The metabolic syndrome is a cluster of interrelated and modifiable risk factors for CVD including abdominal obesity, hypertension, dyslipidaemia and hyperglycaemia(Reference Alberti, Eckel and Grundy1). Diet is the cornerstone in the prevention and therapy of this metabolic disorder, with long-term weight reduction accompanied by an improvement in the metabolic risk profile as the primary therapeutic goal for obese patients. There is general agreement that a high intake of SFA and trans-fatty acids should be considerably reduced, because they increase LDL-cholesterol levels, impair insulin sensitivity and represent a dietary pattern with high energy density (ED), and therefore promote hyperenergetic diets(Reference Lichtenstein, Appel and Brands2–Reference Kant and Graubard5). However, it is still a matter of debate whether saturated and hydrogenated fat should be replaced preferentially by carbohydrates or monounsaturated fat in these patients.
For decades, low-fat, high-carbohydrate diets have been recommended as the most suitable approach for weight reduction with the focus on a high proportion of carbohydrates rather than on their physiological quality(6, Reference Cummings, Parham and Strain7). However, there is now evidence that the carbohydrate quality is more decisive than its quantity. A high intake of refined carbohydrates with a high glycaemic index has not only detrimental effects on the metabolic risk profile by raising serum glucose and TAG levels and decreasing HDL-cholesterol(Reference Hu8–Reference Griel, Ruder and Kris-Etherton10), but is also positively associated with an elevated ED(Reference Bes-Rastrollo, van Dam and Martinez-Gonzalez4), and therefore cannot be recommended for weight reduction. On the other hand, a high intake of fibre-rich carbohydrates is accompanied by a lower metabolic risk and a low ED, and supports weight loss(Reference Jakobsen, Dethlefsen and Joensen11–Reference Roberts, McCrory and Saltzman14).
The second candidate as a substitute for SFA, MUFA, has been shown to exert a variety of beneficial effects on the serum lipid profile, glucose levels and insulin sensitivity as summarised in a recent review(Reference Gillingham, Harris-Janz and Jones15). A high content of MUFA is found in the Mediterranean food pattern, in which the main source of dietary fat is olive oil. Otherwise, this dietary pattern is based predominantly on energy-diluted foods, rich in water and/or fibre (e.g. vegetables, fruits, legumes, whole grains)(Reference Sofi, Abbate and Gensini16). Thus, it is an important exception from other high-fat diets, which tend to have a high energy density. Despite a total fat content of up to 35–40 % of energy (En%), the Mediterranean diet is characterised by a low ED, which facilitates an energy-restricted diet, and leads to a significant weight loss(Reference Shai, Schwarzfuchs and Henkin17, Reference Esposito, Malorino and Ciotola18). Simultaneously, beneficial effects on serum lipid and glucose levels are observed(Reference Due, Larsen and Mu19–Reference Ros21). Thus, this kind of MUFA-rich diet may be a suitable alternative to low-fat diets.
When searching for the optimal dietary regimen, specific food components which may further improve the metabolic risk profile should also be considered. In particular, there is evidence for various protective effects of n-3 fatty acids. These include reduction of serum TAG concentrations, improved insulin sensitivity and decreased blood pressure(Reference Zivkovic, German and Sanyal22). However, these effects have only been shown for the long-chain marine n-3 fatty acids EPA and DHA(Reference Nettleton and Katz23, Reference Riediger, Othman and Suh24). It is largely unknown whether the plant-derived α-linolenic acid (ALA) has a significant preventive potential, too. Possible cardioprotective and anti-inflammatory benefits have recently been reviewed(Reference Stark, Crawford and Reifen25, Reference Robinson, Buchholz and Mazurak26). The effects were described for supplements or flaxseed oil as ALA source(Reference Paschos, Magkos and Panagiotakos27–Reference Barre, Mizier-Barre and Griscti29). Paschos et al. (Reference Paschos, Magkos and Panagiotakos27), for example, found a significant reduction in blood pressure after the intake of 8 g ALA/d from flaxseed oil.
For dietary practice, it is of special interest whether protective effects of ALA can be achieved without supplements but with amounts found in a long-term natural food diet. While a low-fat dietary approach only offers a scarce margin for the intake of vegetable oils as the natural source of ALA, the above-described MUFA-rich dietary pattern provides a suitable option for larger daily amounts of an added vegetable oil. Rapeseed oil is a vegetable oil that provides both a high proportion of MUFA and, simultaneously, about 10 % of ALA.
In the present study, the effects of a rapeseed oil-enriched hypoenergetic diet with a low ED were investigated during a 6-month weight reduction programme in patients with the metabolic syndrome. To identify the specific effects of ALA, the rapeseed oil diet was compared with a control diet rich in olive oil which had a similar MUFA content to ensure that the ALA intake was the only difference between the two dietary approaches.
Methods
Participants
For the present study, a total of 178 interested persons were recruited by advertisement in the local newspaper. To be enrolled in the study, subjects had to meet the diagnosis criteria of the metabolic syndrome according to the definition of the International Diabetes Federation (Table 1)(Reference Alberti, Zimmet and Shaw30). Exclusion criteria were CVD, severe illnesses such as renal failure or liver disease, food allergy or intolerance, pregnancy or lactation, smoking, alcohol abuse and insulin therapy or severe diabetic complications in case of diagnosed type 2 diabetes mellitus. After the first interview, twenty-eight subjects had to be excluded because of not meeting the main criteria. The remaining 150 persons (111 women and thirty-nine men) underwent a screening visit including medical history, anthropometric and biochemical measurements. Of these, ninety-five patients (sixty-five women and thirty men) were enrolled in the study and randomly assigned either to the rapeseed oil-rich diet (RO group) or to the control diet with olive oil (OO group) (Fig. 1). During the study, thirteen patients dropped out due to different reasons, and one person was excluded from the analysis because of multiple drug changes. Thus, the final analysis included eighty-one patients (fifty-five women and twenty-six men); their baseline characteristics are presented in Table 2. The study protocol was approved by the ethics committee of the Ruhr-University Bochum, Germany, and was conducted according to the guidelines laid down in the Declaration of Helsinki. All individuals were informed about the objectives of the study in detail, and written informed consent was obtained from all subjects.
Table 1 Definition of the metabolic syndrome according to the International Diabetes Federation(Reference Alberti, Zimmet and Shaw30)

* European: men ≥ 94 cm, women ≥ 80 cm.
† Or specific treatment for this abnormality.
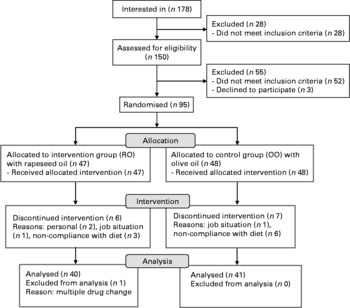
Fig. 1 Flow chart of subject recruitment and withdrawal.
Table 2 Baseline characteristics of the study patients*
(Mean values and standard deviations, n 81)
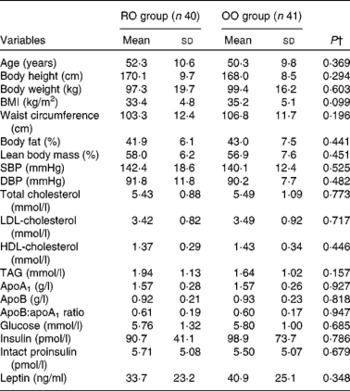
RO, rapeseed oil-rich diet; OO, control diet with olive oil; SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Measurements were made before the start of the intervention. Blood samples were drawn in a fasting state.
† P values were obtained from the unpaired t test.
Study design and dietary intervention
The study was conducted in a parallel design and under outpatient conditions with a 26-week intervention period. Before the study (visit 1), the patients were instructed to complete a 3 d dietary record for estimating their habitual energy and nutrient intake. During the study, at visit 2 (months 3) and visit 3 (months 6), two further dietary records had to be completed for assessing the adherence to the prescribed diets. The dietary records as well as the study diets were calculated using the computer-based nutrient calculation program EBISpro (University of Hohenheim), based on the German Nutrient Data Base Bundeslebensmittelschlüssel, version II.3 (Max Rubner-Institute).
All patients received detailed instructions about the prescribed diets by a trained nutritionist. They obtained instruction booklets indicating the foods to be consumed daily with their respective amounts, as well as non-allowed foods. In addition, they received instructions for preparation and special recipes for use of the study oils and margarines. The participants were provided with kitchen scales for weighing of the prescribed food amounts and with a measuring cup for the daily oil amount. A nutritionist was in close and regular contact with the patients, both at their regular visits at the clinic (see below) and by phone in order to provide support and motivation. Counselling about lifestyle, dietary behaviour and physical activity was identical for both groups.
At visits 1–3, the patients had to visit the clinic for measurement of body weight and composition, and blood pressure, and for obtaining venous blood samples.
According to the official guidelines, the goal for weight reduction during the intervention period was 5–10 % of the baseline body weight(Reference Hauner, Buchholz and Hamann31). To achieve this goal, a daily energy deficiency of 2·0–3·3 MJ is recommended and thus the hypoenergetic diets were calculated with energy deficits in this range. On the basis of the expected energy expenditure of the patients, they were assigned to one of four energy intake levels (5·7, 6·3, 6·9 or 7·8 MJ/d).
All patients ingested mixed diets with conventional foods. Main components of both diets were low-fat foodstuffs, e.g. whole-grain bread and cereals, vegetables, fruits, lean meat, skimmed milk and low-fat dairy products, leading to a similar macronutrient composition of both study diets. Diets were calculated with 42 % of total energy as carbohydrates, 20 % as protein and 38 % as fat. For both diets, targets for SFA were < 10 % and for MUFA 18 % of energy.
The principal sources of fat during the study were refined rapeseed oil (Brökelmann) and a rapeseed-based commercial margarine (Othüna) in the rapeseed oil group, and refined olive oil (Lamotte Oils) and an olive oil-based margarine (Vitaquell) in the olive oil group. The patients were supplied with the respective study oils/margarines for the entire intervention period. They had to consume on average 30 g/d of the study oil plus 20 g/d of margarine, the latter provided in portion packs of 10 g. To compensate for lower linoleic acid content in olive oil compared with rapeseed oil (data not shown), the patients of the former group consumed sunflower oil once per week instead of olive oil. The consumption of fish, fish oil capsules and foods enriched in n-3 fatty acids was not allowed in both groups to ensure that the diets were free of long-chain n-3 fatty acids. The intake of ALA-rich foods, such as walnuts and linseed, was also not allowed throughout the study.
Measurements of anthropometry, body composition and blood pressure
At visits 1–3, the participants were weighed in light clothing without shoes on an electronic scale to the nearest 100 g. Waist circumference was measured with a commercial tape halfway between the last rib and the iliac crest. Height for the calculation of BMI was measured at visit 1 with a wall-mounted stadiometer. For the measurement of body composition, we used bioelectric impedance analysis (Maltron International). It was conducted in horizontal position at the right arm and feet after a 5 min sedentary rest.
Blood pressure was measured in a sitting position at the right upper arm with appropriately sized cuffs after a sedentary rest of 5 min. A fully automated blood pressure monitor was used (Bosch & Sohn), and two measurements at each visit were obtained.
Blood sampling and laboratory measurements
Venous blood samples were drawn after an overnight fast of at least 10 h under standardised conditions. Serum was obtained after 45 min of clotting by centrifugation at 1500 g for 20 min at 4°C and stored at − 80°C until analysis. The biochemical analyses were performed on completion of the study in a series; therefore, all samples from one subject were measured within one analytical run.
Levels of serum total cholesterol, LDL-cholesterol, HDL-cholesterol, TAG, apoA1 and apoB, glucose and insulin were measured on an autoanalyser (Architect System ci8200 series; Abbott Diagnostics) with the following methods: serum total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG and glucose were analysed enzymatically/photometrically; insulin was measured with a chemiluminescence microparticle immunoassay; apoA1 and apoB by immunoturbidimetry. The ELISA method was used to measure concentrations of intact proinsulin (Immundiagnostik) and leptin (DRG Instruments). Inter-assay CV were < 7·0 % for intact proinsulin and < 8·7 % for leptin. Samples were analysed in duplicate with a commercial microplate reader (Tecan).
Statistical analyses
Statistical analyses were performed using SPSS statistics software (SPSS Institute) and were done with patients who completed the study (completers) only. The normal distribution of the data was confirmed by looking at histograms and normal plots of the data, and by performing a Kolmogorov–Smirnov test. Non-normally distributed variables (TAG, glucose, insulin, intact proinsulin and leptin) were transformed with base-10 logarithm to normality. Within-group comparisons were performed using the paired t test. Inter-group interaction was calculated by repeated-measures ANOVA for time × group interaction with the data of visits 1–3 as the within-subject factor (time) and group (RO group v. OO group) as the between-subject factor. Sphericity was tested by Mauchly's test and in cases where the assumption of sphericity did not hold, Huynh–Feldt correction was used. Prevalence data of the metabolic syndrome were analysed by using McNemar's test for the presence of the metabolic syndrome as a dichotomous variable, and inter-group differences of diagnosis criteria of the metabolic syndrome by using the Mann–Whitney U test for unrelated samples. A P value of < 0·05 was considered as significant. Data are presented as means and standard deviations.
Results
Dietary analysis and compliance
Energy intake and nutrient composition of the diets at baseline and during the intervention period is given in Table 3. On the basis of the dietary records, the patients showed good compliance with the prescribed diets. Compared with baseline, both groups significantly reduced their energy intake by 2·3 MJ/d (RO group; P < 0·05) or 2·5 MJ/d (OO group; P < 0·05), respectively, and their diets had a lower ED (P < 0·05). For the changes in energy intake and ED, no significant inter-group differences were observed. In both groups, the dietary intervention led to a distinct decrease in SFA intake (15·8 v. 9·8 En% in the RO group, P < 0·05; 15·1 v. 10·8 En% in the OO group, P < 0·05) and a pronounced increase in MUFA intake (13·6 v. 18·3 En% for the RO group, P < 0·05; 13·6 v. 19·6 En% in the OO group, P < 0·05) when compared with the habitual diets. As calculated, the En% derived from protein, carbohydrates, total fat, SFA and MUFA did not differ significantly between the two groups throughout the study.
Table 3 Energy and nutrient intakes of the patients at baseline and after a 3- and 6-month intervention period
(Mean values and standard deviations, n 81)
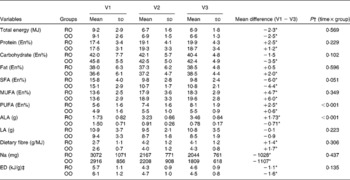
V1, visit 1 (before the study); V2, visit 2 (3 months); V3, visit 3 (6 months); RO, rapeseed oil-rich diet; OO, control diet with olive oil; En%, percentage of energy; ALA, α-linolenic acid; LA, linoleic acid; ED, energy density.
* Mean differences were significantly different from those at baseline (P < 0·05; paired t test).
† P values were obtained from repeated-measures ANOVA.
‡ ED was calculated for solid foods and energy-containing beverages.
In the RO group, the consumption of rapeseed oil led to a mean ALA intake of 3·46 g/d, whereas the OO group ingested 0·78 g/d of ALA (P < 0·001 for time × group interaction; Table 3). The intake of linoleic acid was similar for both groups (10·8 v. 8·5 g/d; P = 0·223).
Weight reduction, body composition and blood pressure
After the 6-month dietary intervention period, body weight of the patients was significantly reduced in both groups ( − 7·8 kg in the RO group v. − 6·0 kg in the OO group), accompanied by reductions in BMI ( − 2·7 v. − 2·1 kg/m2), waist circumference ( − 9·9 v. − 8·9 cm) and body composition, i.e. a decrease in body fat ( − 2·9 v. − 2·0 %) and an increase in lean body mass (+3·0 v.+2·1 %) (P < 0·05 for all parameters; Table 4). For these changes, no significant differences between the groups were observed.
Table 4 Effects of a 6-month intervention with rapeseed oil- (RO) or olive oil-enriched (OO) hypoenergetic diets on body weight, body composition and blood pressure in patients with the metabolic syndrome
(Mean values and standard deviations, n 81)

V1, visit 1 (before the study); V2, visit 2 (3 months); V3, visit 3 (6 months); SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Mean differences were significantly different from those at baseline (P < 0·05; paired t test).
† P values were obtained from repeated-measures ANOVA.
In addition, there was a decline in systolic and diastolic blood pressure ( − 10·0 mmHg in the RO group v. − 8·0 mmHg in the OO group for RR systolic, P < 0·05; − 8·4 mmHg in the RO group v. − 4·4 mmHg in the OO group for RR diastolic, P < 0·05; Table 4). The latter was more pronounced for the RO group when compared with the OO group (P = 0·026 for time × group interaction).
Laboratory measurements
As shown in Table 5, concentrations of total cholesterol and LDL-cholesterol as well as the ratio of apoB:apoA1 significantly decreased during the intervention period, while the values of HDL-cholesterol remained unchanged. In the RO group, there was also a significant decrease in TAG levels ( − 0·45 mmol/l; P < 0·05), which was not found in the OO group ( − 0·20 mmol/l; P>0·05) and which led to a significant difference between the groups (P = 0·020 for time × group interaction). Serum levels of insulin and intact proinsulin were significantly reduced in both groups (P < 0·05 for each parameter). In addition, a significant reduction in serum leptin values was observed throughout the study (P < 0·05). With the exception of serum TAG values, we observed no significant differences for the time × group interaction as measured by repeated-measures ANOVA.
Table 5 Effects of a 6-month intervention with rapeseed oil- (RO) or olive oil-enriched (OO) hypoenergetic diets on the serum lipid and glucose profile and leptin in patients with the metabolic syndrome
(Mean values and standard deviations, n 81)†
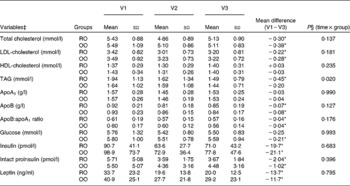
V1, visit 1 (before the study); V2, visit 2 (3 months); V3, visit 3 (6 months).
* P values were significantly different from those at baseline (P < 0·05; paired t test).
† Data of biomarkers are actual means, transformed values were used for statistical analysis.
‡ Variables TAG, glucose, insulin, intact proinsulin and leptin were transformed with base-10 logarithm to normality.
§ P values were obtained from repeated-measures ANOVA.
Prevalence of the metabolic syndrome
Based on the International Diabetes Federation definition for the metabolic syndrome(Reference Alberti, Zimmet and Shaw30), there was a remarkable reduction in the prevalence of the disorder (Fig. 2). According to the inclusion criteria, all patients met the diagnosis criteria at baseline. At the end of the study, the frequency was reduced in both groups to a similar extent (P = 0·856 for group interaction). Only twenty-three patients (58 %) of the RO group and nineteen patients (46 %) of the OO group still met the diagnosis criteria of the metabolic syndrome (for both groups: P < 0·001 for time interaction).
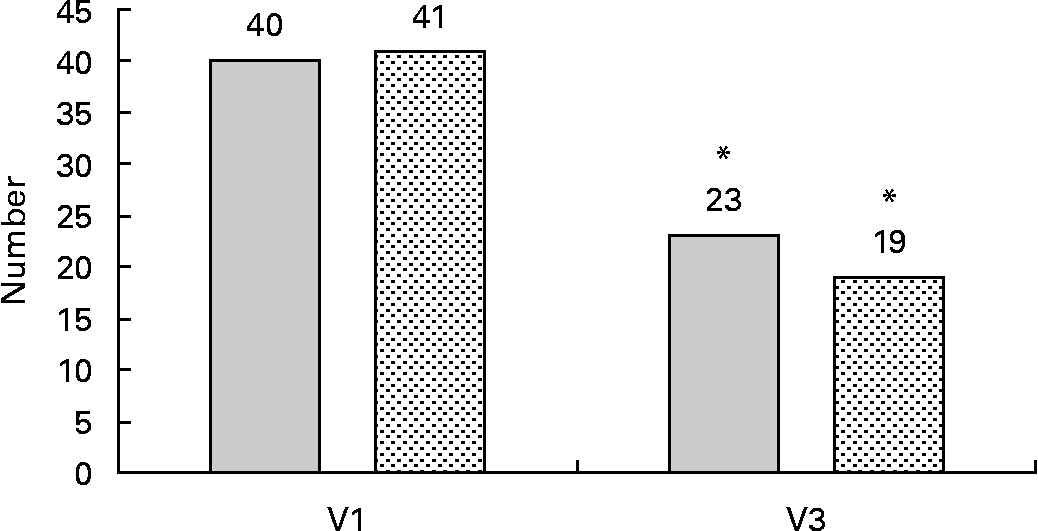
Fig. 2 Number of patients with the metabolic syndrome at the beginning and the end of the 6-month intervention with rapeseed oil (RO; ![]() )- or olive oil (OO;
)- or olive oil (OO; ![]() )-enriched hypoenergetic diets (n 81). * Mean value was significantly different from that at baseline (P < 0·001; McNemar's test). V1, visit 1 (before the study); V3, visit 3 (6 months).
)-enriched hypoenergetic diets (n 81). * Mean value was significantly different from that at baseline (P < 0·001; McNemar's test). V1, visit 1 (before the study); V3, visit 3 (6 months).
Discussion
The objective of the present dietary intervention study was to investigate the effects of a hypoenergetic rapeseed oil-enriched diet on body weight and cardiovascular risk factors in the metabolic syndrome. In addition, focus was put on the question whether specific protective effects for ALA could be identified with a daily amount suitable for long-term nutrition (about 3·5 g/d). For this purpose, the rapeseed oil diet was compared with an olive oil diet with similar nutrient composition but a low ALA content ( < 1 g/d). After the 6-month intervention period, a significant loss in body weight accompanied by a distinct improvement in the overall cardiovascular risk profile of high-risk patients was observed in the rapeseed oil group as well as in the olive oil group. The high ALA intake led to a more pronounced reduction in serum TAG and in diastolic blood pressure compared with the low-ALA olive oil diet, indicating that there may be independent favourable metabolic effects of ALA.
The food pattern in the present study diets was characterised by low-fat foodstuffs such as vegetables, fruits, whole-grain products, lean meat, low-fat milk products, and rapeseed or olive oil as the main source of dietary fat. Despite its total fat content of about 38 En%, the diet had a favourable fatty acid composition, e.g. low SFA and trans-fatty acid contents, but a high proportion of MUFA. In addition, it was rich in dietary fibre and had a low ED. Bes-Rastrollo et al. (Reference Bes-Rastrollo, van Dam and Martinez-Gonzalez4) found that the total fat content of the diet was no determinant for a high ED, but that the ED was positively correlated with the intake of solid fats and refined carbohydrates. Since these products were only allowed in small amounts in this diet, and the intake of low-energy-dense foods rich in water and/or fibre was high, a significant reduction in ED was reached while the total fat content remained unchanged throughout the study. We assume that this low-energy-dense food pattern was mainly responsible for the good compliance of our patients as shown by the low dropout rates during the study. The necessary energy restriction was facilitated by a good practicability in the normal course of life and resulted in a pronounced weight reduction and a loss of body fat. A reduction in dietary ED was also associated with successful weight loss in other studies(Reference Ello-Martin, Roe and Ledikwe32–Reference Rolls, Roe and Beach34). The intensive and ongoing dietary counselling throughout the study may have been a second important factor for the good adherence to the diet, as confirmed by others(Reference Digenio, Mancuso and Gerber35).
With the dietary regimen, we tried to combine the metabolic advantages of different dietary approaches. There is an ongoing discussion about the optimal nutrient composition for dietary treatment of the various risk factor combinations typical for the metabolic syndrome. For the dietary management of hypertension, the low-fat diet of the Dietary Approaches to Stop Hypertension Study (DASH diet) is usually recommended(Reference Appel, Brands and Daniels36). Recently, a carbohydrate-reduced version of the DASH diet and a Mediterranean-style diet have also been emphasised as a therapeutic option(Reference Sacks and Campos37). The decrease in HDL-cholesterol and the increase in serum TAG concentrations often observed with low-fat diets(Reference Griel, Ruder and Kris-Etherton10, Reference Reaven38) can be avoided with a MUFA-rich diet(Reference Estruch, Martínez-González and Corella39, Reference Elhayany, Lustman and Abel40). With regard to LDL-cholesterol, both dietary approaches show comparable reductions(Reference Egert, Kratz and Kannenberg41, Reference Jebb, Lovegrove and Griffin42). MUFA-rich diets also exert favourable effects on serum glucose and insulin levels in patients with insulin resistance or diabetes mellitus when compared with a high intake of refined carbohydrates(Reference Ros21), but have no significant advantages in comparison with a high proportion of fibre-rich carbohydrates(Reference Gerhard, Ahmann and Meeuws43). Our dietary regimen, as described above, was suitable to improve all risk factors. The food pattern was comparable to a Mediterranean-style diet, for which there is a body of evidence that it is effective for weight reduction and ameliorates the global cardiovascular risk in healthy overweight subjects and high-risk patients(Reference Shai, Schwarzfuchs and Henkin17, Reference Estruch, Martínez-González and Corella39, Reference Rallidis, Lekakis and Kolomvotsou44, Reference Rumawas, Meigs and Dwyer45). In agreement with the present findings, Esposito et al. (Reference Esposito, Marfella and Ciotola46) reported a reduced frequency of the metabolic syndrome after a 2-year intervention with a Mediterranean diet compared with a low-fat diet.
With our dietary approach, we were able to show that even with a hypoenergetic diet, it is feasible to ingest a daily amount of about 3·5 g ALA with a long-term natural food diet without supplements. This is two- to threefold of the amount compared with the average intake of 1·0–1·5 g/d in Europe and the USA(Reference Welch, Shakya-Shrestha and Lentjes47, Reference Kris-Etherton, Taylor and Yu-Poth48). In contrast to the well-established cardioprotective effects of the long-chain n-3 fatty acids EPA and DHA(Reference Harris, Miller and Tighe49, Reference Lavie, Milani and Mehra50), there are only a few and conflicting findings on the role of ALA and its optimal dosage for cardiovascular health. Some observational studies have reported an inverse association between ALA intake and CVD(Reference Zatonski, Campos and Willett51–Reference Sala-Vila, Cofán and Pérez-Heras53), which could not be confirmed by a recent intervention trial(Reference Kromhout, Giltay and Geleijnse54). Experimental studies have shown inconsistent results of ALA effects on cardiovascular risk factors(Reference Bloedon, Balikai and Chittams28, Reference Barre, Mizier-Barre and Griscti29, Reference Finnegan, Minihane and Leigh-Firbank55–Reference Schwab, Callaway and Erkkilä57). Zhao et al. (Reference Zhao, Etherton and Martin56) described a decrease in serum total cholesterol, LDL-cholesterol, HDL-cholesterol and TAG after an ALA-rich diet in hypercholesterolaemic subjects compared with an average American diet, whereas Finnegan et al. (Reference Finnegan, Minihane and Leigh-Firbank55) found no significant effects on serum lipids in patients with hyperlipidaemia after ingestion of 4·5 or 9·5 g ALA/d. Bloedon et al. (Reference Bloedon, Balikai and Chittams28) compared the effects of flaxseed-based products with wheat-based products provided with a low-fat diet. The ALA intake in the flaxseed group was 3·4 g/d higher than that in the control group. The flaxseed diet improved insulin sensitivity and reduced serum LDL-cholesterol in hypercholesterolaemic patients. In type 2 diabetes, the intake of 5·5 g ALA/d provided with flaxseed oil did not have any significant effects on glycaemic control(Reference Barre, Mizier-Barre and Griscti29).
Although we found in the present study a significant improvement in the overall cardiovascular risk profile, it must be assumed that these effects were not specific for ALA, because they were similar in the low-ALA control group with otherwise identical nutrient composition and could primarily be attributed to weight loss and the favourable whole dietary pattern as outlined above. However, we observed a significantly more pronounced decline in diastolic blood pressure after the ALA-rich rapeseed oil diet compared with the low-ALA olive oil diet. This finding was confirmed in a Greek study with an ALA intake of 8 g/d, which led to significantly lower systolic and diastolic blood pressure levels compared with a high intake of linoleic acid in normotensive subjects(Reference Paschos, Magkos and Panagiotakos27). In addition, we found a significant decrease in serum TAG concentrations after the high ALA intake which was not observed in the control group. While most studies did not find clear effects of ALA on serum TAG(Reference Finnegan, Minihane and Leigh-Firbank55, Reference Seppänen-Laakso, Laakso and Lehtimäki58–Reference Goyens and Mensink60), Egert et al. (Reference Egert, Kannenberg and Somoza61) also described a significant reduction in TAG values in healthy subjects after ingestion of 4·4 g ALA/d. We speculate from these results that there might be an independent effect of ALA on blood pressure and serum TAG, which needs further investigation.
One limitation of the present study with regard to the ALA-specific effects may be that they were investigated during weight loss. We cannot exclude that catabolic metabolism during weight reduction could have led to other metabolic effects of ALA than those to be observed under stable body-weight conditions and normoenergetic diets. In addition, it would be of interest whether possible effects of ALA were dependent on the total fat content of the diet, on the fatty acid composition, or on the n-6:n-3 ratio, but these questions could not be answered with our design.
With the present study, we provided a contribution to the discussion about the optimal dietary treatment in the metabolic syndrome. In conclusion, a dietary pattern that combines the advantages of a low ED with a high intake of cardioprotective MUFA and ALA can be a feasible and practical approach for long-term dietary treatment in patients with the metabolic syndrome, leading to weight reduction and an improvement in the overall cardiovascular risk profile. More research is needed to investigate the possible independent metabolic effects of ALA in populations with CVD and/or type 2 diabetes compared with healthy controls.
Acknowledgements
This study was supported by the Union for Promoting Oil and Protein Plants e.V. (UFOP) and the International Foundation for the Promotion of Nutrition Research and Nutrition Education (ISFE). U. W., B. S. and D. T. designed the research; Y. H. L.-B. and A. B. conducted the research; A. B. analysed the data; U. W. and A. B. wrote the manuscript; U. W. had primary responsibility for the final content. All authors read and approved the final manuscript. The authors have no conflicts of interest to declare.









