Reactive oxygen species are a by-product of metabolic processes such as mitochondrial ATP generation and inflammation( Reference Murphy 1 ) which impair cellular function by damaging macromolecules, including DNA, proteins and lipids( Reference Apel and Hirt 2 ). The peroxidation of fatty acids in LDL by reactive oxygen species is an early pathogenic step in atherosclerosis( Reference Virdis, Ghiadoni and Giannarelli 3 ). This pathogenic role provides the basis for the investigation of the efficacy of micronutrients with antioxidant capacity (i.e. ascorbic acid, β-carotene and vitamin E) in the prevention of CVD and metabolic disease( Reference Kaliora, Dedoussis and Schmidt 4 ). In vitro studies have shown that the combination of vitamins C and E has synergistic antioxidant effects. Specifically, vitamin C reversed the pro-oxidant state of vitamin E( Reference Rietjens, Boersma and Haan 5 ), and this effect supports the combined administration of vitamins C and E in clinical trials( Reference Plantinga, Ghiadoni and Magagna 6 – Reference Kinlay, Behrendt and Fang 9 ). Observational studies have provided strong evidence for an inverse association between the intake of antioxidant vitamins and the risk of cardiovascular morbidity or mortality( Reference Rimm, Stampfer and Ascherio 10 , Reference Yochum, Folsom and Kushi 11 ). However, randomised controlled trials (RCT) that tested the effects of antioxidant supplements on cardiovascular outcomes have reported conflicting effects( 12 , Reference Collins, Armitage and Parish 13 ). Indeed, some RCT have shown that β-carotene has harmful effects for smokers with respect to lung cancer risk( Reference Omenn, Goodman and Thornquist 14 ) and that vitamin E supplementation has harmful effects on cardiovascular outcomes( Reference Waters, Alderman and Hsia 15 ).
Maintaining a healthy endothelium is important for normal vascular function( Reference Vanhoutte, Shimokawa and Tang 16 ). Abnormal endothelial function (EF) is an early step in atherosclerosis development and is associated with an elevated risk of cardiovascular events, including myocardial infarction and stroke( Reference Gori and Munzel 17 ). Therefore, nutritional interventions which improve EF may help prevent the onset or progression of atherosclerosis( Reference Widlansky, Gokce and Keaney 18 ).
The primary objective of the present study was to conduct a systematic review and meta-analysis of RCT to investigate the effects of supplementation with vitamins C and E on EF measured by either forearm blood flow (FBF) or flow mediated dilation (FMD). In addition, we determined whether the effects on EF were modified by vitamin(s) dose, duration of intervention, participant age or baseline plasma antioxidant vitamin concentrations.
Methods
The present systematic review was conducted according to Cochrane guidelines( Reference Higgins and Green 19 ) and is reported according to PRISMA guidelines( Reference Liberati, Altman and Tetzlaff 20 ). PROSPERO Database registration: CRD42013005602, http://www.crd.york.ac.uk/prospero/
Literature search
We searched four databases (MEDLINE, Embase, Cochrane Library and Scopus) from inception until May 2014 for relevant articles. Additionally, we inspected the reference lists of reviews and included articles from relevant studies. The following search terms were used: vitamin C, ascorbic acid, vitamin E, tocopherol, forearm blood flow, FBF, flow mediated dilation, FMD, hyperaemia, plethysmography, Doppler ultrasound, endothelial function and randomised controlled trials. Full details of the search criteria used in the present study are reported in the online supplementary materials.
Study selection
The following criteria were implemented when identifying articles to be included in the present systematic review: (1) RCT; (2) studies that involved adult participants aged ≥ 18 years; (3) studies that included vitamins C and E given alone or in combination for more than 2 weeks; and (4) studies that reported changes in EF measured using ultrasound (to estimate FMD) or plethysmography (to quantify FBF). No language or time restrictions were applied when searching the databases. Three investigators (A. W. A., C. O. and S. A.) independently screened the titles and abstracts of the articles for eligibility for inclusion. If a consensus was reached, an article was excluded or moved to the next stage (full-text screening), as appropriate. If a consensus was not reached, the article was moved to the next stage, where the full text of selected articles was appraised. Disagreements were resolved by discussion between the reviewers at that stage until a consensus was reached.
Data extraction and quality assessment
The following information was extracted from eligible articles: (1) authors, journal details and year of publication; (2) participants (total number, male:female ratio, age, health status and the use of prescribed drugs); (3) study characteristics (design, methods of randomisation and blinding and report of adverse effects); (4) vitamin C and E supplementation (dose, duration and type of control); (5) EF measurement (FBF or FMD); and (6) circulating concentrations of vitamins C and E before and after intervention.
In addition, we adopted the modified Jadad score (range 0–5) to assess the risk of bias in included studies; a score of ≤ 3 indicates high risk, whereas a score of >3 indicates low risk( Reference Crowther, Lim and Crowther 21 ).
Statistical analysis
Results from EF measurement using FMD and FBF are reported on different scales, and standardised mean differences (SMD) were therefore used as a summary statistic for comparing effect sizes across studies. We undertook data synthesis, including the calculation of effect sizes with 95 % CI, using a random-effects model with inverse variance weighting. For graphical presentation of the EF outcomes, forest plots were generated using the mean and standard deviation at the end of the intervention. For studies that reported changes in EF at two or more time points, the final EF measurement was used in the meta-analysis. Data not provided in the main text or tables were extracted from the figures. For crossover trials, we used the mean and standard deviation separately for the intervention and control conditions. This is regarded as a conservative approach that reduces the power of these studies to show the true effects of the intervention( Reference Elbourne, Altman and Higgins 22 ). Data from studies that reported their results as medians were converted to mean and standard deviation using the formula described by Hozo et al. ( Reference Hozo, Djulbegovic and Hozo 23 ) (online supplementary material). Statistical analyses were performed using STATA 12 (StataCorporation 2011), and a P value of < 0·05 denoted statistical significance.
Subgroup analyses were undertaken to investigate the potential sources of heterogeneity and of the variables that influenced the effect of antioxidant vitamin supplementation on EF. These factors included the outcome (FMD or FBF), age of participants, antioxidant vitamin dose and duration of use and baseline plasma vitamin concentrations. Meta-regression analyses were conducted to discover whether the effect of antioxidant vitamins supplementation on EF was modified by these factors.
Publication bias was evaluated by visual inspection of the funnel plot and by Egger's regression test( Reference Egger, Davey Smith and Schneider 24 ). Heterogeneity between studies was evaluated using Cochrane Q statistics; P>0·1 indicates significant heterogeneity. The I 2 test was also used to evaluate consistency between studies; a value of < 25 % indicates a low risk of heterogeneity, a value of 25–75 % indicates a moderate risk of heterogeneity and a value of >75 % indicates a high risk of heterogeneity( Reference Higgins, Thompson and Deeks 25 ).
Results
Search results
The total number of articles obtained from searching the four databases and after removing duplicates was 2172. The inspection of titles and abstracts yielded 119 articles to be examined in the full-text phase. After full-text examination, forty-six RCT were included in the final analysis (references to these articles are provided in the online supplementary material). Three studies( Reference Franzini, Ardigo and Valtuena 26 – Reference Khan, Ray and Craigie 28 ) were excluded because they administered the antioxidant vitamins as components of complex foods or drinks and not as vitamin supplements, which made it impossible to attribute effects to the vitamins per se and not to other components of the foods or drinks. Full details of the selection process and the reasons for exclusion are summarised in Fig. 1.

Fig. 1 Flow diagram of the process used in selection of the randomised clinical trials included in the present analysis. FMD, flow mediated dilation; FBF, forearm blood flow.
Studies characteristics
The total number of participants was 1817, with a median sample size of twenty-six participants per study (range 7–197). The age of the participants ranged from 23 to 78 years (median 54 years). Seven studies included only males)(S5,S14,S16,S21,S27,S29,S31) and two other studies included only females(S19,S38) (Full references of these studies are reported in the online Supplementary material). In the mixed-sex studies, the proportion of male participants ranged from 38 to 95 %. The median duration of the studies was 56 d (range: 21 d–3 years). The quality of the included studies ranged from 2 to 5, with a median quality score of 3. Descriptions of the study design, outcomes, doses of antioxidant vitamins, health statuses of participants and quality ratings of the included studies are provided in Table 1.
Table 1 Characteristics of the studies included in the meta-analysis

FBF, forearm blood flow; UB; non-blinded; CAD, coronary artery disease; DB, double-blind; FMD, flow mediated dilation; NR, not reported; SB; single-blind; SLE, systemic lupus erythematosus.
* Full references of these studies are reported in the online Supplementary material.
† 1 IU vitamin E = 0·67 mg natural vitamin E.
Some of the articles included results from separate, independent trials that tested the effects of antioxidant supplementation on EF; therefore, the forty-six articles yielded a total of fifty-eight trials to be included in the final analysis. Of these, seventeen trials investigated vitamin C alone, twenty-seven investigated vitamin E alone and fourteen investigated combinations of vitamins C and E.
Meta-analysis
Supplementation with vitamin C alone
Pooling studies that tested the effects of vitamin C supplementation alone (seventeen trials, 478 participants) revealed a significant improvement in EF (SMD 0·25, 95 % CI 0·02, 0·49, P= 0·043). However, these studies were characterised by moderately significant heterogeneity (χ2= 26·9, P< 0·043, I 2= 40·5 %) (Fig. 2). Subgroup analysis demonstrated a significant effect of vitamin C in studies that included participants who were older than 56 years of age (SMD 0·58, 95 % CI 0·16, 0·99, P= 0·007) but no effect in those studies which included participants who were ≤ 56 years (Table 2). Furthermore, meta-regression analysis showed a significant positive correlation between age and the effect of vitamin C supplementation on EF (β 0·023, 95 % CI 0·001, 0·05, P= 0·042) (Fig. 3). Neither subgroup nor meta-regression analyses showed significant modifying effects of the dose or duration of vitamin C supplementation on EF.

Fig. 2 Forest plot showing the effect of vitamin C supplementation on endothelial function. SMD, standardised mean difference. * Full references of these studies are reported in the online Supplementary material.
Table 2 Subgroup analysis conducted on vitamin C supplementation studies included in the meta-analysis
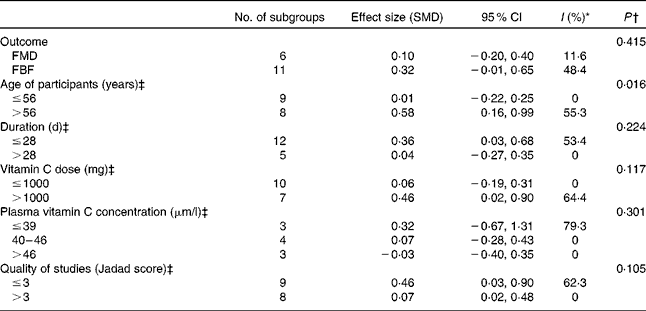
SMD, standardised mean difference; FMD, flow mediated dilation; FBF, forearm blood flow.
* Consistency between studies ( < 25 %, low risk, 25–75 %, moderate risk and >75 % indicates high risk of heterogeneity).
† P for the meta-regression analysis between subgroups.
‡ Groups dichotomised at the median.
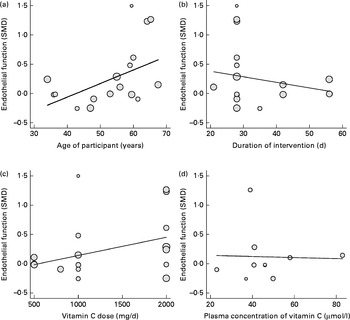
Fig. 3 Association between the: (a) age of participants (β 0·02, P= 0·04), (b) duration (β − 0·01, P= 0·43), (c) dose (β 0·0003, P= 0·10), (d) baseline plasma concentration of vitamin C (β − 0·0009, P= 0·93) and the effect on endothelial function measured by flow mediated dilation and forearm blood flow. Each study is depicted by a ○ where the circle size represents the degree of weighting for the study based on participant numbers in the study. SMD, standardised mean difference.
Supplementation with vitamin E alone
Vitamin E supplementation alone (twenty-seven trials, 742 participants) produced significant improvement in EF (SMD 0·48, 95 % CI 0·23, 0·72, P= 0·0001) (Fig. 4), but there was significant heterogeneity between the studies (χ2= 73·4, P< 0·001, I 2= 64·6 %). Subgroup analysis showed a significantly higher effect of vitamin E supplementation on EF in participants with a baseline plasma vitamin E concentration of less than 20 μm (SMD 1·14, 95 % CI 0·64, 1·64, P= 0·0001) but no effect in those with a baseline vitamin E concentration of >20 μm (Table 3). Additionally, meta-regression analysis demonstrated a significant negative correlation between baseline plasma vitamin E concentration and the effect of vitamin E supplementation on EF (β − 0·03, 95 % CI − 0·06, − 0·001, P= 0·029) (Fig. 5). However, age, vitamin E dose and duration of supplementation had no significant modifying effects on EF.

Fig. 4 Forest plot showing the effect of vitamin E supplementation on endothelial function. SMD, standardised mean difference. * Full references of these studies are reported in the online Supplementary material.
Table 3 Subgroup analysis conducted on vitamin E supplementation studies included in the meta-analysis
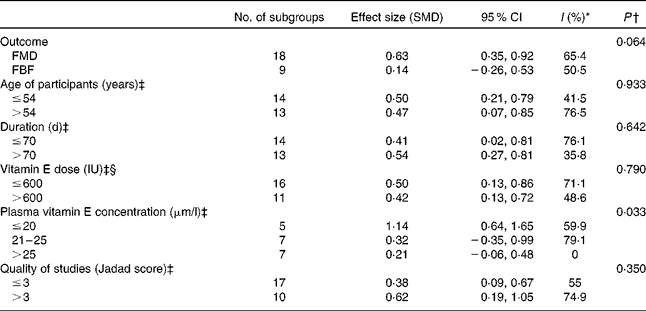
SMD, standardised mean difference; FMD, flow mediated dilation; FBF, forearm blood flow.
* Consistency between studies ( < 25 %, low risk, 25–75 %, moderate risk and >75 % indicates high risk of heterogeneity).
† P for the meta-regression analysis between subgroups.
‡ Groups dichotomised at the median.
§ 1 IU vitamin E = 0·67 mg natural vitamin E.

Fig. 5 Association between the: (a) age of participants (β −0·004, P= 0·67), (b) duration (β −0·0001, P= 0·69), (c) dose (β −0·0002, P= 0·46), (d) baseline plasma concentration of vitamin E (β −0·033, P= 0·03) and the effect on endothelial function measured by flow mediated dilation and forearm blood flow. Each study is depicted by a ○ where the circle size represents the degree of weighting for the study based on participant numbers in the study. SMD, standardised mean difference.
Combined supplements of vitamins C and E
Combined supplements of vitamins C and E (fourteen trials, 597 participants) were ineffective in improving EF (SMD 0·12, 95 % CI − 0·18, 0·42, P= 0·428) (Fig. 6). Furthermore, the heterogeneity between studies was highly significant (χ2= 40·6, P< 0·001, I 2= 68 %). Subgroup and meta-regression analyses failed to uncover any effect of the combined antioxidant vitamins on EF regardless of age, duration or dose of supplemental vitamins or the baseline plasma concentration of these vitamins (Table 4).

Fig. 6 Forest plot showing the effect of combined antioxidant vitamins C and E supplementation on endothelial function. SMD, standardised mean difference. *Full references of these studies are reported in the online Supplementary material.
Table 4 Subgroup analysis conducted on combined vitamins C and E supplementation studies included in the meta-analysis

SMD, standardised mean difference; FMD, flow mediated dilation; FBF, forearm blood flow.
* Consistency between studies ( < 25 %, low risk, 25–75 %, moderate risk and >75 % indicates high risk of heterogeneity).
† P for the meta-regression analysis between subgroups.
‡ Groups dichotomised at the median.
§ 1 IU vitamin E = 0·67 mg natural vitamin E.
Publication bias
Visual inspection of the funnel plots did not show evidence of publication bias for the three meta-analyses (online supplementary Figs. S1–S3). Further, Egger's regression test confirmed the likely absence of publication bias in these meta-analyses (β 1·04, P= 0·547; β − 0·78, P= 0·614; β 1·32, P= 0·327) of supplementation with vitamin C, vitamin E and the combined antioxidants, respectively.
Discussion
Overall, supplementation with either vitamin C or vitamin E separately improved EF significantly in the RCT with the adult human subjects considered in the present review. Furthermore, the effects of individual vitamin supplementation was modified by the age of participants (vitamin C studies) and the baseline plasma concentrations of vitamin E (vitamin E studies). However, the combined administration of both vitamins was not effective in altering EF regardless of study design or participant characteristics.
Evidence from experimental studies has revealed that oxidative stress plays a pivotal role in the aetiology of endothelial dysfunction and atherosclerosis( Reference Gori and Münzel 29 ). In addition, the assessment of endothelial dysfunction has diagnostic and prognostic value in CVD( Reference Fischer, Rossa and Landmesser 30 ).
Proposed mechanisms for the beneficial effects of antioxidant vitamins on EF include increasing NO bioavailability through the up-regulating endothelial NO synthase enzyme and reducing NO inactivation by free radicals( Reference May 31 ). However, experimental studies demonstrated that the rate constant of the reaction of antioxidant vitamins and superoxide is considerably lower than that of the reaction between NO and the free radical superoxide, which makes the antioxidant vitamins ineffective in protecting NO from free radical inactivation( Reference Beckman and Koppenol 32 ). However, there might be alternative molecular mechanisms through which antioxidants vitamins improve EF( Reference Ungvari, Kaley and de Cabo 33 ), including the abrogation of NF-κB-driven systemic chronic inflammation, which can accelerate ageing via the reactive oxygen species-mediated exacerbation of telomere dysfunction and cell senescence( Reference Jurk, Wilson and Passos 34 ). Oxidative stress activates redox-sensitive transcription factors, including the activator protein (AP-1) and NF-κB, which increases the expression of cytokines, adhesion molecules and pro-inflammatory enzymes( Reference El Assar, Angulo and Vallejo 35 ). These factors contribute significantly to the pro-inflammatory microenvironment and facilitate the development of vascular dysfunction( Reference Pashkow 36 ). In middle-aged and older adults, brachial FMD is inversely related to cytokines and adhesion molecules in the circulation, but this relationship appears to be explained largely by traditional coronary risk factors( Reference Vita, Keaney and Larson 37 ). Inhibition of NF-κB signalling improved EF significantly in middle-aged and older adults( Reference Pierce, Lesniewski and Lawson 38 ).
The effect of vitamin C supplementation on EF observed in the present meta-analysis is less than that reported in our recently published meta-analysis of the effect of vitamin C supplementation on EF( Reference Ashor, Lara and Mathers 39 ). However, in that meta-analysis, we included acute studies (2 weeks or less) which were excluded from the present meta-analysis. These acute studies included the use of high intravenous doses of vitamin C for short durations, which may have contributed to the observed higher overall effect of vitamin C supplementation on EF reported earlier( Reference Ashor, Lara and Mathers 39 ).
Combined supplements of antioxidant vitamins
Combined administration of vitamins C and E was ineffective for improving EF. In vitro studies have demonstrated that vitamin C can regenerate oxidized vitamin E, which leads to the restoration of its antioxidant capacity( Reference Traber and Stevens 40 ). This potentially beneficial interaction between the two vitamins provides an underpinning mechanism that supports the design and conducting of RCT that test the efficacy of combined vitamin C and E supplementation on cardiovascular and metabolic outcomes( Reference Cook, Albert and Gaziano 41 , Reference Sesso, Buring and Christen 42 ). However, these studies failed to show beneficial effects of the combined supplements on the investigated outcomes.
Subgroup analyses of large clinical trials (Heart Outcomes Prevention Evaluation (HOPE), Women's Health Study (WHS) and Israel Cardiovascular Events Reduction with Vitamin E (ICARE)) found that administration of vitamin E improved cardiovascular outcomes significantly in diabetic patients with the haptoglobin genotype 2–2( Reference Vardi, Blum and Levy 43 ). In contrast, post hoc analysis of the Women's Antioxidant Vitamin Estrogen (WAVE) study showed that combined vitamin C and E supplementation significantly exacerbated cardiovascular outcomes in a subpopulation with the haptoglobin 2–2 genotype. In transgenic animals with the haptoglobin 2–2 genotype, Asleh & Levy( Reference Asleh and Levy 44 ) reported that the co-administration of vitamin C mitigated the protective effect of vitamin E on HDL.
Human mechanistic studies have found that adding vitamin C to vitamin E may not cause any further reduction in oxidative stress biomarkers( Reference Dietrich, Block and Hudes 45 ). Similarly, in vitro experimental studies demonstrated that the synergistic action of vitamins C and E disappeared under anaerobic conditions and may be converted to a pro-oxidant effect( Reference Kadoma, Ishihara and Fujisawa 46 ). In these studies, monitoring the rate of α-tocopheroxyl radical formation in the presence of co-antioxidants, such as ascorbate, revealed that the rate of α-tocopheroxyl radical formation was enhanced with higher concentrations of vitamin E or under anaerobic conditions( Reference Fujisawa, Ishihara and Atsumi 47 , Reference Kadoma, Ishihara and Okada 48 ).
We recently demonstrated that the combined administration of antioxidant vitamins significantly improved arterial stiffness indices( Reference Ashor, Siervo and Lara 49 ). However, the effect of the combined antioxidant vitamins is largely dependent on the study conducted by Zureik et al. ( Reference Zureik, Galan and Bertrais 50 ), which used minimum doses of the combined vitamins for a duration of 7 years.
Population subgroups that benefit from supplementation
The present meta-analysis showed a greater effect of vitamin C supplementation on EF in older people (Table 2), perhaps because they are more likely to have inadequate micronutrient intakes and therefore have a greater potential to benefit from vitamin C supplementation( Reference Brubacher, Moser and Jordan 51 ). Inadequate intakes may be exacerbated by a reduced capacity for vitamin C absorption with age( Reference Brubacher, Moser and Jordan 51 , Reference Visioli and Hagen 52 ). Furthermore, older people may require more vitamin C than younger adults to combat the greater oxidative stress that results from age-related mitochondrial dysfunction( Reference Puca, Carrizzo and Villa 53 ), but the quantitative needs for vitamin C and other micronutrients by older people are poorly understood.
The present meta-analysis revealed greater effects of vitamin supplementation on EF in those with lower baseline plasma vitamin E concentrations. A previous study showed that those in the lowest quintile of vitamin E status have a higher incidence of CVD( Reference Gey, Puska and Jordan 54 ). Similarly, Iannuzzi et al. ( Reference Iannuzzi, Celentano and Panico 55 ) found that participants in the lowest tertile of vitamin E status have a higher prevalence of carotid plaque. In the SUpplementation en VItamines et Minéraux AntioXydants (SU.VI.MAX) study, Hercberg et al. ( Reference Hercberg, Galan and Preziosi 56 ) found that those with lower baseline concentrations of vitamin C and β-carotene benefitted more from supplementation with antioxidant vitamins and minerals. Furthermore, supplementation with multivitamins for 6 years in a population that had a high prevalence of micronutrient deficiency improved cerebrovascular disease mortality significantly( Reference Mark, Wang and Fraumeni 57 ). These studies support the idea that individual vitamin status may determine the magnitude of the effect of antioxidant vitamin supplementation on EF and may therefore explain some of the inter-individual variation in response to such supplementation.
Implications for public health
In the present meta-analysis, vitamins C and E administered alone showed mild to moderate improvements in EF. However, these positive findings appear paradoxical when they are considered in light of the large RCT performed on individuals with and without CVD which found no benefit from antioxidant vitamin supplementation on major cardiovascular outcomes( 12 , Reference Collins, Armitage and Parish 13 ). Meta-analyses of outcomes from vitamin E supplementation trials demonstrate adverse effects on the risk of stroke in patients with CVD( Reference Miller, Pastor-Barriuso and Dalal 58 , Reference Schurks, Glynn and Rist 59 ). However, there may be specific population subgroups that would benefit from supplementation with these vitamins, including older people and those with a habitually low (baseline) vitamin status. Further research aimed at identifying those who have the potential to benefit would be valuable and might offer an objective basis for individualising such interventions.
Limitations
Two of the major limitations of the present meta-analysis are the relatively small sample size of the majority of the included studies and the significant heterogeneity observed between the included studies. However, the individual trials were designed to investigate the effects of antioxidant vitamins in population groups with variable age and health status (e.g. hypertensive, hypercholesterolemic or diabetic patients) that may have been the potential cause of the observed heterogeneity. In addition, the various methods and procedures implemented in measuring EF may add to the significant heterogeneity observed in those meta-analyses.
The primary outcome of the present meta-analysis was EF, whereas previous meta-analyses have focused on major cardiovascular outcomes, including angina, acute myocardial infarction, stroke or cardiovascular death( Reference Miller, Pastor-Barriuso and Dalal 58 , Reference Schurks, Glynn and Rist 59 ). The present study's focus on EF facilitates investigation of the effects of antioxidant vitamin interventions on an early CVD precursor, because abnormal EF is closely linked to the development and progression of atherosclerotic lesions and can contribute to plaque instability and subsequent cardiovascular complications( Reference Deanfield, Halcox and Rabelink 60 ). This focus on early events in the pathway to CVD may help identify opportunities for early interventions. A recent meta-analysis of fourteen cohort studies, which included a total of 5500 participants, estimated that a 1 % increase in brachial artery FMD (one of the indices of EF used in the present study) was associated with a 13 % reduction in the risk of future cardiovascular events( Reference Inaba, Chen and Bergmann 61 ).
Conclusions
The present meta-analysis has shown that supplementation with either vitamin C or vitamin E has a moderate protective effect on EF. The effect size of the interventions was greater in those with lower baseline vitamin E status and in older people, who are more likely to experience micronutrient deficiency and elevated oxidative stress. However, the lack of benefit with combined vitamin C and E interventions is a clear demonstration of the complexity of the relationship between antioxidant vitamin supplementation and CVD-related outcomes. The results emphasise the potential importance of a personalised approach to interventions with these vitamins when attempting to enhance primary or secondary prevention of CVD. The next logical step to advance this research would be to conduct secondary analyses of existing datasets from appropriate RCT to test whether benefits from supplementation with vitamins C and E will be observed in participants with greater oxidative stress and/or lower levels of circulating vitamin concentrations at baseline.
Supplementary material
To view supplementary material for the present article, please visit http://dx.doi.org/10.1017/S0007114515000227
Acknowledgements
A. W. A. is funded by the Ministry of Higher Education and Scientific Research of Iraq. J. L. and J. C. M. acknowledge support from the LiveWell Programme, which is funded through a collaborative grant from the Lifelong Health and Wellbeing (LLHW) initiative and managed by the Medical Research Council (MRC) (grant number G0900686).
A. W. A. drafted the manuscript; A. W. A., M. S. and J. C. M. conceived the idea for the study and developed the search strategy; A. W. A., C. O. and S. A. conducted the search; A. W. A., M. S. and J. L. summarised the data; all authors contributed to the data analysis, verification, writing and revising the manuscript.
The authors declare that there are no conflicts of interest.












