Aflatoxin is a fungal toxin produced by Aspergillus flavus and Aspergillus paraciticus ( Reference Sabran, Jamaluddin and Abdul Mutalib 1 ), found in many food items such as nuts, cereals, and spices and herbs( Reference Reddy, Farhana and Salleh 2 ). Chronic aflatoxin exposure is linked to the development of hepatocellular carcinoma (HCC)( 3 ). Global statistics indicate that more than fifty-five billion people suffer from uncontrolled exposure to aflatoxins( Reference Strosnider, Azziz-Baumgartner and Banziger 4 ). Moreover, unawareness of the public about aflatoxin-contaminated foods has been reported by some studies( Reference Jolly, Bayard and Awuah 5 – Reference Leong, Rosma and Latiff 8 ). The occurrence of aflatoxin in foodstuffs is prevalent in many developing countries in Africa and Asia. Aflatoxicosis is prevalent at an alarming rate in these countries and many cases have been reported and have caused deaths( Reference Lye, Ghazali and Mohan 9 – Reference Kensler, Roebuck and Wogan 11 ). In Malaysia, the consumption of aflatoxin-contaminated noodles caused acute hepatic encephalopathy in thirteen children, as up to 3 mg of aflatoxin was suspected to be present in a single serving of the contaminated noodles( Reference Lye, Ghazali and Mohan 9 ).
Although strict food regulations imposed by many countries were successful in eliminating or limiting the exposure, it is believed that some aflatoxin-contaminated foods still persist in the human food resources( Reference Sabran, Jamaluddin and Abdul Mutalib 1 ) and aflatoxin-contaminated foods cannot be neglected. Therefore, secondary prevention steps such as dietary or clinical interventions are developed as they are able to reduce aflatoxin bioavailability in the body or ameliorate aflatoxin-induced damage( Reference Liu and Wu 12 ). Despite the effectiveness of the non-nutritional adsorbents such as clay and activated C to bind aflatoxin and prevent aflatoxin absorption, there should also be other more practical alternatives.
The concept of probiotic-mediated detoxification has been proposed and supported by several findings from in vitro and in vivo animal studies( Reference El-Nezami, Kankaanpaa and Salminen 13 – Reference Nikbakht Nasrabadi, Jamaluddin and Abdul Mutalib 16 ) as one of the dietary approaches to prevent human exposure to aflatoxin. Although the mechanism by which probiotic bacteria bind to aflatoxin is unclear, it is suggested that the bacteria adsorb aflatoxins to the surface of the bacterial cell wall through a weak binding associated with hydrophobic interactions between the peptidoglycan layers( Reference Hernandez-Mendoza, Guzman-De-Pena and Gonzalez-Cordova 14 ) and consequently limit the intestinal uptake. Lactobacillus casei Shirota (LcS) is one of the potential probiotics capable of doing so. Findings from in vitro studies( Reference El-Nezami, Kankaanpaa and Salminen 13 , Reference Kabak and Ozbey 15 ) using this bacterium led us to conduct an experiment on a murine model, where LcS significantly reduced aflatoxin blood serum levels in aflatoxin-induced rats( Reference Nikbakht Nasrabadi, Jamaluddin and Abdul Mutalib 16 ). The outcomes of the animal study were valuable( Reference Nikbakht Nasrabadi, Jamaluddin and Abdul Mutalib 16 ), but the application and effectiveness of LcS in humans are not yet completely understood. Nevertheless, a couple of clinical studies showed that using a mixture of probiotics effectively reduced the biomarkers of aflatoxin( Reference El-Nezami, Mykkänen and Kankaanpää 17 , Reference El-Nezami, Polychronaki and Ma 18 ), and these findings show that probiotic bacteria have the potential to act as adsorbents of aflatoxins in humans.
In the present study, two biomarkers of aflatoxin B1 (AFB1) – namely, serum aflatoxin B1-lysine adduct (AFB1-lys) and urinary aflatoxin M1 (AFM1) – were investigated. These aflatoxin-specific biomarkers are used in many epidemiological studies( Reference Romero, Ferreira and Dias 19 – Reference Polychronaki, Wild and Mykkänen 22 ) and are reliable molecular biomarkers for the study of human exposure to aflatoxin( Reference Kensler, Roebuck and Wogan 11 ) as well as the surrogate efficacy biomarkers of aflatoxin for the assessment of different therapeutic/intervention agents and techniques in human intervention trials( Reference Wang, Afriyie-Gyawu and Tang 23 – Reference Tang, Tang and Xu 25 ). When humans are exposed to AFB1 through the diet( Reference Sabran, Jamaluddin and Abdul Mutalib 1 ), the toxin is absorbed via a passive diffusion( Reference Hsieh and Wong 26 ) in the intestinal tract, primarily in the duodenum. A study involving human volunteers found that AFB1 equivalents’ absorption into systemic circulation was rapid with peak concentrations achieved within approximately 1 h( Reference Jubert, Mata and Bench 27 ), in agreement with data found in rats, where AFB1 was absorbed quickly in the small intestine( Reference Coulombe and Sharma 28 ). Once absorbed, AFB1 is distributed via blood( Reference Wilson, Ziprin and Ragsdale 29 , Reference Kumagi 30 ). In rats, AFB1 is concentrated in the liver 30 min after an intraperitoneal dosage, but it can take up to 24 h with an oral dose( Reference Wogan 31 ). In the liver, AFB1 is metabolised by CYP450 enzymes, where the metabolites can be excreted or interact with other biological molecules such as DNA and proteins( Reference Wang, Shen and He 32 – Reference Partanen, El-Nezami and Leppänen 34 ). AFB1-lys adduct is formed when AFB1 is metabolised into a reactive epoxide (AFB1-8,9-epoxide), where it can further react with serum albumin (ALB) to form a long-lived lysine adduct( Reference Kensler, Roebuck and Wogan 11 ). Regarding AFM1 formation, AFB1 undergoes hydrolysis where the metabolite is excreted via urine( Reference Mykkänen, Zhu and Salminen 33 ). It is found that 1·2–2·2 % of ingested AFB1 is converted into AFM1 and excreted via urine in humans( Reference Zhu, Zhang and Hu 35 ), whereas the conversion rate varies between animals depending on their ability to metabolise AFB1 ( Reference Egmond 36 ).
Each biomarker has a characteristic half-life within the body( Reference Williams, Phillips and Jolly 37 ). The detection of urinary AFM1 reflects short-term exposure, probably 1–3 d( Reference Kensler, Roebuck and Wogan 11 ) and varies day to day depending on the amount of ingested aflatoxin-contaminated foods( Reference Williams, Phillips and Jolly 37 ). Conversely, AFB1-lys has a half-life in the body of 30–60 d( Reference Williams, Phillips and Jolly 37 ) and its detection integrates exposure over a longer period( Reference Kensler, Roebuck and Wogan 11 , Reference Williams, Phillips and Jolly 37 , Reference Mohd Redzwan, Rosita and Mohd Sokhini 38 ). Indeed, these two biomarkers serve as elegant tools to provide very useful information on the extent of human exposure to aflatoxin. Of great significance is a positive correlation between serum AFB1-lys and urinary AFB1-DNA adduct, a pro-mutagenic DNA lesion of aflatoxin exposure( Reference Kensler, Roebuck and Wogan 11 , Reference Cupid, Lightfoot and Russell 39 ), which is linked to the development of HCC( 3 , Reference Kensler, Roebuck and Wogan 11 ).
In the present study, we hypothesised that probiotic bacteria can provide a barrier that can limit aflatoxin absorption by binding to aflatoxin, where an aflatoxin–bacteria complex is formed and excreted eventually via the faeces. Therefore, the objective of this study was to determine whether the supplementation of fermented milk drink containing probiotic LcS could prevent aflatoxin absorption and subsequently reduce the circular production of serum AFB1-lys and urinary AFM1.
Methods
Study design
Ethics approval for this study was obtained from the Ethics Committee for Research Involving Human Subjects Universiti Putra Malaysia (UPM), Malaysia, and all the procedures were carried out in accordance with the Helsinki Declaration of 1975, as revised in 2008, and the Nuremberg Code 1946. The present study was randomised, double-blind, cross-over, placebo-controlled intervention with two phases. Each phase ran for 4 weeks with 2 weeks of wash-out period between the phases. The duration of intervention of 4 weeks/phase was chosen for this study as the concentration of AFB1-N 7-guanine adduct was found to reduce significantly as early as during the 3rd week of intervention with probiotics( Reference El-Nezami, Polychronaki and Ma 18 ). In fact, its concentration reduced by 55 % after 5 weeks of intervention( Reference El-Nezami, Polychronaki and Ma 18 ). Although a longer period of intervention is recommended, such trials can be costly and require high compliance from the subjects.
Sample size rationale
Data from an intervention study using a mixture of probiotics to reduce the concentrations of the AFB1-N 7-guanine adduct( Reference El-Nezami, Polychronaki and Ma 18 ) were referred to calculate the sample size. Following a 5-week intervention study, the concentrations of the AFB1-N 7-guanine adduct were reduced in the probiotic group, as the mean AFB1-N 7-guanine adduct was 0·19 ng/ml compared with the placebo group of 0·46 ng/ml. Based on this information, the mean difference between the groups (d) of 0·27 ng/ml was obtained. The pool standard deviation (sd pooled) at baseline was used as the best alternate standard deviation for the sample size calculation according to the formula by Rosnow & Rosenthal( Reference Rosnow and Rosenthal 40 ), and the calculated sd pooled was 1·11. The value was then used to estimate within-subject variance (sd w ) for a cross-over study according to the formula (sd w =sd pooled×(1−ρ)), where ρ is the Pearson’s correlation coefficient estimated between two measures on the same subject. Assuming that there is a relatively modest correlation of 0·5 (ρ=0·5), the sd w would be half of the sd pooled (1·11×(1−0·5)=0·555)( Reference Julios 41 ). Based on a formula for cross-over studies( Reference Julios 41 ) and using the values of d and sd w , with α of 0·05 and power of 80 %, the required sample size was sixty-six subjects. To allow for a drop-out rate of 10 %, a total of seventy-two subjects were needed from the pool of subjects who participated in the screening and had detectable levels of urinary AFM1.
Study population
Subjects were recruited from among the employees at a faculty in UPM. Before the start of the intervention, a screening process was conducted and 160 subjects were recruited, for which the data have been published elsewhere( Reference Mohd Redzwan, Rosita and Mohd Sokhini 20 ). The inclusion criteria for the intervention study were as follows: subjects with detectable levels of urinary AFM1 (>0·005 ng/ml), healthy with no chronic diseases, not pregnant or planning to get pregnant and voluntary participation. Moreover, subjects who were allergic to fermented milk containing probiotic bacteria, lactose intolerant and with gastric problems or liver and kidney injuries were excluded. As the selection of subjects was based on those exposed to aflatoxin through the detection of urinary AFM1, they did not represent the general aflatoxin exposure in Malaysia.
A total of ninety-eight from 160 subjects involved in the screening stage had detectable levels of AFM1, and seventy-six subjects had AFM1>0·005 ng/ml. The subjects’ medical history was evaluated and none of them had any chronic disease, allergy to fermented milk (contained probiotics) and lactose intolerance. Two subjects were excluded due to pregnancy and three subjects did not agree to participate in the intervention. Finally, only seventy-one subjects agreed to participate in the intervention. Signed informed consent was obtained before the start of the intervention. Subjects were randomly divided into two groups. As the total number of subjects was not even (i.e. seventy-one subjects), one group had thirty-six subjects and the other had thirty-five subjects. Subjects provided blood samples for the analysis of liver and kidney functions before the intervention began.
Study protocol
Before the start of the intervention, a controller (a third party who was not involved in the study) was appointed to hold the information on the types of treatment (probiotic or placebo drinks) that each group will receive during the intervention. Researchers and subjects were blinded and the controller determined the order of interventions ((1st (probiotic) – washout – 2nd (placebo)) or (1st (placebo) – washout – 2nd (probiotic))) using colour code – namely, Blue and Yellow. For the first 4 weeks of the intervention (1st phase), one group was supplied with fermented milk drinks containing LcS (probiotic drinks) and the other group with placebo drinks. After 2 weeks of wash-out period, the intervention was crossed over, where subjects who received placebo drinks before the wash-out period were given probiotic drinks and vice versa and the intervention continued for another 4 weeks (2nd phase).
To ensure compliance, both drinks were given to the subjects on a daily basis twice a day (after breakfast and lunch). However, for weekend consumption, four bottles (probiotic or placebo drinks) were given on every Friday afternoon. The drinks were given in an ice box to maintain the refrigerated temperature. To ensure that subjects consumed the drinks and complied with the study protocol, a few reminders were made by calling and/or texting the subjects during the weekend.
Throughout the intervention, subjects were asked to consume their normal diets. Samples (5 ml) of fasting blood and morning urine were collected at baseline and every 2 weeks (2nd, 4th, 6th, 8th and 10th week). Serum was separated from the blood by centrifugation at 3000 g for 10 min and maintained at −80°C. An aliquot of 15 ml urine was also maintained at −80°C.
Subjects’ food intake was examined using a 2-d food record. Subjects were taught by the researchers and enumerators to record their food intakes properly. They were asked to record details such as the type and amount of foods/drinks, brand, preparation methods and recipes in a booklet provided to them. Household measuring cups were also provided to the subjects to assist the recording process. The food record was collected when the subjects gave their blood and urine samples, and it was examined and checked by enumerators to make sure that they had recorded their food intake properly. At the end of each phase, subjects also received an FFQ comprised of several food items such as cereals, nuts, milk and dairy products and spices that are frequently reported in the literature to be contaminated with aflatoxin. Subjects were required to tick their frequency of intakes during the 4-week intervention in both phases based on seven scales – namely, ‘2–3 times/d’, ‘once a d’, ‘2–3 times/week’, ‘once a week’, ‘2–3 times/month’, ‘once a month’ or ‘not eating’ in order to study the frequency of food intakes between the two phases.
Probiotic and placebo drinks
Both probiotic and placebo drinks were prepared in a plastic bottle with a volume of 80 ml by Yakult (Malaysia) Sdn. Bhd. The probiotic and placebo drinks were identical in appearance, taste and colour. The ingredients were fructose, maltitol, skimmed milk powder, glucose and permitted flavouring. The manufacturer confirmed that the probiotic drinks contained at least 3·0×1010 colony-forming units (CFU) of LcS, whereas the placebo drinks did not contain any probiotic bacteria. Therefore, the only difference between these two drinks was the presence or absence of LcS.
Analysis of the 2-d food record
Nutrition analysis software, Nutritionist Pro™ Diet Analysis (Axxya Systems) with food data bank from the USA, was used to analyse the food records collected from the subjects. The reference data used were the Nutrient Composition of Malaysian Foods (4th edition)( Reference Tee, Mohd Ismail and Mohd Nasir 42 ), which was more representative of local foods compared with other sources abroad. The total energy intake was also calculated for carbohydrates, proteins and fat.
Analysis of the FFQ
Two sets of FFQ were given to the subjects in order to study the frequency of food intake between the two phases based on seven scales as mentioned earlier. A score of 6 for ‘2–3 times/d’ to 0 for ‘not eating’ was computed for each scale to estimate the frequency of food intake of the subjects during the intervention. The higher score computed from the FFQ indicated that the foods were consumed more frequently and vice versa.
Analysis of serum aflatoxin B1-lysine adduct
The concentrations of serum AFB1-lys were measured as described by Mohd Redzwan et al.( Reference Mohd Redzwan, Rosita and Mohd Sokhini 38 ). In brief, serum was digested with PRONASE® Protease (Calbiochem) for 3 h at 37°C, followed by purifications steps using Oasis® MAX Cartridge (Waters). The digested sample was allowed to pass the cartridge by gravity, followed by sequential washing steps with HPLC grade water (J.T. Baker), 70 % HPLC grade methanol (Honeywell Burdick & Jackson) in water, 1 % ammonium hydroxide (EMD Chemicals Inc.) in methanol and 100 % HPLC grade methanol. AFB1-lys was eluted with 2 % formic acid (Fluka) in methanol, evaporated to dryness and reconstituted with 25 % HPLC grade methanol in water before HPLC analysis. A reversed-phase HPLC analysis was carried out using a 1200 series of liquid chromatography system with a quaternary pump (Agilent Technologies). The chromatographic separation was performed using a ZORBAX® Eclipse XDB-C18 column, 250×4·6 mm, 5 μm (Agilent Technologies), connected to a guard column (Security GuardTM). The mobile phases were 20 mm-ammonium phosphate monobasic, pH 7·2, and HPLC grade methanol, and the separation was carried out in a linear gradient profile. A sample of 100 μl was injected to the HPLC system with a flow rate of 1 ml/min and the peak of AFB1-lys was detected in a fluorescence detector (FLD) with wavelength of 405 nm (excitation) and 470 nm (emission). To identify the peak of AFB1-lys adduct in the samples, blank human serum spiked with AFB1-lys standard (courtesy of Prof Wang’s Laboratory) was used as the control. The limit of detection (LOD) was 0·05 ng/ml AFB1-lys. The peak of AFB1-lys was detected at a retention time of 13·1 min. Serum AFB1-lys was expressed as pg/mg ALB.
Analysis of urinary aflatoxin M1
The extraction procedures and analysis of urinary AFM1 were based on the methods of Mohd Redzwan et al.( Reference Mohd Redzwan, Jamaluddin and Mohd Sokhini 43 ). In brief, 5 ml of urine was adjusted to an acidic pH with 0·5 ml of 1 m-ammonium formate (pH 4·5) (Sigma-Aldrich) and the volume was increased to 10 ml with ultrapure water obtained from Milli-Q system (Thermo Scientific Barnstead) and vortexed. Subsequently, the sample was passed by gravity through the Immunoaffinity Column (IAC), EASI-EXTRACT® AFLATOXIN (R-Biopharm Rhône Ltd) followed by washing twice with 10 ml PBS (pH 7·4). Air pressure was applied to remove any residual PBS, and AFM1 was eluted from the IAC at a flow rate of 1 drop/s with 2 ml HPLC grade methanol (Merck) and collected in an amber glass vial (Fisher Scientific). The eluate was evaporated to dryness with purified N gas and reconstituted with 500 μl mobile phase. A reversed-phase ultra HPLC (UHPLC) analysis was carried out using an Agilent 1290 Infinity liquid chromatography system with a binary pump and an auto sampler coupled with an FLD (Agilent Technologies). The column used was the ZORBAX RRHD Eclipse Plus C18 column, 150×2·1 mm, 1·8 μm, connected to a UHPLC fast guard column, Eclipse Plus C18, 2·1×5·5 mm (Agilent Technologies). The mobile phase was water–acetonitrile–methanol (6·5:2·5:1, v/v/v %) and was of HPLC grade (Merck). The chromatographic separation was performed isocratically at a flow rate of 0·2 ml/min, with 20 μl volume of injection, and the column’s temperature was maintained at 40°C. The wavelength of FLD was fixed at 365 and 435 nm for excitation and emission, respectively. For quality control purpose, AFM1 standard (Trilogy Analytical Laboratory Inc.) and/or spiked AFM1 urine were injected for every six injections (i.e. six samples) during the analysis in order to assure the correct identification of AFM1 peak in the samples. The LOD was 0·018 ng/ml AFM1. AFM1 was eluted at 5·6 min, and urinary AFM1 was expressed as pg/µmol creatinine.
Statistical analyses
Data were analysed using SPSS version 17.0 software (SPSS Inc.). Data from both the groups (Blue and Yellow) were combined and analysed. As the study design was a cross-over study and each subject consumed probiotic and placebo drinks throughout the duration of the intervention, data were also analysed individually to see whether the order of probiotic intervention had an impact on the outcomes. Reduction of serum AFB1-lys and urinary AFM1 was the primary outcome of this study. In addition, the difference in the concentrations of serum AFB1-lys and urinary AFM1 between the treatments was the secondary outcome investigated here.
Before further analyses were conducted, data were checked for normality. For data that were normally distributed – that is, energy intake, macronutrients intake, dietary fibre intake and serum AFB1-lys – parametric analysis was performed. Paired sample t test was used to compare all the above variables between each time point. Besides, independent t test was used to compare the food intake between subjects in the Blue and Yellow groups when they were given probiotic drinks. As for the FFQ administered during the intervention, paired sample t test was used to compare the frequency of intakes within the groups between the two treatments, whereas independent t test was used to compare the frequency of intakes between the groups for each treatment. In order to study the effect of treatment over 4 weeks of intervention on the concentration of serum AFB1-lys, ANOVA for repeated measurements was applied.
Conversely, data of urinary AFM1 were not normally distributed, and therefore non-parametric analysis was used. The Wilcoxon signed-rank test was used to compare the concentrations of urinary AFM1 between each time point. To investigate the effect of treatments over 4 weeks of intervention on the concentration of urinary AFM1, the Friedman’s test was used. On the other hand, correlation analyses were carried out between aflatoxin biomarkers and foods that are possible sources of aflatoxins and also between subjects’ food consumption. The level of significance was assigned at P value<0·05. After all the analyses were completed, the orders of intervention ((1st (probiotic) – washout – 2nd (placebo)) or (1st (placebo) – washout – 2nd (probiotic))) were revealed by the controller to the researchers.
Results
Baseline characteristics of the subjects
The recruitment of subjects through the screening stage began in January 2012 and was completed in March 2012, and some of the data have been published elsewhere( Reference Mohd Redzwan, Rosita and Mohd Sokhini 7 ). Of the seventy-two subjects required, only seventy-one were successfully recruited, and 50·7 % of them were males (n 36) and the rest were females (n 35). The mean age of the subjects was 34·34 (sd 9·70) years, ranging from 23 to 57 years, and was not significantly different between males (34·08 (sd 10·35) years) and females (34·60 (sd 9·12) years). Overall, subjects’ BMI was 25·26 (sd 5·50) kg/m2. Females (25·58 (sd 5·50) kg/m2) had slightly higher BMI than males (24·95 (sd 5·58) kg/m2) but the difference was not significant. In all, thirty-three subjects were overweight and obese and had BMI>25 kg/m2. Analysis of aspartate aminotransferase, alanine transaminase, alkaline phosphatase, γ-glutamyl transpeptidase, total bilirubin, blood urea nitrogen and creatinine indicated that subjects had normal liver and kidney functions before the start of the intervention.
The subjects’ socio-demographic characteristics, urinary AFM1 concentration and the intake of food groups that are possible sources of aflatoxin measured during the screening stage are shown in Table 1. There were no significant differences in socio-demographic characteristics between the two groups. No differences in the intakes of food groups known to be the most common sources of dietary aflatoxin exposure were also observed between the groups. Based on findings obtained during the screening stage, all the subjects had similar intake of foods and were assumed to have homogeneous dietary exposure to aflatoxin before the start of the intervention.
Table 1 Subjects’ socio-demographic and data on food intake and urinary aflatoxin M1 (AFM1) obtained from the screening stage (Mean values and standard deviations)
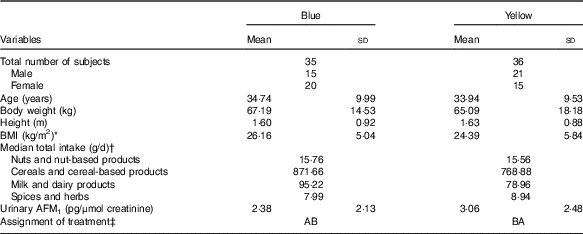
* BMI is calculated based on the formula (body weight (kg)/height2 (m2)).
† Subjects’ food intakes were obtained from the FFQ administered during the screening stage.
‡ AB=(1st (probiotic) – washout – 2nd (placebo)); BA=(1st (placebo) – washout – 2nd (probiotic)).
Adherence to the study protocol
All the subjects who participated in this study completed both phases of the intervention. Although some of them had reported taking antibiotics during the intervention (n 8), they were still provided with either probiotic or placebo drinks during the intervention period with the intention to treat. A flow diagram reflecting the subjects’ passage during the intervention is illustrated in Fig. 1. Five subjects were taking antibiotics when they were being provided the probiotic drinks. Finally, considering the effect of antibiotics on the activity of probiotic bacteria, data from only sixty-six subjects (71−5; thirty-four from the Blue group and thirty-two from the Yellow group) were used for further statistical analyses.

Fig. 1 Flow chart of subjects’ progression in the intervention study. AFM1, aflatoxin M1.
Frequency of intakes of foods that are possible sources of aflatoxin
Table 2 shows the intake frequency of fifteen food items that are possible sources of aflatoxin obtained through an FFQ given to the subjects at the end of each phase. The outputs measured from the FFQ were the frequency of intake and not the amount of foods, which was different from the FFQ survey conducted during the screening stage. Overall, subjects had significantly (P<0·05) higher frequency of breads and spices and herbs intakes when they were supplemented with probiotic drinks than during the placebo intervention period. As for the within-group analysis, no significant differences on the frequency of intakes were observed in the Blue group. On the other hand, subjects in the Yellow group had significantly (P<0·05) higher frequency of breads and liquid milk intakes during the 4 weeks of intervention with the probiotic drinks than with the placebo drinks. Nevertheless, the frequency of intakes of these foods was not significantly different between the two groups when the subjects were supplemented with the probiotic and placebo drinks, respectively.
Table 2 Food frequency intakes of foods that are possible sources of aflatoxin during the intervention (Mean values and standard deviations)

UHT, ultra high temperature pasteurized.
* P<0·05.
† Food frequency scores range from 0 (not eating at all) to 6 (eat 2–3 times/d).
‡ No significant differences in food frequency between the groups, respectively, during the probiotic and placebo consumption period.
§ P values obtained from paired samples t test.
Subjects’ food consumption
There were significant differences in energy and macronutrient intakes during the consumption of probiotic and placebo drinks, respectively (Table 3). While consuming the placebo drinks, the only difference observed was the intake of protein as subjects consumed significantly high-protein diets during the 4th week of intervention compared with the baseline. Conversely, energy and macronutrient intakes were found to be significantly higher during the 4th week of probiotic intervention compared with the baseline. To support these findings, within-group analysis was performed and the results are presented in Table 4.
Table 3 Energy, macronutrient and dietary fibre intake of all subjects (Mean values and standard deviations; n 66)

Mean value was significantly different from that at baseline: * P<0·05, ** P<0·01 (paired samples t test).
Table 4 Energy, macronutrient and dietary fibre intakes of subjects from the Blue and Yellow groups (Mean values and standard deviations)

* Significantly different (P<0·05) when compared with the baseline based on paired samples t test.
** Significantly different (P<0·05) when compared with the 6th week (i.e. baseline value for 2nd phase) based on paired samples t test.
In the Blue group, the intakes of energy, macronutrients and dietary fibre were not significantly different throughout the 4-week intervention period, either with probiotic or placebo drinks. However, the 4th week carbohydrate intake was significantly higher compared with the baseline intake when the subjects consumed probiotic drinks. In contrast, subjects in the Yellow group had significantly higher intakes of protein during the 4th week of intervention with placebo compared with the baseline intakes. More prominent differences were observed during the 2nd phase – that is, during the consumption of probiotic drinks. The energy intake was significantly higher at the last week of intervention, which saw an increase of 16·4 % energy intake from 7937 kJ (1897 kcal) to 9247 kJ (2210 kcal). Regarding macronutrient intakes, the intakes of carbohydrates and fat were significantly higher compared with the 6th week intakes (i.e. baseline intake for 2nd phase). In addition, the subjects had significantly higher dietary fibre intakes compared with the baseline intakes (Table 4).
We carried out further analysis to compare the food intakes of subjects in the Yellow and Blue groups during the probiotic consumption period. We found that subjects in the Yellow groups had significantly higher intake of macronutrients compared with subjects in the Blue group (Table 5).
Table 5 Comparison of energy, macronutrients and dietary fibre intakes during the probiotic consumption period (Mean values and standard deviations)

* Consumed probiotic drinks before the wash-out period (1st phase).
† Consumed probiotic drinks after the wash-out period (2nd phase).
‡ P values are obtained from the independent t test indicating significant difference.
Modulation of serum aflatoxin B1-lysine adduct
Overall, we collected 426 serum samples (seventy-one subjects×6 times of collection) and all of them had detectable concentrations of AFB1-lys. We found no significant difference in the concentrations of AFB1-lys during the 4 weeks of intervention with probiotic and placebo drinks. However, further analysis conducted within the groups showed some promising findings. As for subjects in the Blue group, the consumption of placebo did not change the concentrations of AFB1-lys. Nevertheless, when the subjects were given probiotic drinks, the outputs of ANOVA for repeated measurements indicated that the concentrations of AFB1-lys were significantly different (F 2,66=4·283; P=0·035, partial η 2=0·115) during the 4 weeks of intervention. The pair-wise comparison further showed that the concentrations were significantly lower after 2 weeks of intervention (P=0·048), with a percentage reduction of 17·63 %. Although not significant (P=0·332), the concentrations of AFB1-lys at the end of the intervention (4th week) was lower compared with the baseline. In fact, the 4th week concentrations of the two treatments were significantly different (P=0·005) (6·35 (sd 2·41) pg/mg ALB, placebo v. 5·48 (sd 2·25) pg/mg ALB, probiotic). The same analyses were performed for data obtained from subjects in the Yellow group as well, and we found no significant differences in the concentrations of AFB1-lys for both treatments over the 4 weeks of intervention (Table 6).
Table 6 Concentration of serum aflatoxin B1-lysine adduct (AFB1-lys) adduct at different time points for both treatments (Mean values, standard deviations and ranges)

a,b Means values with unlike superscript letters were significantly different compared with the baseline for each treatment arm (P<0·05).
* Significantly different when compared with the placebo at the same time point (P<0·05).
† P values are obtained from ANOVA for repeated measurement.
Modulation of urinary aflatoxin M1
A total of 426 urine samples were collected, but only 153 samples were positive for AFM1. Due to the high rate of non-detectable values of AFM1 in the urine samples, the concentration of negative samples was expressed as half of LOD( Reference El-Nezami, Polychronaki and Ma 18 ) (i.e. 1/2×0·018 ng/ml=0·009 ng/ml). Overall, the intervention did not change the concentrations of urinary AFM1. In addition, within-group analysis also indicated no significant differences in the median urinary AFM1 concentration among subjects in the Blue group across the three different time periods (i.e. baseline, 2nd and 4th week) when the subjects consumed the probiotic (χ 2=1·647, df=2; P=0·439) or placebo drinks (χ 2=2·529, df=2; P=0·282). Although not significant, a decreasing trend in median urinary AFM1 concentrations was observed between the 2nd and 4th week with probiotic intervention. A similar observation was found in the Yellow group when the subjects were given probiotic (χ 2=4·759, df=2; P=0·093) and placebo drinks (χ 2=1·688, df=2; P=0·430) (Table 7).
Table 7 Concentrations of urinary aflatoxin M1 (AFM1) at different time points for both treatments (Medians, mean values and standard deviations)

a Mean values with unlike superscript letters were significantly different compared with the baseline for each treatment arm (P<0·05).
* P values are obtained from the Friedman’s test.
Association between aflatoxin biomarkers and frequency of intakes of foods that are possible sources of aflatoxin
As shown in Table 8, several significant associations were found between aflatoxin biomarkers and the frequency of intakes of foods that are possible sources of aflatoxin. Significant and negative associations were found between the 2nd week AFB1-lys and the intake frequency of rice and glutinous rice (r −0·263), all types of nuts (r −0·266), chocolate and malt drinks (r −0·258) and liquid milk (r −0·291), whereas the 2nd week urinary AFM1 concentration was significantly correlated with the frequency of nut-based foods intake (r 0·258) during the probiotic consumption period. Conversely, the intake frequency of nuts-based foods and cheese, respectively, were the only foods items that significantly correlated with the baseline (r 0·271) and 4th week (r −0·242) urinary AFM1 concentrations during the placebo consumption period.
Table 8 Association between aflatoxin biomarkers and frequency of foods that are possible sources of aflatoxin during the intervention

AFB1-lys, aflatoxin B1-lysine adduct; UHT, ultra high temperature pasteurized; AFM1, aflatoxin M1.
* P<0·05, ** P<0·001.
† Pearson’s correlation coefficient.
‡ Spearman’s correlation coefficient.
Within-group analysis also showed some significant associations between the two studied variables. In the Blue group, the baseline AFB1-lys was significantly correlated with the intake frequency of all types of nuts (r −0·341), whereas the 2nd week and 4th week urinary AFM1 concentrations, respectively, were significantly correlated with the intake frequency of two food items (2nd week: rice and glutinous rice, r 0·342; powdered milk, r 0·340 and 4th week: peanut, r 0·341; nuts-based foods, r 0·396) during the probiotic intervention period. Meanwhile, the intake frequency of noodles and pasta and chocolate and malt drinks during the placebo intervention period were found to be significantly correlated with the baseline (r 0·384) and 4th week (r 0·310) urinary AFM1 concentrations, respectively. As for the Yellow group, we found that the 2nd week AFB1-lys level was significantly and inversely correlated with the intake frequency of chocolate and malt drinks (r −0·365) and cheese (r −0·506) when the subjects were supplemented with the probiotic drinks. In addition, the frequency of liquid milk consumption was also significantly correlated with the 4th week urinary AFM1 concentration (r −0·465) during the 4 weeks of probiotic intervention. Nonetheless, we did not find any significant correlations between the two studied variables during the placebo consumption period.
Association between aflatoxin biomarkers and food consumption
No significant associations between aflatoxin biomarkers and food consumption were found in the Blue group (data are not shown here). As for the Yellow group, there was no correlation between baseline and 2nd week aflatoxin biomarkers and food consumption, and thus the data are not presented here. Nevertheless, some positive and significant correlations (P<0·05) were observed between the 4th week aflatoxin biomarkers and the consumption of macronutrients and dietary fibre. Indeed, the correlation coefficients showed a medium effect size (correlation coefficient>0·3) between the two variables (Table 9).
Table 9 Correlations between the 4th week aflatoxin biomarkers and macronutrients and dietary fibre intakes among subjects in the Yellow group during the probiotic consumption periodFootnote †
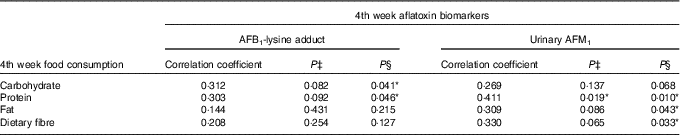
AFB1-lys, aflatoxin B1-lysine adduct; AFM1, aflatoxin M1.
* P<0·05.
† Pearson’s correlation analysis was conducted for correlation between AFB1-lys adduct and macronutrients and dietary fibre intakes, whereas Spearman’s correlation analysis was performed for urinary AFM1 and macronutrients and dietary fibre intakes.
‡ P value at two-tailed test.
§ P value at one-tailed test. The test was conducted due to the non-normal distribution of urinary AFM1.
Discussion
Aflatoxin exposure in Malaysia is considered to be higher compared with other countries in South East Asia( Reference Liu and Wu 12 , Reference Mohd-Redzwan, Jamaluddin and Abd-Mutalib 44 ). To the best of our knowledge, this is the first study conducted in humans investigating the effectiveness of probiotic LcS in reducing the concentrations of serum AFB1-lys and urinary AFM1. The application of probiotics as potential decontaminating agents of aflatoxin is highly promising and may be of immense value in reducing the exposure of this food-borne contaminant in humans. This is evident as a 5-week intervention study with a mixture of probiotics reduced significantly urinary AFB1-N 7-guanine adduct concentration by 55 %( Reference El-Nezami, Polychronaki and Ma 18 ). This finding was the basis for deciding the present study’s duration of 4 weeks. Overall, the intervention did not change both aflatoxin biomarkers’ concentrations but the concentrations were reduced in certain subjects who participated in this study. In particular, a significant reduction in the concentrations of serum AFB1-lys was detected and a decreasing trend in the median urinary AFM1 concentration was observed with probiotic drink supplementation among the subjects in the Blue group, but not in the Yellow group. Through these findings, it can be said that the order of probiotic intervention may influence the outcomes obtained here as the efficiency of LcS as an aflatoxin adsorbent was observed in the 1st phase rather than in the 2nd phase of intervention.
This study is one among the few( Reference El-Nezami, Mykkänen and Kankaanpää 17 , Reference El-Nezami, Polychronaki and Ma 18 ) that investigated the effect of probiotic bacteria as potential adsorbents of aflatoxin in humans. Furthermore, to make a comparison, for example, the data obtained by El-Nezami et al. ( Reference El-Nezami, Polychronaki and Ma 18 ) can explain a few dissimilarities. First, El-Nezami et al. ( Reference El-Nezami, Polychronaki and Ma 18 ) used a mixture of probiotics, whereas the present study provided subjects with fermented milk drinks containing LcS. Both studies provided almost similar concentrations of bacteria. Hernandez-Mendoza et al.( Reference Hernandez-Mendoza, Garcia and Steele 45 ) indicated that Lactobacillus strains had different ability to bind AFB1 in vitro. Nevertheless, the effectiveness of LcS treatment was observed in reducing the blood serum concentrations of AFB1 in aflatoxin-induced rats( Reference Lewis, Onsongo and Njapau 10 , Reference Nikbakht Nasrabadi, Jamaluddin and Abdul Mutalib 16 ). As such, differences in the strains of probiotics used could be the possible reason for the observation found here compared with the findings presented by El-Nezami et al. ( Reference El-Nezami, Polychronaki and Ma 18 ).
Second, the biomarker of aflatoxin investigated was different, as El-Nezami et al. ( Reference El-Nezami, Polychronaki and Ma 18 ) measured AFB1-N 7-guanine adduct concentrations, which is a DNA adduct of aflatoxin. The adduct is a short-term biomarker as it is unstable and undergoes rapid de-purination and excretion via urine( Reference Egner, Groopman and Wang 46 ). Indeed, in a pharmacokinetics study of AFB1 involving human volunteers, the excretion of AFB1 metabolites via urine occurred rapidly with >94 % of total urine AFB1 equivalents produced within the first 24 h( Reference Jubert, Mata and Bench 27 ). However, the concentrations of the AFB1-N 7-guanine adduct measured reflected the rate of AFB1-8,9-epoxide formation as well as the competing pathways of AFB1-8,9-epoxide reactions with DNA v. glutathione, other macromolecular targets and water( Reference Kensler, Roebuck and Wogan 11 ). As the AFB1-N 7-guanine adduct and aflatoxin biomarkers, especially urinary AFM1 concentrations, measured in this study have different metabolic pathways, it can be assumed that differences in the biological activities may contribute to the outcomes found in our study.
Although El-Nezami et al.( Reference El-Nezami, Polychronaki and Ma 18 ) found that AFB1-N 7-guanine adduct was significantly reduced within 3–5 weeks of intervention, it can be postulated that a 4-week intervention study is too short to observe the effects of LcS intervention on the concentrations of serum AFB1-lys and urinary AFM1. It is evident as shown by Wang et al. ( Reference Wang, Afriyie-Gyawu and Tang 23 ) that a significant reduction of these two aflatoxin biomarkers was only observed after 12 weeks of intervention with NovaSil clay (Engelhard Chemical Corporation). Moreover, a 4-week intervention with NovaSil clay did not change the concentrations of AFB1-ALB adduct and urinary AFM1 concentrations( Reference Wang, Afriyie-Gyawu and Tang 23 ), which are the same aflatoxin biomarkers investigated in the present study. Considering that probiotic LcS used in this study works in a similar manner as NovaSil clay( Reference Wang, Afriyie-Gyawu and Tang 23 ) by binding the aflatoxin molecules, it can be assumed that the 4-week intervention duration may not reveal any significant reduction of aflatoxin biomarkers, preferably serum AFB1-lys and urinary AFM1, but a long intervention period may do so. Indeed, a 25-d intervention with yogurt containing Lactobacillus rhamnosus GR-1 did not find any significant reduction of blood metal levels as compared with the long-term consumption of probiotics( Reference Bisanz, Enos and Mwanga 47 ). The authors also suggested that probiotic consumption does not have a fast-acting effect, but rather acts over the longer term( Reference Bisanz, Enos and Mwanga 47 ).
LcS from the fermented milk has the capacity to survive in the gastrointestinal tract as up to 51·2 % ingested bacteria can be found in the ileum( Reference Oozeer, Leplingard and Mater 48 ). Ileum is the last section of the small intestine and the absorption of aflatoxin mostly occurs in the small intestine, where the binding of aflatoxin by bacteria occurs predominantly in the duodenum( Reference Hernandez-Mendoza, Rivas-Jimenez and Garcia 49 ). Assuming that the survival rate of LcS is equivalent to 51·2 %( Reference Oozeer, Leplingard and Mater 48 ) and the initial concentration of bacteria is 3×1010 CFU/bottle, consumed by the subjects during the intervention, it can be calculated that approximately 1·46×1010 CFU/LcS is still available to prevent the absorption of aflatoxin. The number of bacteria is still sufficient to decrease aflatoxin level as a minimum of 2–5×109 CFU/ml of bacteria is required to remove AFB1 significantly in vitro ( Reference Hernandez-Mendoza, Garcia and Steele 45 ).
Our intervention did not significantly reduce the concentration of aflatoxin biomarkers; however, of the two groups analysed, subjects in the Yellow group did not show any effect towards the probiotic intervention compared with the Blue group. The main route of aflatoxin exposure is through the diets( Reference Sabran, Jamaluddin and Abdul Mutalib 1 , Reference Mohd-Redzwan, Jamaluddin and Abd-Mutalib 44 ) and it can be assumed that subjects in the Yellow group were exposed to aflatoxin when they were provided with the probiotic drinks as they had significantly higher frequency of intake of two food items (breads and liquid milk) that are possible sources of aflatoxin (Table 2). Moreover, the intake frequency of some of these foods were also found to be significantly and conversely associated with the concentrations of aflatoxin biomarkers (Table 8). In other words, high intake of foods that are possible sources of aflatoxin corresponds to low concentration of aflatoxin biomarkers during the probiotic intervention period among subjects in the Yellow group. Based on these findings, the application of probiotic LcS theoretically could prevent aflatoxin absorption from the aflatoxin-contaminated foods consumed by the subjects and consequently reduce circular production of serum AFB1-lys and urinary AFM1. However, it was not observed, especially, in the Yellow group with the probiotic intervention. Due to this, we can presume that there may be other factors that can affect the number and/or efficiency of LcS present in the small intestine as aflatoxins are not adsorbed by the bacteria, where the toxin is available for intestinal absorption.
A possible explanation could be due to the dietary intakes of subjects during the intervention. In this study, positive associations were found between urinary AFM1 and fat and protein intakes among subjects in the Yellow group (Table 9) and the intakes of these macronutrients were significantly higher compared with the baseline intakes while they were consuming probiotic drinks (Tables 4 and 5). Nyathi et al. ( Reference Nyathi, Dube and Hasler 50 ) and Hasler et al. ( Reference Hasler, Dube and Nyathi 51 ) showed that high intakes of fat enhanced the activity of detoxification pathways of AFB1 in animal models. A high-fat diet also increased cytochrome 1A1 and 2B1 activities and reduced the amount of AFB1 available for hepatic macromolecular binding( Reference Hasler, Dube and Nyathi 51 ). With regard to protein intake, a high-protein diet can stimulate hepatic β-oxidation process( Reference Bortolotti, Kreis and Debard 52 ), which involves CYP2E1 and CYP4A enzymes, sources of pro-oxidants in liver cell lines( Reference Robertson, Leclercq and Farrell 53 ). Hepatocyte-derived cell lines with high levels of CYP2E1 enzyme produced high levels of GSH( Reference Robertson, Leclercq and Farrell 53 ) and GSH is important during the detoxification process of aflatoxin( Reference Wang, Shen and He 32 ). Moreover, high intakes of these macronutrients can also affect the secretion of bile acids, as a high-fat diet increases the levels of luminal bile juice in rats( Reference Suzuki and Hara 54 ). Bortolotti et al. ( Reference Bortolotti, Kreis and Debard 52 ) found that the total concentration of bile acid increased about 50 % after consumption of high-fat diets. In fact, deoxycholic acid, chenodeoxycholic acid and cholic acid increased significantly in healthy men following a hyperenergetic high-fat, high-protein diet( Reference Bortolotti, Kreis and Debard 52 ). As the survivability of probiotic bacteria in the gastrointestinal tract can be influenced by the concentration of bile juice( Reference Sahadeva, Leong and Chua 55 ), the ability of LcS to adsorb aflatoxin is affected and becomes limited. As such, there will be more absorption of aflatoxin in the small intestine. Subsequently, the detoxification of AFB1 into AFM1 is favoured due to the high intakes of fat and protein among the subjects. As a result, a high concentration of AFM1 was observed in the Yellow group during the 4 weeks of intervention with the probiotic drinks.
Another scenario that can be explained is the correlation between dietary fibre and urinary AFM1 concentrations (Table 9). Indeed, the intake was significantly higher at the 4th week of intervention, about 28 % increment compared with the baseline intake during the period of probiotic consumption among subjects in the Yellow group (Table 4). The indirect effect of dietary fibre could be related to the secretion of mucin, the main component of intestinal mucus( Reference Montagne, Peil and Lallès 56 , Reference Johansson, Ambort and Pelaseyed 57 ). For example, Gratz et al. ( Reference Gratz, Mykkänen and Ouwehand 58 ) showed the ability of intestinal mucus to alter the binding of aflatoxin by bacteria in vitro. Mucus pre-incubation significantly reduced AFB1 binding by 23·8 % and 61·2 %, respectively, for L. rhamnosus GG and mixture of L. rhamnosus LC-705 and Propionibacterium freudenreichii ssp. shermanii JS( Reference Gratz, Mykkänen and Ouwehand 58 ). In fact, in vivo animal studies showed the increment of mucin content after the animals were fed a high-dietary fibre diet( Reference Fuller and Cadenhead 59 – Reference Morita, Tanabe and Ito 63 ). To relate the above explanation with ours, the supposed binding of aflatoxin by LcS might be affected due to the excretion of mucus as a result of high-dietary fibre intake, and thus diminished the ability of LcS to bind aflatoxin. Due to this, there will be more absorption of aflatoxin in the small intestine. As explained previously, aflatoxins are susceptible to the detoxification pathway where AFM1 is produced predominantly. As a result, it was reflected on the concentrations of aflatoxin biomarkers measured, especially urinary AFM1, among subjects in the Yellow group (Table 7).
As explained above, with dietary influence on the detoxification of aflatoxin (the excretion of AFM1 in urine) and the ability of LcS to bind aflatoxin, the concentrations of serum AFB1-lys can be assumed to decrease over the 4 weeks of intervention in the Yellow group. However, the concentration was unchanged and did not show any decreasing trend throughout the 4 weeks of intervention with LcS (Table 6). AFB1-lys is formed through the binding of AFB1-epoxide with protein ALB during the Phase II metabolism( Reference Kensler, Roebuck and Wogan 11 , Reference Wogan 31 ). It is possible that the concentration of AFB1-lys measured during the 4 weeks of intervention reflects aflatoxin exposure during the 2 weeks of wash-out period, as ALB has half-life of about 20 d( Reference Christopher, Jiang and Sabbioni 64 ). Other studies have also indicated that the half-life of AFB1-lys could be between 30 and 60 d( Reference Williams, Phillips and Jolly 37 ). Therefore, the concentration of AFB1-lys measured during the 4-week intervention could be due to the ‘carry over’ effect because of a short wash-out period between the phases. It is likely that the subjects were exposed to aflatoxin during the wash-out period, and therefore the concentration of AFB1-lys measured in the 2nd phase among subjects in the Yellow group may reflect exposure during that time.
Limitations and conclusion
This study found that diets may play a major role on two major aspects – namely, the survival of probiotic LcS in the gastrointestinal tract and the metabolism of aflatoxin. It is very challenging in any intervention study to control the diet of subjects, especially when the duration of the intervention is long. The vastly different diet of the subjects, consumed during the 1st and 2nd phase of intervention, may provide an explanation for the observation found in the present study. As humans are exposed to aflatoxins mainly through the diets, it is also very difficult to have a pool of subjects with homogeneous exposure of aflatoxin. Moreover, the short wash-out period could be one of the limitations of the present study. Practically, the duration of the wash-out phase should be based on the half-life of aflatoxin biomarkers measured in the study.
It can be concluded that a 4-week intervention with fermented milk drink containing LcS did not change the concentrations of aflatoxin biomarkers. Nevertheless, the intervention had some effects on subjects who participated in this study. The potential of LcS as an adsorbent of aflatoxin was observed among subjects in the Blue group. Within 2 weeks of intervention, the concentrations of serum AFB1-lys reduced significantly, with a percentage reduction of 17·63 %. In fact, the 4th week concentration of serum AFB1-lys was significantly different between the treatments. Although not significant, a decreasing trend of urinary AFM1 concentration was observed within the last 2 weeks of the intervention. These observations, therefore, suggest that LcS can be used as one of the dietary approaches to prevent human exposure to aflatoxin. Therefore, a longer intervention period is recommended to investigate the effect of continuous consumption of fermented milk drink containing LcS in reducing the concentration of aflatoxin biomarkers. In addition, faecal analysis should also be performed. Besides, the effect of dietary macro and microcomponents on the binding ability of aflatoxin by LcS warrants an in-depth research using in vitro and in vivo models.
Acknowledgements
This research is a part of S. M. R.’s PhD Thesis submitted to UPM in fulfilment of the requirement for the degree of Doctor of Philosophy. S. M. R. thanks the Ministry of Education, Malaysia, for the scholarship received under MyBrain15 programme (MyPhD) and UPM for the financial aid for research attachment at the University of Georgia, USA. The authors thank the subjects in the study for their contributions.
This research was financially supported by Yakult Honsha Co. Ltd and managed by UPM Holdings Sdn. Bhd. (project no. 278).
S. M. R. contributed to the study design, analysis, data interpretation and writing of the manuscript. M. S. A. M., Z. A. and R. J. contributed to the experimental design, data analysis and interpretation and provided constructive comments and advices in preparing the manuscript. J.-S. W. and M.-S. K. aided in the analysis of AFB1-lys at the University of Georgia, USA. N. 'A. A. R. analysed the 2-d food record. E. N. N. helped in preparing and reviewing the manuscript.
None of the authors has any conflicts of interest.













