Resveratrol (3,4′,5 trihydroxystilbene) is a polyphenolic secondary metabolite produced within plants in response to a range of environmental stressors( Reference Fremont 1 ). Previous investigations in young, healthy humans have demonstrated significantly increased cerebral blood flow (CBF) after acute resveratrol supplementation( Reference Kennedy, Wightman and Reay 2 ), which is probably mediated by the ability of resveratrol to modulate nitric oxide synthesis( Reference Gresele, Pignatelli and Guglielmini 3 ). In line with this, oral consumption has been shown to enhance endothelium-dependent relaxation in rats( Reference Rivera, Morón and Zarzuelo 4 , Reference Rush, Quadrilatero and Levy 5 ), and to improve flow-mediated dilatation in overweight/obese humans( Reference Wong, Howe and Buckley 6 ). An increase in blood-borne neural metabolic substrates such as oxygen( Reference Moss, Scholey and Wesnes 7 ) and glucose( Reference Scholey, Harper and Kennedy 8 ) has been shown to enhance aspects of cognitive performance in healthy, young humans. However, to date, there is no evidence that cognitive function is modulated during acute, resveratrol-mediated increases in CBF.
One potential explanation for this lack of cognitive effects is the rapid metabolism and poor bioavailability of oral resveratrol( Reference Walle, Hsieh and DeLegge 9 ), which might reduce its potential bioactivity. Pharmacokinetic studies have demonstrated plasma C max levels of resveratrol metabolites between 0·9 and 3·7 µm following a single oral dose of 500 mg of resveratrol( Reference Boocock, Faust and Patel 10 ), with levels of the parent compound at trace or undetectable concentrations( Reference Kennedy, Wightman and Reay 2 , Reference Boocock, Faust and Patel 10 – Reference Marier, Vachon and Gritsas 13 ) after acute, bolus supplementation. Conversely, results from three preclinical chemopreventive efficacy papers suggest that repeated low daily doses of resveratrol (up to 2 mg/kg) are sufficient to produce peak plasma concentrations of aglycone resveratrol of up to 2 µm, potentially exerting beneficial chemopreventive effects( Reference Gescher and Steward 14 ) possibly as a result of a cumulative increase in plasma levels of resveratrol.
Thus, the current study investigated the effects of 28-d supplementation with 500 mg of resveratrol in healthy adults, with the hypothesis being that daily consumption of this polyphenol, over an extended period, may increase bioavailability in terms of plasma levels and may potentiate any effects on cognitive performance and CBF. In the current study, continuous-wave near-IR spectroscopy (CW-NIRS) was used to monitor acute changes in CBF in the prefrontal cortex during the performance of cognitive tasks that activate this brain region. This technique was combined with transcranial Doppler (TCD) sonography, applied to the middle cerebral artery (MCA), which provides a measure of acute and chronic changes in global CBF velocity (CBFV) and which has been converged successfully with NIRS previously( Reference Ide, Horn and Secher 15 ). Resveratrol has previously been shown to interact with a number of diffuse, health-related parameters such as antioxidant and anti-inflammatory status( Reference Jia, Zhu and Misra 16 , Reference Donnelly, Newton and Kennedy 17 ), monoamine oxidase-A and B (MAO-A/B) activity( Reference Xu, Wang and You 18 ) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha PGC-1α ( Reference Liu, Li and Liu 19 ) production. Hence, the current study also assessed health, mood and sleep parameters via questionnaires.
Methods
Participants
All participants reported themselves to be in good health and free from illicit drugs, alcohol, prescription medication and herbal extracts/food supplements at each assessment. Participants confirmed that they would also abstain from the latter for the duration of the study and that any changes in medication or health status would be reported to the researcher when they occurred. Participants who had suffered a head injury, neurological disorder or neuro-developmental disorder were excluded from participation, as were those who did not have English as their first language, or had any relevant food allergies or intolerances, digestive problems, smoked tobacco, drank excessive amounts of caffeine (more than 600 mg/d as assessed by a caffeine consumption questionnaire), took illicit social drugs, were pregnant, seeking to become so or were breast-feeding.
The study received ethical approval from the Northumbria University Psychology Department (within the Faculty of Health and Life Sciences) ethics committee (reference: SUB16_EW_1010; date approved 11 November 2010) and was conducted according to the Declaration of Helsinki (1964). All participants gave their written informed consent before their inclusion in the study.
See Table 1 for participant composition (broken down per analysis).
Table 1 Participant compositionFootnote *

NIRS, near-IR spectroscopy; TCD, transcranial Doppler; GHQ, General Health Questionnaire; POMS, Profile of Mood States; PSQI, Pittsburgh Sleep Quality Index.
* In all, sixty participants were originally recruited to take part in all aspects of assessments apart from the blood pressure measurement, which utilised only thirty participants because of the potential disruption this may have caused to NIRS measurement. Reasons for excluding data from analyses include: technical problems with equipment (affecting aspects of twelve cognitive performance data sets, fourteen NIRS, fourteen TCD recordings (namely not being able to locate a consistent, 5-min, blood flow trace in the latter and data which were outside of the calculated standard deviations of this cohort, and may suggest an ill-fitting headband, with regards NIRS) and six blood pressure readings) and participants not complying with proper completion of measures/omitting to respond (affecting aspects of seven cognitive performance data sets, seven responses from the GHQ, six from the POMS, seven from the PSQI, five from the food consumption questionnaire and three from the treatment guess response).
Treatments
Over the course of this 28-d supplementation study, participants received either 500 mg of pure trans-resveratrol (TransmaxTM by BiotiviaTM with a guaranteed purity of 98 %, also containing 10 mg of piperine/ capsule) or an inert placebo (methyl cellulose) once daily, with the treatment allocation dictated by Latin square. Participants were instructed to consume their daily capsule in the morning and preferably with breakfast.
Participants consumed their first and last capsule of treatment during the two laboratory visits and were instructed to self-supplement every day in the interim. Participants kept a treatment log during this time, noting down the time of capsule consumption every day. A treatment pot containing thirty-two capsules was given to each participant at the end of visit 1, which was enough for 28 d of supplementation plus extra in case of loss, continued supplementation because of unforeseen circumstances and to verify compliance.
All treatments were administered in identical green vegetarian capsules with the BiotiviaTM logo and presented in identical white treatment pots with only the participant number to identify them. All treatments were produced by BiotiviaTM, prepared by the lead investigator and coded by a third party who had no further involvement in any aspect of the study. No member of the investigational team was aware of the contents of the capsules until a blind-data review was completed.
Measures of cerebral blood flow (CBF)
Two complementary techniques were used as follows.
Acute changes in CBF-NIRS: NIRS is non-invasive brain imaging technique predicated on the absorption by oxygenated HB (oxy-Hb) and deoxygenated Hb (deoxy-Hb) of differing wavelengths of IR light, introduced through the intact scalp/skull. CW-NIRS can be used to assess acute changes in local CBF, as indexed by concentration changes in total Hb during a single continuous recording session; see Kennedy et al.( Reference Kennedy, Wightman and Reay 2 ) for a full description of the methods used here. Given that CW-NIRS generates concentration change data that is intrinsically baseline-adjusted to the concentration immediately before the first data point in the recording session, it cannot be used to quantify gross changes in CBF parameters that take place between two separate recording sessions. In this instance, the change from baseline data generated by the NIRS system was subjected to a second baseline adjustment by creating ‘change from baseline’ data with respect to the 10 min of NIRS data collected immediately before the treatment; this provided a more accurate baseline measure of immediately pre-treatment NIRS parameters. All subsequent NIRS data were collapsed into 2-min epochs (twenty resting-period epochs spanning 0–40 min and twenty task-period epochs spanning 40–80 min).
Chronic changes in CBF-TCD: given the inability of CW-NIRS to measure chronic changes in CBF parameters, a second measure of CBF was also used. TCD sonography is a non-invasive method of measuring CBFV through the basal intracerebral vessels through the intact skull( Reference Markus 20 ), and it was used at pre- and post-dose time points on day 1 and day 28. Pulses of ultrasound penetrate the skull at a number of ‘acoustic windows’, which include temporal, orbital, foraminal and submandibular, insonating vessels at particular depths, with the returning ‘echo’ displayed as a Doppler waveform( Reference Nicoletto and Burkman 21 ). The mean velocity, peak systolic velocity, diastolic velocity and pulsatility index (all centimetre per second) of the insonated vessel are provided, indicating the speed of the flow of blood and the variability of blood velocity.
TCD has been used to investigate blood flow abnormalities in a number of haematological, for example, stroke risk in sickle cell patients( Reference Adams, McKie and Carl 22 ), and vascular, for example, cerebrovascular reactivity in degenerative and vascular dementia( Reference Vicenzini, Ricciardi and Altieri 23 ), disorders, as well as investigating the relationship between brain activity (in response to cognitive tasks) and blood flow velocity in healthy participants( Reference Harders, Laborde and Droste 24 ) and the CBFV response to pharmacological interventions, for example, caffeine( Reference Jones, Herning and Cadet 25 ), and drugs, for example, in cocaine abusers( Reference Herning, King and Better 26 ).
In the current study, CBFV was measured with participants sitting in a reclined position in a quiet room. A trans-temporal acoustic window was used for assessment of the right MCA using pulsed TCD (Digi-LiteTM; Rimed) with a 2-MHz probe held in place by a light, mounted head frame. This device provides mean velocity, peak systolic velocity, diastolic velocity and pulsatility index information every 30 sec, equating to approximately 10 values across the 5-min recording used here, for each of the four aforementioned variables. These were averaged to give 1 value for that time point (before and after dose on day 1 and before and after dose on day 28) for statistical analysis.
Cognitive tasks
The computerised battery of cognitive tasks (which all, to a greater or lesser extent, activate the prefrontal cortex: serial subtractions( Reference Kazui, Kitagaki and Mori 27 ); rapid visual information processing (RVIP)( Reference Coull, Frith and Frackowiak 28 ); 3-Back( Reference Jansma, Ramsey and Coppola 29 )) were delivered on a laptop using the Computerised Mental Performance Assessment System (COMPASS, University of Northumbria) software, and they comprised
serial subtractions (2 min each of serial 7, 13 and 17 s)
RVIP (2 min)
Both the serial subtraction and RVIP task are described in detail in Kennedy et al.( Reference Kennedy, Wightman and Reay 2 ).
3-Back: the 3-Back version of this task was used in this paradigm, and it required participants to indicate whether the letter presented on screen was also present three letters back in the letter sequence. Participants must respond by pressing the ‘yes’ or ‘no’ button on the response box, to each letter, as quickly as they can. This task lasts for 2 min, and it is scored for accuracy and reaction time.
Questionnaires
Food consumption questionnaire
A non-validated food consumption questionnaire was used to collect information on the general diet of participants (e.g. ‘How many portions of fruit and vegetables did you eat on an average day in the past week?’) and specifically polyphenol/resveratrol consumption (e.g. ‘In the entire previous week, on how many occasions have you eaten a portion of berries or grapes?’). The questionnaire consisted of thirteen questions, with several questions also relating to compliance (e.g. ‘Was treatment consumed with breakfast and/or before 9:30 am every day in the past week?’) and medication (‘Have you consumed any medication in the past week? If so, please state the medication, dose, when taken and for what reason’). This researcher-created questionnaire has no reliability/sensitivity measures, and it was used solely as a tool to detect any gross changes in the consumption patterns of participants, which might affect outcome measures. The researcher noted no salient dietary or medication changes across the study for any of the participants.
General Health Questionnaire
The General Health Questionnaire (GHQ)( Reference Goldberg 30 ) used in the current study was the twenty-eight-item scaled version, which assesses somatic symptoms, anxiety and insomnia, social dysfunction and severe depression. The twenty-eight items are scored from 0 to 3, with participants indicating the frequency or extent to which they have experienced a number of issues, such as ‘Have you recently been having hot or cold spells?’, in the previous week. The items combine to assess the four aforementioned sub-scales, and the total possible score (when these four sub-scales are collated) ranges from 0 to 84, with higher scores representing more negative symptoms.
Profile of Mood States
The Profile of Mood States (POMS) is a well-validated questionnaire of mood states and their fluctuations both in the clinical and research settings( Reference McNair, Lorr and Droppleman 31 ). Participants rated sixty-five adjectives (e.g. unhappy, considerate), in terms of how much they had felt each one in the past week, using a five-point scale from ‘not at all’ to ‘extremely’. Scores from these sixty-five items (which includes seven dummy adjectives) are combined to give six global scores of ‘tension’, ‘depression’, ‘anger’, ‘fatigue’, ‘confusion’ and ‘vigour’. A total mood disturbance score can also be calculated by adding the scores from the first five of these global scores and subtracting ‘vigour’.
Pittsburgh Sleep Quality Inventory
The Pittsburgh Sleep Quality Inventory (PSQI) is a well-validated subjective measure of the quality and pattern of sleep( Reference Buysse, Reynolds and Monk 32 ). The current study tailored this questionnaire to assess sleep during the past ‘week’ rather than ‘monthly’ as per the original. The PSQI assesses seven factors – subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction – via questions regarding sleep timings and zero to three-point scales in which participants rate whether they have experienced a number of issues (e.g. ‘During the past week, how often have you had trouble sleeping because you have had bad dreams?’) from ‘not during the past week’ to ‘3 or more times in the past week’. A global sleep score is created by totalling the seven subfactor scores, with higher scores indicating poorer sleep quality.
Treatment guess
During the day 28 visit, participants were asked to guess which treatment they thought they had been taking for the duration of the study and to explain any reasons for that guess.
Procedure
This investigation required participants to attend the laboratory for an initial training/screening session and then on two separate occasions, 28 d apart, for laboratory-based testing sessions. Participants were required to supplement themselves with one capsule per d in the interim.
Upon arrival at both day 1 and day 28, laboratory visit participants completed four questionnaires: a food consumption questionnaire; the GHQ; POMS; and the PSQI. All questionnaires were answered in relation to the previous 7 d and completed every 7 d during the supplementation period. After filling in the questionnaires, participants then gave a blood pressure (BP) reading or an intravenous blood sample (fifteen participants provided blood samples; see below for more information and the demographics of the seven participants from the resveratrol condition entered into the analysis), which was immediately followed by a 5-min rest. A 5-min recording of cerebral perfusion in the MCA was then taken with TCD. The NIRS headband was then positioned onto the forehead of the participant to monitor CBF in the prefrontal cortex throughout the session. Once a reliable trace was identified, participants commenced 20 min (×2 repetitions of the battery) of baseline cognitive tasks. The first of these repetitions acted as a ‘refresher’, attenuating any practice effects, and the second was used to create change from baseline data for the analysis of cognitive outcome data. A 10-min rest period then followed, with NIRS data averaged across this period and used as an accurate, immediately pre-treatment baseline for the calculation of change from baseline data for the post-treatment periods. During this 10-min resting period, participants watched a non-arousing DVD. Participants then consumed the first day’s treatment and continued to watch the DVD for a further 40-min absorption period. After this period, a BP reading was taken in those who did not provide a blood sample previously and 40 min of post-dose tasks commenced. After completion of the task, a further BP reading was taken from the relevant participants, followed by a short break before the second TCD recording was conducted. After the TCD recording, participants were either free to leave the laboratory or provided a final blood sample if they were part of the aforementioned sub-section of participants. The timelines and running order of the testing sessions are shown in Fig. 1.

Fig. 1 Upon arrival, participants completed four questionnaires (a food consumption questionnaire, the General Health Questionnaire, Profile of Mood States and the Pittsburgh Sleep Quality Index), which they answered in relation to the previous 7 d and completed every 7 d during the supplementation period. Participants then gave a blood pressure (BP) reading or an intravenous blood sample, which was immediately followed by a 5-min rest. A 5-min recording of cerebral perfusion in the middle cerebral artery was then taken with the transcranial Doppler (TCD). The near-IR spectroscopy (NIRS) headband was then positioned, and 20 min of baseline tasks commenced. A 10-min rest then followed, during which participants watched a non-arousing DVD. Participants then consumed their treatment capsule and continued to watch the DVD for a further 40-min absorption period. A BP reading was then taken from a sub-sample of participants and 36 min of post-dose tasks commenced. The NIRS headband was removed and a further BP reading taken, followed by a short break, before the second TCD recording was conducted. Following the TCD recording, the aforementioned sub-section of participants provided a blood sample and left the laboratory. RVIP, rapid visual information processing.
Bioavailability assessment
Participants
Complete sample sets comprising all four time points were obtained from fifteen participants (eight from placebo and seven from resveratrol; ten females and five males; mean age 19·87 years; range 18–25 years). All participants were asked, at the beginning of the study, if they would provide blood samples as part of the investigation: the above fifteen participants represent those who agreed to this aspect of the study and for whom all four samples could be collected in full. The seven resveratrol participants included in the analysis comprised six females and one male, with a mean age of 19·43 years, ranging from 18 to 21 years.
Venous blood samples were collected before consuming the day’s treatment, and 110 min post dose in this sub-sample of participants using 4·7-ml monovettes (containing lithium heparin) (Sarstedt AG & Co.). Samples were centrifuged at 2500 rpm for 15 min at 20ºC to yield plasma, which was then stored at –80°C until analysis.
The preparation of samples and LC-MS analysis are as per a previous study conducted by this laboratory( Reference Wightman, Reay and Haskell 33 ).
Statistics
The analyses of TCD, plasma, questionnaire, behavioural and treatment guess data were conducted with IBM SPSS Statistics 19.0 for Windows (SPSS Inc.). NIRS data were analysed with Minitab 16 for Windows (Minitab Inc.).
Questionnaire data analysis
Questionnaire data (GHQ, POMS and PSQI) for each of the four post-dose weekly completions were analysed as change from baseline (the questionnaire scores obtained on day 1 before treatment) for each individual variable/sub-component by a mixed (day (×4): 7, 14, 21, 28, by treatment (×2): 500 mg resveratrol and placebo) ANOVA with Bonferroni-corrected post hoc Student’s t tests conducted if a significant main and/or interaction effect was evinced here.
Treatment guess analysis
Treatment guess data were analysed by χ 2 test.
Transcranial Doppler
The raw data for each of the four TCD variables (mean velocity, peak systolic velocity, diastolic velocity and pulsatility index) were analysed by a mixed (treatment (×2): 500 mg resveratrol and placebo, by time (×4): baseline day 1, post-dose day 1, pre-dose day 28 and post-dose day 28) ANOVA.
Plasma analysis
The raw data for each of the four forms of plasma resveratrol (resveratrol-3-sulphate, resveratrol-4'-O-glucuronide, resveratrol-3'-O-glucuronide and ‘total metabolites’; which is the sum of the three metabolites) were analysed via ANOVA with time as a factor (×4: baseline day 1, post-dose day 1, pre-dose day 28 and post-dose day 28).
Cognitive task data and blood pressure analysis
The cognitive task and BP measures produce data that can be analysed to assess both acute (potential treatment effects within day 1), pure-chronic (chronic treatment-related effects that have taken place across the 28-d supplementation period but before taking the day 28 treatment) and superimposed acute/chronic (the difference in ‘acute’ effects between day 1 and day 28) effects of resveratrol. To adequately analyse the ‘acute’, ‘pure chronic’ and ‘superimposed acute/chronic’ effects of the treatments, two separate ANOVA were conducted:
-
1. Pure chronic effects
To ascertain whether any pure chronic effects of resveratrol supplementation had taken place, pre-dose data on day 28 were converted to change from day 1 pre-dose baseline and analysed via one-way ANOVA to compare performance between treatments.
-
2. Acute, chronic and superimposed effects
To ascertain whether any acute and/or superimposed chronic effects of resveratrol supplementation had taken place, data were converted to change from baseline with respect to the pre-treatment scores on the first day of treatment (day 1) and analysed via a repeated measures ANOVA (treatment (resveratrol/placebo,×repetition (×4 for cognitive data and ×2 for BP), by day (day 1/28)).
Both ANOVA were used in order to tease apart acute effects restricted to day 1 (treatment×day interactions with significant effects restricted to day 1), acute effects across both day 1 and day 28 (main effect of treatment and/or a treatment×repetition interaction) and a superimposed acute/chronic effect (treatment×day interaction with significant effects restricted to day 28 (interpreted with reference to the pure chronic ANOVA results)). If any such main and/or interaction effects were observed, then Bonferroni-corrected post hoc Student’s t tests were conducted to assess where these differences lie. This analysis plan has proven sensitivity in detecting the acute and chronic effects of ginseng in healthy, human participants previously( Reference Reay, Scholey and Kennedy 34 ).
Near-IR spectroscopy analysis
NIRS data were converted to ‘change from baseline’ (calculated from the 10-min pre-treatment resting period) and averaged across 2-min epochs during the 40-min ‘rest/absorption’ and 40-min cognitive task performance period. ANOVA (treatment group×2-min epoch×day) was conducted on these data, with planned comparisons of data from each epoch being made between placebo and 500 mg of resveratrol (resulting in forty planned comparisons for oxy-HB, deoxy-Hb and total-Hb) using t tests calculated with the mean squares error from the ANOVA( Reference Keppel 35 ). A significant result on this ANOVA was not used as a prerequisite for carrying out and interpreting the planned comparisons, and it is therefore not presented here. However, in order to reduce the potential for type I errors, all planned comparisons were Bonferroni corrected, and only those planned comparisons associated with a consistent pattern of significant effects are interpreted and reported herein.
Results
Compliance
Potential compliance ranged from 0 to 114 % (the upper limit reflecting thirty-two capsules consumed over 28 d). Average compliance was 101 %, with a range of 78·5–114·3 %. Data from one participant with 78·5 % compliance (who provided blood samples in the placebo condition only) were excluded from analysis, because they were below a pre-set level of 80 %, making average compliance 101·4 % with a range of 92·9–114·3 %.
Treatment guess
χ 2 test revealed no significant difference between treatment guesses in the two treatment groups: χ 2=0·766; df=1; P=381.
Near-IR spectroscopy parameters
Total Hb
Planned comparisons revealed that, on day 1, levels of total-Hb were significantly higher after resveratrol, compared with placebo, during the 2-min epochs spanning 35–38 min post dose (35/36 min (P=0·003), 37/38 min (P=0·008)) in the absorption period and the epochs spanning 75–78 min (75/76 min (P=0·008), 77/78 min (P=0·005)) of the post-dose task period. No significant differences were found between resveratrol and placebo on day 28.
Oxygenated Hb
Planned comparisons revealed that, on day 1, levels of oxy-Hb were significantly higher in the resveratrol condition, compared with placebo, during the 2-min epochs commencing 23 (P=0·002), 27 (P=0·005), 33 (P=0·002), 35 (P=0·001) and 37 (P=0·009) min post dose in the absorption period and the epochs spanning 41–44 (41/42 (P=0·006), 43/44 (P=0·001)), 53–54 (P=0·001), 61–68 (P=0·0008, 0·001, 0·007 and 0·001, respectively), 71–72 (P=0·003), 75–78 (75/76 (P=0·0002) and 77/78 (P=0·0002)) min of the post-dose task period. No significant differences were found between resveratrol and placebo on day 28.
Deoxygenated Hb
Planned comparisons revealed that, on day 1, levels of deoxy-Hb were significantly higher in the placebo condition, compared with resveratrol, during the 2-min epochs commencing 27 (P=0·001), 29 (P=0·006) and 35 (P=0·003) min post-treatment in the absorption period and the epochs commencing 43 min (P=0·004), and spanning 51–54 min (51/52 min (P=0·0002), 53/54 min (P=0·004)), and those spanning 61–72 min (61/62 min (P=0·001), 63/64 min (P=0·003), 65/66 min (P=0·0005), 67/68 min (P=0·002), 69/70 min (P=0·004), 71/72 min (P=0·0008), respectively) and those spanning 75–80 min (75/76 min (P=0·001), 77/78 min (P=0·003), 79/80 min (P=0·004), respectively) min of the post-dose task period. No significant differences were found between resveratrol and placebo on day 28.
Mean total-, oxy- and deoxy-Hb levels for placebo and resveratrol, across day 1 and day 28, are shown in Fig. 2.
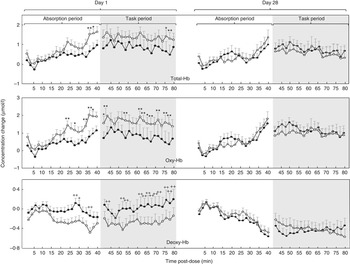
Fig. 2 Concentration changes from baseline in levels of (top) total Hb (Total-Hb), (middle) oxygenated Hb (Oxy-Hb) and (bottom) deoxygenated Hb (Deoxy-Hb) averaged across two-min epochs during a 40-min absorption period and subsequent 40 min of cognitive task performance following placebo or 500 mg of resveratrol on day 1 and day 28 (n 46). ![]() , Placebo;
, Placebo; ![]() , 500 mg of resveratrol. Values are means, with standard errors represented by vertical bars. Significance planned comparisons (Bonferroni corrected) between resveratrol and placebo of data from each 2-min epoch: * P<0·05 and ** P<0·01.
, 500 mg of resveratrol. Values are means, with standard errors represented by vertical bars. Significance planned comparisons (Bonferroni corrected) between resveratrol and placebo of data from each 2-min epoch: * P<0·05 and ** P<0·01.
Transcranial Doppler parameters
No significant acute chronic or gross chronic effects were observed with any of the four TCD parameters (Mean velocity, peak systolic velocity, diastolic velocity and pulsatility index).
Cognitive task performance
-
1. Pure chronic ANOVA
The results of the ANOVA on day 28 pre-dose data (converted to change from day 1 baseline) comparing performance between 500 mg of resveratrol and placebo demonstrated a significant effect of treatment for the 3-Back task in terms of the percentage of correct responses (F 1,40=8·60; P=0·006), with better performance in the resveratrol condition as compared with placebo.
-
2. Acute, chronic and superimposed ANOVA
The results of the treatment×repetition×day ANOVA are as follows. Note that, for brevity, only those significant main and/or interaction effects involving treatment are described here, but see online Supplementary Materials for all ANOVA F and P value tables.
7s incorrect
Analysis revealed a main effect of treatment (F 1,39=6·40; P=0·016) (with the mean for number of serial 7s incorrect responses for placebo, overall, higher than the mean for 500 mg resveratrol) and a day×repetition×treatment interaction (F 3,117=0·260; P=0·034). Post hoc comparisons (Bonferroni corrected) revealed a significant difference on day 1 at repetition 4 (P=0·005) and trends for differences on day 1 at repetition 2 (P=0·073) and on day 28 at repetition 3 (P=0·070). The mean number of incorrect responses was lower in the 500-mg resveratrol condition in all three cases.
17s correct
The ANOVA revealed an interaction between day×treatment×repetition (F 3,117=3·45; P=0·019). Post hoc comparisons revealed significant differences on day 28 at repetition 1 and repetition 3 (both P=0·04), with the mean number of serial 17s correct completions being higher in the placebo condition in both cases.
17s incorrect
The ANOVA showed a main effect of treatment (F 1,39=5·79; P=0·021) (with the mean number of 17s subtraction incorrect responses, overall, being higher in the placebo condition as compared with 500 mg of resveratrol). An interaction between repetition×treatment (F 3,117=3·55; P=0·017) was also observed. With regard to the repetition×treatment interaction, post hoc comparisons revealed only one significant comparison between treatments at the fourth repetition on day 28. Here, the mean number of incorrect responses was higher (P=0·003) in the placebo condition.
General health
There were no significant treatment-related differences on the GHQ or its subcomponents.
Sleep
There were no significant treatment-related differences on the PSQI or its subcomponents.
Mood
A significant treatment effect was observed for the ‘fatigue’ measure alone (F 1,52=9·37; P=0·003), which was derived from the POMS questionnaire. Further analysis with Bonferroni-corrected post hoc Student’s t tests demonstrated that subjective ratings of fatigue were significantly lower for resveratrol on day 7 (P=0·04), day 21 (P=0·013) and day 28 (P=0·001). A move towards a trend was also evinced for day 14 (P=0·097). See online Supplementary Materials for average weekly ratings on POMS questionnaire and ANOVA F and P value tables.
Blood pressure
-
1. Pure chronic ANOVA
The results of the ANOVA on day 28 pre-dose BP measurements (converted to change from day 1 baseline) comparing readings between 500 mg of resveratrol and placebo demonstrated only a significant effect for diastolic BP (F 1,28=5·86; P=0·022), with levels being higher in the resveratrol condition.
-
2. Acute, sub-chronic and superimposed ANOVA
No significant effects were observed for systolic BP or heart rate. For diastolic BP, a significant interaction between treatment×day was evinced (F 1,22=6·61; P=0·017), which revealed only one significant comparison in the placebo condition between day 1 and day 28 at the 40-min post-dose measurement (P=0·46). Here the mean was higher overall on day 28 compared with day 1.
See online Supplementary Materials for BP values and ANOVA F and P value tables.
Plasma analysis (total metabolite levels)
6A significant effect of time was observed (F 1·35,8·10=7·50; P=0·02) for levels of total resveratrol metabolites (the sum of resveratrol 3-O-sulphate and resveratrol-4'- and 3'-O-glucuronides), with pair wise comparisons revealing that day 1 post-dose levels were higher than day 1 baseline (P=0·023), that day 28 pre-dose levels were higher than day 1 baseline (P=0·033) and that day 28 post-dose levels were higher than both day 1 baseline (P=0·003) and day 28 pre-dose levels (P=0·005). All three metabolites followed this same pattern of significance, and thus, for brevity, only total metabolite levels are reported here.
No resveratrol (in any form) was found in baseline samples on day 1, indicating that all volunteers did not consume resveratrol-containing products before the study. No aglycone resveratrol was quantifiable in plasma at any time point, on either day. Resveratrol 3-O-sulphate was the predominant metabolite in all volunteers, contributing 73–77 % of total metabolites. The 4'- and 3'-O-glucuronide forms evinced roughly equal contributions to the remaining metabolites in circulation.
Mean plasma concentration values (µm) for resveratrol metabolites at baseline and post-dose (110 min after administration) on day 1 and, after daily 500-mg consumption, on day 28 are shown in Fig. 3.

Fig. 3 Plasma concentrations of resveratrol metabolites(![]() , total metabolites;
, total metabolites; ![]() , sulphate;
, sulphate; ![]() , 4’ glucuronide;
, 4’ glucuronide; ![]() , 3’ glucuronide) at baseline and post-dose (110 min post-administration of 500 mg trans-resveratrol) on day 1 and day 28. Values are means (n 7), with standard errors represented by vertical bars.
, 3’ glucuronide) at baseline and post-dose (110 min post-administration of 500 mg trans-resveratrol) on day 1 and day 28. Values are means (n 7), with standard errors represented by vertical bars.
Discussion
In summary, the results here show that although a single dose of 500 mg of trans-resveratrol can modulate CBF parameters in the frontal cortex in a pattern consistent with increased blood flow, supplementation for 28 d does not result in any clear improvements in cognitive function, despite an increase in plasma metabolite levels. However, there was evidence of significantly reduced fatigue and higher diastolic BP following extended supplementation. No modulation of subjective sleep quality, health or chronic CBF was observed.
The chronic 28-d dosing paradigm used in the current paper was designed to address the potential ineffectiveness of resveratrol at eliciting cognitive performance effects after acute, bolus supplementation( Reference Kennedy, Wightman and Reay 2 , Reference Wightman, Reay and Haskell 33 ). The hypothesis was that chronic consumption of resveratrol might increase exposure to resveratrol – a polyphenol with known low bioavailability following acute administration( Reference Walle, Hsieh and DeLegge 9 ). This increased exposure may be expected to enhance the biological activity of resveratrol, specifically, of importance here, those with direct and/or indirect effects on cognitive function. However, analysis demonstrated that the only cognitive task measure to evince a pure chronic effect (derived by the comparison of changes in performance between resveratrol and placebo between day 1 baseline and day 28 before dose) was N-Back % correct: that is, after 28 d of supplementation, participants in the 500-mg resveratrol condition completed significantly more correct 3-Back responses before taking their day’s treatment, as compared with placebo. No effects on this measure were observed after consumption of treatment on day 1 or day 28, nor were any effects observed on the other accuracy sub-measure assessed here. The results of acute and chronic/superimposed analysis revealed that, on day 28, participants in the resveratrol condition performed slower, achieving less correct responses on the serial 17 subtractions task. However, on day 1 and day 28, participants in the same condition also performed more accurately (less incorrect responses) on the serial 7 and serial 17 subtraction tasks. Although these results suggest a speed accuracy trade-off, closer inspection of these significant main effects highlights an inconsistent and difficult-to-interpret pattern, with the effects on the serial 7 task restricted to the fourth task battery repetition on day 1 only and the first, third and fourth repetitions, on day 28, for the effects on the serial 17 subtraction task, where both higher and lower performance was seen in the resveratrol condition. Because of the lack of any clear pattern of results in both the acute and chronic effects of resveratrol on cognition here (and indeed the previous two studies assessing the effects of resveratrol on cognitive function), it is important to regard these results with caution. It may be that the relatively small sample here is masking a real effect, or a clearer effect, of resveratrol or it may be that a number of type I errors have inflated expectations. Nevertheless, only a tightly controlled, cross-over study with greater power would be able to address this issue.
The current study demonstrates that 500 mg of trans-resveratrol is able to augment the CBF response to cognitive task demands, relative to placebo, after acute, oral administration to healthy human participants. This acute augmentation manifested in small, significantly higher levels of total-Hb, indicative of increased CBF, at the ends of the absorption- and post-dose task periods and a consistent pattern of significantly higher levels of oxy-Hb across some of the absorption- and post-dose task periods following the first dose of resveratrol on day 1. Levels of deoxy-Hb were also significantly lower in the resveratrol condition, as compared with placebo. This latter finding is directly opposite to that reported previously( Reference Kennedy, Wightman and Reay 2 , Reference Wightman, Reay and Haskell 33 ), and it is contrary to the hypothesis that resveratrol would facilitate increased oxygen extraction because of its reported effects on oxidative phosphorylation( Reference Lagouge, Argmann and Gerhart-Hines 36 ). No clear reason for this anomalous finding can be offered at present, but it may be notable that although the previous two aforementioned resveratrol/NIRS studies by this laboratory were cross-over studies the current study is the first to use a between-subjects design, and this may introduce an unanticipated degree of variability in CBF parameters. In contrast to day 1, the consumption of the resveratrol treatment on day 28 was not found to have an acute effect on any of the CBF parameters. As noted above, CW-NIRS generates concentration change, rather than quantitative data, and therefore it only provides a measure of acute changes in haemodynamics during each discrete recording session. It therefore provides no direct measure of any changes that have taken place between recording sessions, in this case as a consequence of chronic resveratrol supplementation. The lack of an effect here may then reflect several distinct possibilities. It may, of course, reflect a simple attenuation of the acute effects seen following the first dose of resveratrol on day 1. However, it could equally reflect either the raised levels of resveratrol metabolites seen before treatment on day 28, which may have precluded a further acute effect of an additional dose on day 28, or it may indicate that a gross (undetected) change in CBF parameters had already taken place, attenuating the possibility of any additional acute effects of the day 28 treatment.
In the current study, TCD was also incorporated to provide a measure of chronic CBF. This technique provides an absolute quantitative measure of CBF (in this case as indexed by CBFV in the right MCA), which was intended to elucidate any gross chronic changes in CBF as a consequence of resveratrol supplementation. No significant changes in CBFV were observed with TCD, suggesting a simple absence of modulation of CBF by resveratrol. However, this interpretation should be tempered by several considerations. The first is that the recording period was much shorter (at 5 min) than for NIRS, and it was undertaken entirely at rest, with no data collected during the period of task performance, during which resveratrol has been shown to have its most pronounced effects. Second, although the NIRS was used to measure local changes in CBF in the upper layers of the frontal cortex during tasks that activate this brain area, the right MCA supplies the entire right side of the cortex. Given this, any vasodilatory effects restricted to the locality of neural activity (in this case the prefrontal cortex) may have been swamped in the gross blood flow. Potential reasons for a lack of significant CBFV changes include the relatively short recording period with the TCD: 5 min, yielding only two measurements per minute, which may simply be to narrow a window to detect effects. The TCD recording periods were also conducted during times of minimal cognitive demand (pre and post the cognitive task periods) and, as such, metabolic substrate demands would have been less during these periods and an increase in the haemodynamic response unnecessary. Ideally, the TCD and NIRS would both have been used to record concomitantly throughout the absorption and cognitive task periods. Unfortunately, because of the physical constraints of the equipment used here, this was not possible.
The current study does, however, report vascular effects of resveratrol in the periphery on day 28, with the analysis of pure chronic effects (derived by comparing change from day 1 baseline BP measurements between resveratrol and placebo to pre-treatment on day 28) demonstrating higher diastolic BP in resveratrol-supplemented participants. No pre-treatment baseline differences in BP readings nor acute effects of treatment within day 1 or day 28 were observed. This finding is intuitively unexpected, as resveratrol has previously been shown to be a vasodilator( Reference Wong, Howe and Buckley 6 , Reference Wong, Berry and Coates 37 ) – a phenomenon associated with lowered BP. Whether resveratrol can act as a vasoconstrictor is, at present, unknown, but it may be noteworthy that structurally similar polyphenols, such as the tea polyphenol epigallocatechin-3-gallate (EGCG), can act both as both vasodilators and vasoconstrictors depending on the dose and the time of assessment( Reference Alvarez, Campos and Justiniano 38 ). EGCG has also been investigated with regard to its cognitive and CBF effects in humans, with a single dose of 135 mg leading to a significant reduction in CBF as compared with placebo, which might indeed be suggestive of vasoconstriction.
No significant differences between treatments, or within-treatment changes, were observed with subjective perceptions of general health (as assessed by the GHQ) or sleep (as assessed by the PSQI). With regard to subjective perceptions of mood, the only variable on the POMS questionnaire that evinced any significant difference was ‘fatigue’, which remained significantly lower across the entire 28-d period in the resveratrol condition, as compared with placebo. Little research exists regarding the effects of polyphenols on mood, but this anti-fatigue effect may find an explanation in in vitro and animal work, which reports the ability of resveratrol to inhibit MAO-A/B activity. This inhibition was reported to lead to an increase in monoamine neurotransmitter concentrations, namely 5-hydroxytryptophan (5-HT), noradrenaline and dopamine, with a concomitant improvement in mood, which was similar to that seen with imipramine and fluoxetine in mice( Reference Xu, Wang and You 18 ). Interestingly, quercetin, another red wine polyphenol, also shows anti-fatigue activity through increased energy expenditure and endurance capacity in mice( Reference Stewart, Soileau and Ribnicky 39 , Reference Davis, Murphy and Carmichael 40 ) and power output in elite male cyclists when part of a cocktail of supplemented compounds( Reference MacRae and Mefferd 41 ). Mechanisms include increased blood flow because of vasorelaxation( Reference Chen and PaceAsciak 42 ) and oxygenation, with Davis et al.( Reference Davis, Murphy and Carmichael 40 ) also reporting SIRT-mediated increases in mitochondrial gene expression in brain and skeletal muscles. Both mechanisms are shared with resveratrol( Reference Lagouge, Argmann and Gerhart-Hines 36 , Reference Chen and PaceAsciak 42 ) and could explain the increased energy levels seen here. It is worth noting here that, although there was no statistically significant difference in baseline (before dose on day 1) levels of fatigue between resveratrol and placebo participants, the baseline values were nevertheless numerically higher in the former group (8·04 compared with 5·54, respectively), which might suggest that this effect represents a return to normal levels for the resveratrol group following an unusually high baseline.
Analysis of the plasma samples, taken from a sub-sample of seven participants from the resveratrol condition on day 1, demonstrated increases in acute resveratrol metabolite levels post dose very similar to those seen in a previous study conducted by this laboratory( Reference Kennedy, Wightman and Reay 2 ). Pre-dose levels of metabolites on day 28 were also significantly higher than pre-dose levels on day 1, suggesting that chronic consumption results in an accumulation of resveratrol metabolites in plasma. They subsequently increased following the day 28 treatment, and again ended at a significantly higher level than that post dose on day 1. Pre- and post-dose levels of resveratrol on day 28 were significantly higher than baseline levels on day 1 and, within day 28, post-dose levels were significantly higher than pre-dose levels. Taken together, these findings suggest (hence their presence before treatment administration on day 28) that this may amplify the increase following acute administration (hence numerically higher levels at day 28 post dose compared with day 1 post dose). The fact that the day 1 baseline mean levels were 0 does render this comparison statistically problematic. However, disregarding statistical significance, the fact that metabolites were present on day 28 (considering that levels were 0 at baseline on day 1) is indicative that an increase in plasma levels of resveratrol had taken place. This novel finding of accumulating levels of resveratrol metabolites as a consequence of chronic administration certainly warrants further investigation with larger samples, as previous acute dose research does not suggest that plasma metabolites should still be present beyond 24 h( Reference Walle, Hsieh and DeLegge 9 ), or certainly not at the pre-dose levels seen here on day 28( Reference Boocock, Faust and Patel 10 ). It may be possible that these effects are the result of some other, unknown, factor/s – for instance, the consumption by participants of more resveratrol-containing products or an additional resveratrol capsule before attending the laboratory on day 28. However, this seems unlikely, and is argued against by the participants’ treatment diaries and a capsule count.
The methodology of the current study had a number of strengths and limitations. The nature of the paradigm, namely the time frame involved and the use of equipment that dictates individual testing (i.e. the NIRS and TCD), necessarily means that the sample size is somewhat restricted for outcome measures such as cognitive performance, which ideally require a larger sample than the physiological measures. In this study, the issue was exacerbated by the loss of a number of sets of data (due largely to an equipment failure), which reduced the number of cognitive performance data sets. This renders interpretation of the cognitive data more difficult, but an argued strength of this paper is the caution with which the authors have regarded such data. Another limitation relates to the equipment utilised here to measure CBF. As noted above, CW-NIRS only generates acute concentration change data, and therefore the question that it was used to address on day 28 of the current study was ‘Are the acute haemodynamic effects of the single dose of resveratrol taken on day 28 the same, or different, to those seen following the first dose taken on day 1?’. The results showed that there were no acute effects on day 28, and thus they were different. However, the difficulty in interpreting this finding further is that this could reflect an attenuation of the acute effects over time, but it could equally be the result either of the raised levels of resveratrol metabolites already seen before taking the day 28 treatment, or indeed unmeasured chronic effects on CBF. To address the last of these points, TCD was incorporated as a measure of chronic changes of absolute CBFV, but this measure showed no effect, although again this could be due to methodological issues (including measuring at rest, rather than during task performance, and the diffuse rather than local nature of the measurement). It would therefore be advantageous to revisit the question of the chronic effects of resveratrol on CBF using the more recently introduced ‘quantitative’ NIRS, which, as the name suggests, generates quantitative, rather than concentration change, data. In terms of strengths, the current paper incorporated a range of methodologies in order to answer the hitherto unaddressed question as to whether resveratrol can engender chronic cognitive effects. This is also the first paper to show that repeated consumption of resveratrol can lead to cumulative plasma levels at a dose that is recommended by many over-the-counter resveratrol products.
In conclusion, the current study reports that chronic, 28-d supplementation of 500 mg of trans-resveratrol results in significantly reduced fatigue and higher diastolic BP, but it does not modulate sleep, health or chronic CBF. The single, chronic, cognitive effect evinced by resveratrol and the confusing pattern of acute effects should be treated with caution. This study is the first to suggest that chronic resveratrol consumption could result in cumulative plasma levels in healthy humans after oral administration.
Acknowledgements
The direct study costs (participant payments and treatments) of the research described herein were funded by BiotiviaTM Longevity Bioceuticals. Biotivia had no role in the design, analysis or writing of this article.
All of the authors (E. L. W., C. F. H.-R., J. L. R., G. W., T. D., W. Z. and D. O. K.) were actively involved in the planning of the research described herein and in writing the paper. E. L. W. collected the data. G. W., T. D. and W. Z. planned and carried out the analysis of the plasma samples. All authors contributed to and reviewed the final publication.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515003037







