirtshals, Denmark, BioMar A/S's trial station, Hirtshals, Denmark and Risø DTU, Roskilde, Denmark for their invaluable help during the experimentation and analytical work. The present study was part of K. S. E.'s PhD study, sponsored by DTU Aqua and BioMar A/S, Denmark. The authors' responsibilities were as follows: all authors helped to plan the research; K. S. E. was responsible for the biological trials; K. S. E. performed the laboratory work; K. S. E. analysed the data; all authors helped to interpret the results; K. S. E. wrote the manuscript; all authors helped to proofread the manuscript. There are no conflicts of interest to report.