The metabolic syndrome, a concurrence of at least three of the parameters such as overweight and abdominal fat distribution, dyslipidaemia (elevated TAG and/or reduced HDL-cholesterol), arterial hypertension, impaired glucose metabolism, and chronic low-grade inflammation, has gained awareness and interest. It is directly correlated with the development and progression of atherosclerotic vascular disease as well as with type 2 diabetes mellitus, and identifies individuals at a higher risk of CVD than the general population( Reference Alberti, Eckel and Grundy 1 – Reference Grundy 3 ). Importantly, the close relationship between the metabolic syndrome, endothelial dysfunction and hypertension is linked to elevated cardiovascular risk, coronary artery disease and mortality( Reference Di Pino, Alagona and Piro 4 – Reference Suzuki, Hirata and Elkind 6 ).
Recently, a number of circulating endothelial and inflammatory markers have been identified and implicated in the pathogenesis of atherosclerosis and CVD( Reference Hope and Meredith 7 , Reference Hope and Meredith 8 ). Cellular adhesion molecules including intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and endothelial selectin are generally accepted to be associated with the pathogenesis of atherosclerosis and are the indicators of endothelial dysfunction preceding clinical events( Reference Hope and Meredith 8 ). These endothelial markers are elevated in obesity and in patients with metabolic syndrome traits( Reference Hope and Meredith 8 – Reference Palomo, Jaramillo and Alarcon 10 ). Similarly, inflammatory markers including high-sensitivity (hs) C-reactive protein (CRP), TNF-α and IL-6 are associated with atherosclerosis, and have also been shown to be elevated in obesity( Reference Alexopoulos, Katritsis and Raggi 11 – Reference Khosravi, Ka and Huang 13 ). YKL-40, also known as human cartilage glycoprotein 39 or chitinase-3-like protein 1, is an inflammatory glycoprotein involved in endothelial dysfunction by promoting chemotaxis, cell attachment and migration, reorganisation and tissue remodelling in response to endothelial damage( Reference Rathcke and Vestergaard 14 ). Elevated circulating YKL-40 levels have been found to be associated with cardiovascular as well as all-cause mortality( Reference Rathcke and Vestergaard 14 – Reference Harutyunyan, Gotze and Winkel 16 ). In addition, previous studies have reported that patients with type 2 diabetes mellitus exhibit elevated plasma levels of YKL-40 compared with healthy control subjects( Reference Nielsen, Erikstrup and Johansen 17 , Reference Hempen, Kopp and Elhenicky 18 ). Recent studies have suggested that YKL-40 might act not only as a potential biomarker for endothelial dysfunction, but also for atherosclerosis, insulin resistance and type 2 diabetes mellitus( Reference Rathcke, Johansen and Vestergaard 19 ).
A large body of epidemiological data and evidence from randomised controlled human trials has demonstrated cardioprotective effects of the marine n-3 fatty acids EPA and DHA( Reference Saravanan, Davidson and Schmidt 20 – Reference Wang, Harris and Chung 22 ). For example, EPA and DHA have been shown to reduce serum TAG concentrations, to lower arterial blood pressure (BP), to reduce inflammation, and to improve vascular endothelial function( Reference Egert and Stehle 23 – Reference Egert, Kannenberg and Somoza 27 ). It is largely unknown whether the plant-derived α-linolenic acid (ALA) has a significant preventive potential, too. Possible cardioprotective and anti-inflammatory benefits of dietary ALA have been reviewed recently( Reference Stark, Crawford and Reifen 28 – Reference Rodriguez-Leyva, Dupasquier and McCullough 30 ). Potential effects have been examined with different ALA dosages and under isoenergetic and thus weight-stable study conditions. To the best of our knowledge, there is no human trial investigating ALA-specific cardioprotective effects during energy restriction and body-weight loss. Therefore, in the present study, we examined, for the first time, the effects of an energy-restricted diet enriched with ALA on the biomarkers of vascular function and inflammation in patients with metabolic syndrome traits. We have previously reported the beneficial effects of this diet regimen on the parameters of lipid and glucose metabolism( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ). A range of subject baseline parameters, diet intake characteristics and also some results are re-presented here, in order to better relate them to the newly presented biomarkers.
Patients and methods
Patients
Details of the study design, dietary interventions, and patient recruitment, enrolment and randomisation have been described previously( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ). In brief, 150 interested volunteers aged 18–70 years with overweight attended a screening examination that included physical assessments (height, body weight, BP, waist and hip circumferences), clinical assessments (e.g. serum lipids and lipoproteins, glucose), medical history and a dietary questionnaire.
Participants who had the following traits of the metabolic syndrome were included: central obesity (waist circumference ≥ 94 cm for men and ≥ 80 cm for women) plus two of the following criteria: (1) fasting serum TAG concentrations of ≥ 1·7 mmol/l; (2) reduced serum HDL-cholesterol ( < 1·03 mmol/l in men; < 1·29 mmol/l in women); (3) elevated BP (systolic ≥ 130 mmHg; diastolic ≥ 85 mmHg); (4) fasting plasma glucose ≥ 6·5 mmol/l( Reference Baxheinrich, Stratmann and Lee-Barkey 31 , Reference Alberti, Zimmet and Shaw 32 ). Exclusion criteria were as follows: smoking; insulin-dependent diabetes mellitus; liver, gastrointestinal or inflammatory diseases; history of cardiovascular events; use of anti-obesity medications or anti-inflammatory drugs; cancer; pregnancy or breast-feeding; alcohol abuse; necessity for a medically supervised diet.
A total of ninety-five patients (thirty male and sixty-five female) were included in the study. Of these, thirteen patients dropped out due to different reasons, and one participant was retrospectively excluded because of multiple drug changes. Thus, the final analysis included eighty-one patients (twenty-six male and fifty-five female)( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ). Baseline characteristics are presented in Table 1.
Table 1 Baseline characteristics of the overweight-to-moderately obese men and women who completed the study( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ) * (Mean values and standard deviations)
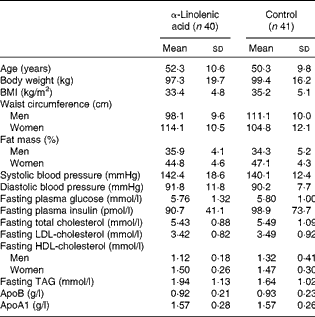
* The two groups did not differ significantly with regard to any of these variables (P>0·05; independent-samples t tests or Mann–Whitney U test).
All study procedures were approved by the ethics committee of the Ruhr-University Bochum, located at HDZ NRW in Bad Oeynhausen, Germany, and were in compliance with the Declaration of Helsinki. All participants provided informed written consent. The study was registered at http://www.germanctr.de/ and http://apps.who.int/trialsearch/ as DRKS00006232.
Study design and dietary intervention
The study was a randomised controlled dietary intervention study and was conducted under outpatient conditions over a 26-week intervention period( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ). In a parallel design, patients were randomised to an energy-restricted diet (see below) enriched with ALA (ALA diet; approximately 3·4 g ALA/d) or an energy-restricted control diet (approximately 0·9 g ALA/d) (Table 2). The principal sources of dietary fat during the intervention period were either refined rapeseed oil (ALA, 7 % of total fatty acids) and a rapeseed oil-based commercial margarine for the ALA diet (Goldina; ALA, 6 % of total fatty acids), or refined olive oil (Henry Lamotte Oils; ALA, 0·5 % of total fatty acids) and a commercial olive oil-based margarine (ALA, 0·5 % of total fatty acids, mOlivo; Vitaquell) in the control group. The plant oils and the margarines were used for the preparation of all meals and snacks. The ALA diet was identical to the control diet in all respects except the principal sources of dietary fat. The consumption of fish, fish oil capsules and foods enriched with n-3 fatty acids was not allowed in both intervention groups to ensure that the diets were free of long-chain n-3 fatty acids( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ).
Table 2 Composition of the habitual diet and the study diets* (Mean values and standard deviations)
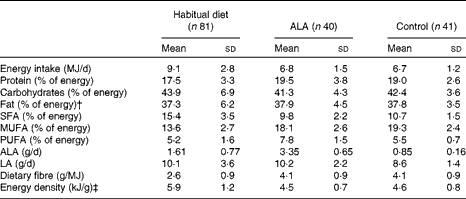
ALA, α-linolenic acid; LA, linoleic acid.
* Data were calculated from 3 d food diaries; composition data of the study diets were calculated from the protocols of weeks 12 and 26.
† Total fat contains approximately 95 % fatty acids, the other approximately 5 % is made up of glycerol and other lipids.
‡ Energy density was calculated for solid foods and energy-containing beverages.
Before the intervention period, the patients were instructed to complete a 3 d dietary record for estimating their habitual energy and nutrient intake. During the intervention period (weeks 12 and 26), two further dietary records were completed for assessing the adherence to the prescribed diets. The dietary records as well as the study diets were calculated using the computer-based nutrient calculation program EBISpro (University of Hohenheim) based on the German Nutrient Data Base Bundeslebensmittelschlüssel, version II.3 (Max Rubner-Institut).
All patients received detailed instructions about the prescribed diets, instructions for food preparation and special recipes for use of the study oils and margarines. In addition, patients were provided with kitchen scales for weighing the prescribed food amounts and with a measuring cup for the daily oil amount. A nutritionist was in close and regular contact with the patients. Counselling about lifestyle, dietary behaviour and physical activity was identical for both intervention groups( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ).
The goal for body-weight reduction during the intervention period was 5–10 % of the baseline body weight. To achieve this goal, hypoenergetic diets with a mean daily energy deficit in the range of 2·0–3·3 MJ were calculated. On the basis of the expected energy expenditure of the patients, they were assigned to one of four energy intake levels (5·7, 6·3, 6·9 or 7·8 MJ/d)( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ). The intervention diets were calculated with 42 % of total energy as carbohydrates, 20 % of energy as protein and 38 % as dietary fat (Table 2). The main components of both diets were low-fat foodstuffs, e.g. whole-grain bread and cereals, vegetables, fruits, lean meat, skimmed milk and low-fat dairy products as described previously( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ).
Measurements of anthropometry, body composition and arterial blood pressure
Body height was determined using a wall-mounted stadiometer to the nearest 0·5 cm during the first examination. Waist circumference was measured to the nearest 0·5 cm midway between the lowest rib and the iliac crest, while the participant was at minimal respiration. Body composition was determined by bioelectric impedance analysis (Maltron International Limited). BP measurements were obtained with a fully automated BP monitor (Bosch+Sohn) as described previously( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ).
Blood sampling
Venous blood samples were obtained at baseline (week 0) and after 12 and 26 weeks of consuming the energy-restricted diets. All samples were taken after an overnight fast of at least 10 h under standardised conditions. Serum and plasma were obtained by centrifugation at 1500 g for 20 min at 4°C, and stored at − 80°C until analysis. All laboratory measurements were performed blinded, without any knowledge of the intervention group. Serum samples were used for the determination of lipid parameters, insulin, glucose, hs-CRP, hs-TNF-α, hs-IL-6, soluble vascular cell adhesion molecule-1 (sVCAM-1), soluble intercellular adhesion molecule-1 (sICAM-1), soluble endothelial selectin (sE-selectin), asymmetric dimethylarginine (ADMA) and YKL-40. EDTA plasma was used for the measurements of big endothelin-1; citrate plasma was used for the analysis of fibrinogen.
Measurements of serum lipid, glucose, insulin and high-sensitivity C-reactive protein concentrations
Fasting serum concentrations of total cholesterol, LDL-cholesterol, HDL-cholesterol, TAG, apoB and apoA1, glucose and insulin were measured using the autoanalyser Architect System (ci8200 series; Abbott Diagnostics) as described previously( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ). Serum concentrations of hs-CRP were determined using an automated high-sensitivity immunoturbidimetric test.
Measurements of fibrinogen, high-sensitivity TNF-α, high-sensitivity IL-6, soluble vascular cell adhesion molecule-1, soluble intercellular adhesion molecule-1, soluble endothelial selectin, asymmetric dimethylarginine, big endothelin-1 and YKL-40 concentrations
Plasma fibrinogen was measured by the Clauss assay using the Sysmex CA-7000 autoanalyser (Siemens Healthcare Diagnostics). Commercial ELISA assays were used to measure serum concentrations of hs-IL-6 (R&D Systems Europe), hs-TNF-α (R&D Systems), sE-selectin (R&D Systems), sVCAM-1 (R&D Systems), sICAM-1 (R&D Systems), ADMA (Immundiagnostik AG), YKL-40 (Quidel) and plasma concentrations of big endothelin-1 (Biomedica). The samples were analysed in duplicate with a commercial microplate reader (Tecan). All inter-assay CV were ≤ 10 %.
Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences, version 11.5 (SPSS, Inc.). The distribution of variables was analysed by checking histograms and normal plots of the data, and normality was tested by means of Kolmogorov–Smirnov and Shapiro–Wilk tests. Baseline characteristics of the groups were compared by means of independent-samples t tests or Mann–Whitney U test. Changes in anthropometric parameters and blood parameters were compared by repeated-measures ANOVA, with the data of weeks 0, 12 and 26 as the three levels of the within-subject factor (time) and treatment (ALA v. control) as the between-subject factor. Repeated-measures ANOVA were conducted with log-transformed variables if the residuals were non-normally distributed, which was the case for hs-CRP, sVCAM-1, sICAM-1, sE-Selectin, YKL-40, hs-TNF-α and hs-IL-6. All tests were two-tailed, and P values ≤ 0·05 were considered significant. Pearson's correlation coefficient was used to evaluate the relationships between different variables.
Results
Body weight, fat mass and arterial blood pressure
As reported previously, body weight decreased throughout the study by 7·8 (se 6·2) kg in the ALA group and by 6·0 (se 4·8) kg in the control group, changes that were significant overall but that did not differ significantly between the treatment groups (P< 0·001 for time; P= 0·155 for time × treatment interaction; Fig. 1)( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ). Body fat mass decreased by a mean of 5·8 (se 4·5) kg in the ALA group and by 4·2 (se 4·4) kg in the control group. This change was significant overall (P< 0·001 for time), but did not differ significantly between the two intervention groups (P= 0·263 for time × treatment interaction; data not shown). In addition, systolic and diastolic BP significantly decreased throughout the study (systolic BP, − 10·0 (se 13·0) mmHg in the ALA group v. − 8·0 (se 14·6) mmHg in the control group, P< 0·001 for time; diastolic BP, − 8·4 (se 9·3) mmHg in the ALA group v. − 4·4 (se 6·6) mmHg in the control group, P< 0·001 for time). The latter was more pronounced for the ALA group when compared with the control group (P= 0·026 for time × treatment interaction). Resting systolic and diastolic BP at baseline and the changes in systolic and diastolic BP from baseline to after intervention were correlated with the individuals with higher baseline BP, demonstrating greater reductions in systolic and diastolic BP in response to the intervention diets (total study group: systolic BP, r − 0·567, P< 0·001; diastolic BP, r − 0·489, P< 0·001). In addition, changes in body fat mass correlated with changes in systolic (r 0·26, P< 0·05) and diastolic BP (r 0·362, P< 0·001).
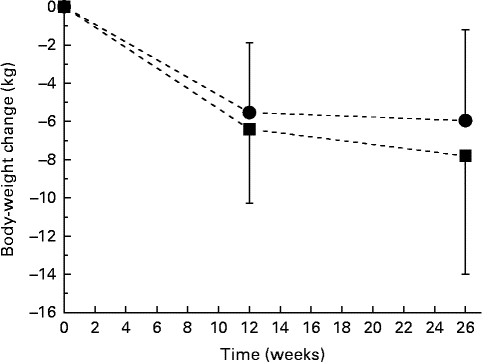
Fig. 1 Body-weight changes during 26 weeks of energy restriction with two different diets: α-linolenic acid (ALA) diet (![]() , n 40); control diet low in ALA (
, n 40); control diet low in ALA (![]() , n 41). Values are means, with their standard errors represented by vertical bars. There was a significant effect for time (P< 0·001), but no significant diet×time interaction effect (P= 0·155).
, n 41). Values are means, with their standard errors represented by vertical bars. There was a significant effect for time (P< 0·001), but no significant diet×time interaction effect (P= 0·155).
Biomarkers of inflammation and endothelial function
Serum hs-CRP, hs-TNF-α and hs-IL-6 concentrations decreased significantly over time, but there was no effect of treatment group on this change (Table 3). The plasma concentrations of fibrinogen neither changed significantly throughout the study nor differed in any way between the two dietary groups (Table 3). The serum concentrations of sVCAM-1 and plasma big endothelin-1 slightly increased throughout the study, with no effect of treatment group on these changes (Table 3). In contrast, serum sICAM-1, sE-selectin and ADMA decreased significantly over time, but decreases were independent of the intervention (Table 3). During both treatments, serum YKL-40 concentration significantly decreased over time. The decrease in the concentration of YKL-40 was significantly more pronounced in the ALA group than in the control group (Table 3).
Table 3 Fasting biomarkers of inflammation and endothelial function in overweight-to-moderately obese men and women at baseline (week 0) and after 12 and 26 weeks of consuming an energy-restricted diet rich in α-linolenic acid (ALA; n 38) v. a control diet low in ALA (n 39) (Mean values and standard deviations)

RM-ANOVA, repeated-measures ANOVA; hs, high sensitivity; CRP, C-reactive protein; sVCAM-1, soluble vascular cell adhesion molecule-1; sICAM-1, soluble intercellular adhesion molecule-1; sE-selectin, soluble endothelial selectin; ADMA, asymmetric dimethylarginine; YKL-40, human cartilage glycoprotein 39 or chitinase-3-like protein 1.
Serum concentrations of hs-CRP, hs-TNF-α, hs-IL-6, sICAM-1, sE-selectin, ADMA and YKL-40 at baseline and the changes in these biomarkers from baseline to after intervention were significantly correlated with the individuals with higher baseline concentrations, demonstrating greater reductions in these biomarkers in response to the intervention diets (hs-CRP, r − 0·756; hs-TNF-α, r − 0·632; hs-IL-6, r − 0·601; sICAM-1, r − 0·448; sE-selectin, r − 0·649; ADMA, r − 0·518; YKL-40, r − 0·834; P< 0·001 for all such values). In addition, changes in body fat mass correlated with changes in the concentrations of sICAM-1 (r 0·284, P< 0·05), sE-selectin (r 0·411, P< 0·001), hs-CRP (0·377, P< 0·001) and YKL-40 (r 0·324, P< 0·01).
Baseline systolic and diastolic BP did not significantly correlate with the biomarkers of inflammation and endothelial function. In addition, changes in BP variables did not significantly correlate with changes in the biomarkers of inflammation and endothelial function.
Discussion
The aim of the present randomised controlled dietary intervention study in overweight and moderately obese patients with metabolic syndrome traits was to investigate the effects of an energy-restricted diet rich in ALA on vascular function and systemic inflammation. After a 26-week intervention period, a distinct improvement in the biomarkers of endothelial function, resting BP and inflammation in the ALA group as well in the control group was detected. The high ALA intake led to a more pronounced reduction in serum YKL-40 concentration and diastolic BP compared with the intake of low-ALA control diet, indicating that there may be independent favourable physiological effects of ALA during weight loss. To our knowledge, this type of investigation has not been reported previously.
The observed decrease in systolic and diastolic BP during loss in body fat mass is similar to the results from previous weight-loss studies using different energy-restricted diets( Reference Wycherley, Brinkworth and Keogh 33 – Reference Keogh, Brinkworth and Noakes 35 ). A meta-analysis by Neter et al. ( Reference Neter, Stam and Kok 36 ) of twenty-five randomised controlled trials showed a reduction in systolic and diastolic BP of − 1·05 and − 0·92 mmHg/kg body-weight loss, respectively. Although the exact mechanism of the relationship between obesity and elevated BP and the effect of body-weight loss on BP is unknown, there are several plausible biological pathways. For example, the adipose renin–angiotensin–aldosterone system is overactivated in obese individuals, and renin activity and aldosterone concentrations are higher in obese than in lean subjects( Reference Frigolet, Torres and Tovar 37 ). In addition, activity of the sympathetic nervous system is increased in hypertensive, obese individuals, which could induce obesity-related renal effects( Reference Masuo, Rakugi and Ogihara 38 ). Decreased insulin sensitivity and hyperinsulinaemia as part of the metabolic syndrome might also form an essential link between obesity, sympathetic nervous activation and hypertension, and energy restriction has been shown to improve insulin sensitivity( Reference Frigolet, Torres and Tovar 37 , Reference Soare, Weiss and Pozzilli 39 ). As published previously( Reference Baxheinrich, Stratmann and Lee-Barkey 31 ), fasting serum concentrations of insulin and intact proinsulin were significantly reduced in both dietary groups in the present study. The improvement of the metabolic situation in terms of insulin resistance and homoeostatic model assessment of insulin resistance (HOMA-IR) may have an impact on YKL-40 levels, too.
The magnitude of the hypotensive effect observed in the present study and previously during body-weight loss( Reference Neter, Stam and Kok 36 ) is certainly clinically relevant, and is expected to considerably reduce the risk of CVD in our patients. For example, a reduction in systolic BP of 10 mmHg – comparable to the present results – lowered the risk of myocardial infarction by 21 % in the UK Prospective Diabetes Study( 40 ).
A significantly more pronounced decline in diastolic BP was observed after the intake of the ALA-rich diet than after the intake of the control diet. We speculate from these results that there might be an independent effect of ALA on BP. This finding was confirmed in a Greek study in normotensive, dyslipidaemic patients and under isoenergetic study conditions with an ALA intake of 8 g/d, which led to significant lower resting systolic and diastolic BP compared with a high intake of linoleic acid( Reference Paschos, Magkos and Panagiotakos 41 ). In contrast, other human trials in subjects with the risk of CVD did not report a BP-lowering effect of ALA( Reference Bemelmans, Broer and Feskens 42 – Reference Singer, Berger and Wirth 44 ). The physiological mechanisms by which dietary ALA might lower BP are not well understood. It is assumed that the long-chain n-3 dietary fatty acids EPA and DHA at high doses ( ≥ 3 g/d) can modulate BP mainly through their effects on the renin–angiotensin–aldosterone system, their effects on peripheral sympathetic tone, the reduction in plasma viscosity, the production of endothelial NO and the production of 3-series PG with vasoactive effects( Reference Cabo, Alonso and Mata 45 ). ALA can be converted to EPA and DHA by elongation and desaturation, but the extent of this conversion is not clear and at best very limited( Reference Goyens, Spilker and Zock 46 , Reference Goyens, Spilker and Zock 47 ). It, therefore, remains unclear whether the BP-lowering effects during the intake of an ALA diet can be ascribed to dietary ALA, or through its conversion to the long-chain n-3 fatty acids EPA and/or DHA.
In the present study, a number of markers of endothelial function and inflammation (e.g. sICAM-1, sE-selectin, hs-CRP, hs-TNF-α, hs-IL-6) were improved probably mainly as a result of body fat loss during the two energy-restricted diets accounting for decreased endothelial cell activation. Similar findings have been reported in several previous studies after weight loss( Reference Wycherley, Brinkworth and Keogh 33 – Reference Keogh, Brinkworth and Noakes 35 , Reference Ziccardi, Nappo and Giugliano 48 ). For example, Ziccardi et al. ( Reference Ziccardi, Nappo and Giugliano 48 ) found reductions in the concentrations of IL-6, TNF-α, sVCAM-1 and sICAM-1 following a body-weight loss of 10 kg (10 %) in obese women. The levels of sICAM-1 predict future cardiovascular events in healthy subjects, and the reduction in the concentrations of these markers leads to a reduction in the risk of CVD( Reference Ridker, Hennekens and Roitman-Johnson 49 ). We found small but significant increases in the concentrations of sVCAM-1 and big endothelin-1 (Table 3), which we are unable to explain. However, we believe that these small increases are unlikely to be biologically important.
Diet-induced body-weight loss was able to significantly reduce the circulating YKL-40 concentrations in our overweight-to-obese patients with metabolic syndrome traits. Similar results have been reported recently in obese patients with type 2 diabetes mellitus( Reference Catalan, Gomez-Ambrosi and Rodriguez 50 ). In addition, we found a positive association between YKL-40 and body fat. The biological function of YKL-40 is not yet clear; however, the present results strengthen the view for an involvement of YKL-40 in low-grade inflammation associated with abdominal fat distribution and the development of the metabolic syndrome. We observed a significantly more pronounced decrease in circulating YKL-40 concentration after the intake of the ALA-rich diet than after the intake of the control diet. To the best of our knowledge, this finding has not been reported previously. We speculate from the present results that there might be an independent effect of dietary ALA on the regulation of YKL-40.
In the present study, endothelial-derived adhesion molecules did not correlate with BP, emphasising that factors regulating endothelial function differ at least in part from those regulating BP.
In conclusion, the present study indicates that in overweight-to-obese patients with metabolic syndrome traits, both vascular function and inflammation are improved during body-weight loss. In addition, the ALA diet alone was associated with significant reductions in diastolic BP and serum YKL-40 concentration. These effects of ALA may provide novel mechanisms by which ALA may affect vascular compliance.
Acknowledgements
The present study was supported by the Union for the Promotion of Oil and Protein Plants (UFOP) and the International Foundation for the Promotion of Nutrition Research and Nutrition Education (ISFE). UFOP and ISFE had no role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: U. W., B. S., S. E. and D. T. designed the research; Y. H. L.-B. and A. B. conducted the research; A. B. and S. E. analysed the data; S. E. wrote the manuscript; B. S. and U. W. had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has any conflict of interest to declare.






