Nutritional management during the pre-weaning period can have short-term effects on health, mortality rate, growth efficiency and farm economics, as well as long-term effects on milk production(Reference Godden1). Therefore, it has been indicated that improving the pre-weaning growth rate through providing more milk or encouraging a greater intake of starter concentrate can have long-term effects on the productivity of farm replacement heifers(Reference Bhatti, Ali and Nawaz2). Conversely, implementation of the intensified or accelerated rearing programmes for dairy calves can increase rearing costs due to the high milk consumption that may negatively affect overall farm profitability(Reference Drackley3). However, the calves have been encouraged to feed more solid feed rather than liquid feed to stimulate gastrointestinal development and reduce the potential for diarrhoea and other diseases(Reference Khan, Bach and Weary4).
Generally, carbohydrates prepare the primary source of energy in starter diets for young calves. Although some research indicated negative effects of supplemental fat on dairy calf growth performance, this was mainly due to the detrimental effects of fat on nutrient digestibility, ruminal fermentation and microbial activity(Reference Ghorbani, Kazemi-Bonchenari and Hossein Yazdi5,Reference Yousefinejad, Fatahnia and Kazemi-Bonchenari6) ; however, positive results have been obtained when calves received different supplemental fat, such as essential fatty acid (FA)(Reference Hill, Bateman and Aldrich7) or n-3 FA, supplied through linseed oil (LSO) in starter diets(Reference Kazemi-Bonchenari, Dehghan-Banadaky and Fattahnia8). Discrepancies among studies that have evaluated fat incorporation in calves’ diets can be related to many factors, such as fat feeding levels, fat delivery methods through a liquid or solid feed, starter processing methods or individual FA profiles of the supplemented fat(Reference Bhatti, Ali and Nawaz2). Regardless of all the aforementioned items, the availability rate of supplemented FA to rumen microbes has a pivotal role in the responses of ruminants to supplemented fat. The high ruminal availability of supplemental FA for rumen microbes can reduce microbial activity, microbial protein synthesis, fibre digestibility and even looser faecal score that can cause a high diarrhoea rate in milk-fed calves(Reference Ghorbani, Kazemi-Bonchenari and Hossein Yazdi5). This can be more detrimental when calves are supplemented with ruminally unprotected unsaturated FA (UFA)(Reference Ghorbani, Kazemi-Bonchenari and Hossein Yazdi5,Reference Yousefinejad, Fatahnia and Kazemi-Bonchenari6) that can mask the favourable effects of the UFA. Saponification of FA with Ca salts is a strategy used to reduce the ruminal availability of supplemental FA for rumen microbes. This strategy allows the animal to profit from UFA sources, such as soyabean oil (SBO) and fish oil (FO), with few or no negative effects of supplemented fats on ruminal fermentation and microbial activity. To the best knowledge of the author, there is no work evaluating the effect of FA supplied through SBO and FO in the form of FA saponification with Ca salt in dairy calves in hot conditions.
Heat stress generally worsens the growth and health status of the calves because of the increase in energy requirement as well as a reduced appetite and weakness of the immune function(Reference Hyeok, Piao and Park9). Fat supplementation, especially rumen-protected sources, is one of the leading nutritional strategies to combat the metabolic effects of heat stress(Reference Hyeok, Piao and Park9). Some researchers have reported that increasing dietary fat supplied through essential FA in pregnant cows could have positive growth responses in dairy calves in different metabolic and nutritional statuses(Reference Garcia, Greco and Favoreto10). Recent studies have indicated that exposure to high environmental temperatures triggers some inflammatory signalling pathways in cattle(Reference Hyeok, Piao and Park9,Reference Min, Zheng and Zhao63) . The n-6 and n-3 FA are the essential FA and they are necessary for normal plasma membrane fluidity and neural development(Reference Kalupahana, Goonapienuwala and Moustaid-Moussa11) which can be more critical in the hot season. These FA are also considered to be the precursors to eicosanoids, which act as signalling molecules in regulating inflammatory pathways and regulating the function and secretion of adipose tissue by binding to PPAR(Reference Kalupahana, Goonapienuwala and Moustaid-Moussa11). The n-3 FA are known for their anti-inflammatory properties, whereas the n-6 FA are pro-inflammatory substances(Reference Ballou and DePeters12). Various studies, essentially performed in rodents, have confirmed that the FA profile of the phospholipid portion of immune cells is influenced by the diet, and also that alterations in the ratio of n-6 and n-3 FA can alter the activity of immune cells(Reference Calder13). Regardless of the favourable effect of high dietary n-3 FA on animal growth performance and immunity, this kind of FA can also modulate pro-inflammatory agents produced through adipose tissue in human studies(Reference Kalupahana, Goonapienuwala and Moustaid-Moussa11). These findings prompt animal nutritionists to incorporate higher n-3 FA sources in animal diets to provide an optimised immune function, consequently improving animal growth performance, especially in high tension conditions.
Feeding a high quantity of maize grain and soyabean meal in the starter diet of dairy calves can make a high n-6: n-3 FA ratio, such as 15:1, as reported by Hill et al. (Reference Hill, Bateman and Aldrich7). Based on this, it can be hypothesised that supplemental linoleic acid may aid the critical inflammatory response of the calf when exposed to environmental pathogens and stressors because linoleic acid works as a precursor of pro-inflammatory mediators such as cytokines and eicosanoids(Reference Calder14). The minor replacement of coconut oil with porcine lard in milk replacer increases the intake of linoleic and α-linolenic acids and can improve calf growth performance and some facet of immunity(Reference Garcia, Greco and Favoreto10). Dairy calves suffer from diarrhoea in the early weeks of life due to the low immune function and their high susceptibility to pathogens, and this is more obvious in dairy farms during hot conditions(Reference Godden1,Reference Yousefinejad, Fatahnia and Kazemi-Bonchenari6,Reference Ballou and DePeters12) . Thus, we assumed that a greater n-3:n-6 FA ratio in starter feed of dairy calves may improve the immunity of animals under hot conductions.
The effect of supplementation of a UFA source (protected through ruminal metabolism) in dairy calves experiencing heat stress is unknown. Consequently, the present study aimed to assess the effect of different UFA sources on Holstein’s calves during thermal stress. We hypothesised that the rumen protection of UFA, such as by n-6 FA and n-3 FA, through saponification would affect the calf’s response to heat-stress conditions. Thus, the present study aims to evaluate and compare the effects of n-6 FA supplied through SBO and n-3 FA supplied through FO and their equally mixed values on the growth performance, feeding behaviour, blood metabolite, liver enzymes, acute-phase proteins and inflammatory factors in dairy calves reared under hot conditions.
Materials and methods
Animal welfare
All the study protocols, including animal welfare, management and experimental procedures, and sampling methods, in the present study, were approved by the International Animal Care and Ethics Committee of the Iranian Council of Animal Care(15).
Weather conditions
The temperature and humidity index (THI) was calculated according to Herbut and Angrecka(Reference Herbut and Angrecka16). The mean monthly THI for the study location (Pars-Abad Moghan, Iran) in June, July and August of 2018 was calculated as 78·8, 76·7 and 75·7, respectively (Table 1). The highest and lowest monthly temperatures were 37·68 and 20·47°C, respectively. This index is formulated based on environmental temperature data and it is highly correlated with the body temperature of cows exposed to heat stress(Reference Gaughan, Mader and Holt17). Bohmanova et al. (Reference Bohmanova, Misztal and Cole18) reported that the use of the THI index helps estimate cows’ milk production in the South East of the USA. According to the THI data obtained, our calves were kept in moderate thermal stress conditions(Reference Moran19). For the THI calculation, the daily temperature and relative humidity were collected every 30 min using a data logging digital humidity/temperature meter (TROTEC-BL30 Climate Data Logger; Trotec GmbH).
Table 1. Minimum and maximum temperature and temperature–humidity index (THI) during experimental period (from June until August 2018)
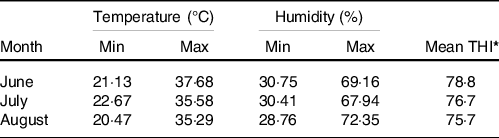
*THI Tdb + (0.36 * Tdp) + 41.2 (Tdb = dry bulb temperature, T dp = dew point temperature).
Calves and experimental design
The present study was conducted at Moghan Agro-Industrial and Livestock Co. during June, July and August of 2018 (Table 1). The animals were cared for according to the Guide for the Care and Use of Agricultural Animals in Research and Teaching(20). Forty Holstein calves with a mean body weight (BW) of 39·67 (sd 3·5 kg), ten calves per group, from healthy multiparous dams with a normal dry period, were randomly kept in separate bedded hutches (2·2 × 1·1 m) in an open area equipped with sunshade, from 3 to 65 d of age. High-quality colostrum was fed to the amount of 6 l within the first 12 h of life (3 l of colostrum within 2 h of life and 3 l in a second feeding). The calves were checked to have normal health conditions and randomly assigned to the experimental diets on day 3 of age. The experimental diets were formulated to meet the current NRC(21) nutrient requirements. The treatments were: (1) no fat supplementation (CON); (2) the starter feed supplemented with SBO rich in n-6 FA (3 %, DM basis); (3) the starter feed supplemented with FO rich in n-3 FA (3 %, DM basis) and (4) the starter feed with an equal amount of SBO and FO (1·5 % each, DM basis) (MIX). To avoid the negative effects of various FA sources on ruminal fermentation and microbial activity, both supplemented SBO (PersiaFat-Omega-6®) and FO (PersiaFat-Omega-3®) were ruminally protected, obtained through saponification with Ca salts that were provided by the Kimiya Danesh Alvand Company (Table 2).
Table 2. Chemical composition and fatty acid profile for supplemental fat sources in the present study*
(Mean values and standard deviations)
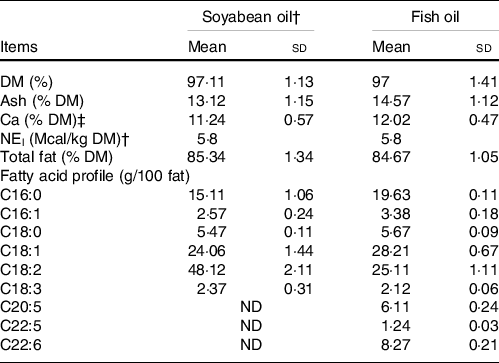
* Chemical composition and fatty acid profiles were determined in three replicates.
† Provided by the manufacturer based on digestibility studies and calculations based on NRC (2001) equations.
‡ Ca salts of fatty acids were provided by Kimiya Danesh Alvand Co.
The milk was fed two times per day (at 08.00 and 16.00 hours) via pre-washed and sanitised stainless-steel buckets. The calves had unlimited water and starter diet possession throughout the experimental period. They received 4 l of the whole milk/d from days 3 to 30, 6 litre/d from days 31 to 50, 4 litre/d from days 51 to 60 and 2 litre/d milk (once a day), during the morning feeding, from days 61 to 64 of age. Eventually, they were weaned at 65 d of age. All of the calves were weaned on day 65 of age based on a gradual 5-d weaning schedule. The daily offered milk (38°C) was weekly analysed for nutrients (MilkoscanTM S50-75610; FOSS,) and the average milk composition was as follows: 3·3% fat, 3·5 % crude protein (CP), 4·2 % lactose and 13% total solids.
The ingredients, chemical composition and FA profile of the experimental diets are presented in Table 3. Weekly samples of starter feed were collected and analysed for DM (protocol no. 934·01), CP (protocol no. 988·05) and ether extracts (protocol no. 954·02), according to AOAC(22). Dietary fibre fractions (neutral-detergent fibre and acid-detergent fibre) were analysed according to Van Soest et al. (Reference Van Soest, Robertson and Lewis23). Medium chopped alfalfa hay (containing 16·6 % CP, 46·3 % neutral-detergent fibre and 1·72 % ether extract, DM basis) was added to the starter feed (10 % based on starter DM basis) at 21 d of age for all experimental groups. The starter diets were presented at 09.00 hours after the refusals were weighed to compute the feed intake throughout the study.
Table 3. Ingredient and nutrients composition of starter and mixed diets in experimental groups*,†,‡
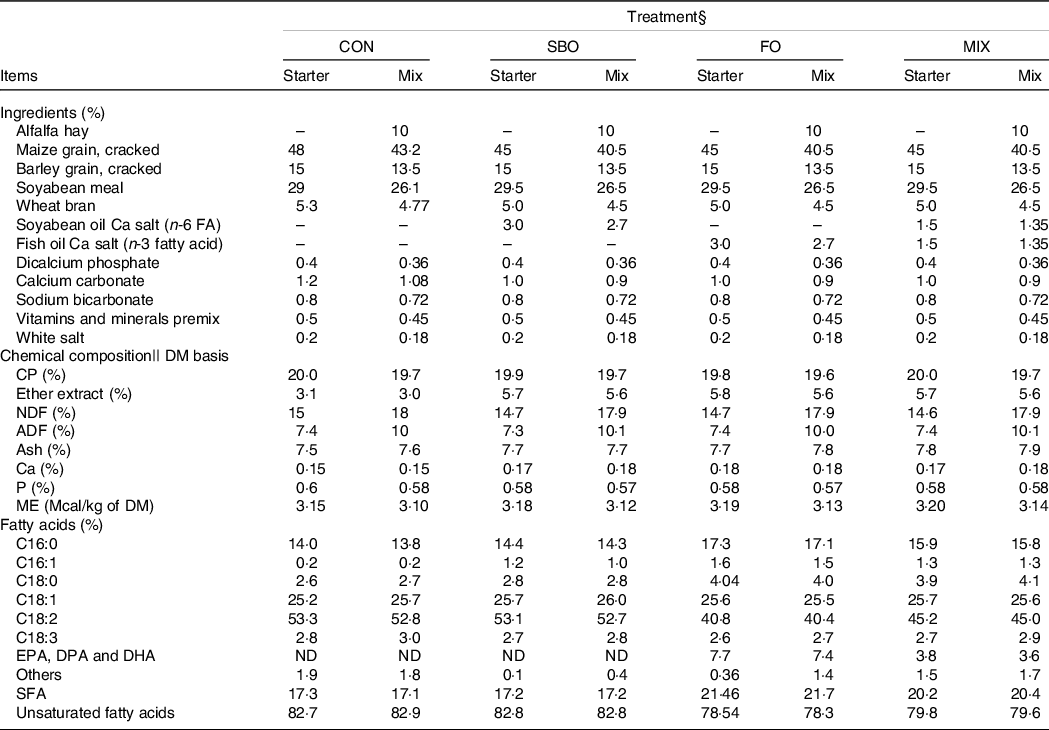
EPA (20:5n-3); DPA (22:5n-3); DHA (22:6n-3).
* Calculations based on NRC (2001) equations.
† The Ca salts of soyabean oil and Ca salts of fish oil (Persia fat) were kindly provided by Kimiya Danesh Alvand Co.
‡ Mineral–vitamin pre-mix contains: 75 mg as Retinolof vitamin A, 1.25 mg of vitamin D, 1.25 mg of vitamin E, 120 g of Ca, 20 g of P, 20.5 g of Mg, 186 g of Na, 7.7 g of Zn, 2.25 g of Mn, 1.25 g of Fe, 3 g of S, 14 mg of Co, 1.25 g of Cu, 56 mg of I and 10mg of Se.
§ Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
|| Chemical composition and fatty acid profiles of the starter and mix diets were laboratory analysed in three replicates.
Measurements and analytical methods
Calf starter intake was recorded daily by weighing the differences between how much starter diet was offered and how much refused. BW was measured immediately after birth, at the beginning of the study (day 3) and then at 7-d intervals using a digital weighbridge. Feed efficiency (FE; kg of BW gain/kg of total DM intake (starter DM intake + milk DM intake)) was also calculated.
Body length, body barrel, wither height, pin height and hip width, and hip to pin width were measured using a caliper and were recorded at days 3, 30 and 65 of the experiment.
The respiratory rate (breaths/min) was recorded by counting the movements of the abdominal muscles in the flanks during respiration(Reference Peña, Risco and Kunihiro24), while the calves were in a lying posture, over 4 h during two consecutive days leading to 30 and 65 d of the experiment(Reference Kovács, Kézér and Ruff25). After each of the respiratory rate recordings, rectal and ear skin temperature was also measured using a digital and IR thermometer, respectively (ZOTEK-GM320; Zotek tools). In addition, the rectal temperature was measured weekly before the morning milk feeding meal.
The health score of the calves was recorded daily according to Wisconsin Madison’s calf health chart (2003). Faecal samples were collected by rectal palpation into sterile containers on days 58–60 to count the colony number of Lactobacillus, E. coli and Salmonella species(Reference Sanders26) using specific growth media in a veterinary microbiology laboratory (Mabna Laboratory). Faecal samples were also obtained twice a day before a.m. and p.m. milk feeding time on six consecutive days (from days 55 to 60) through rectal palpation to determine apparent nutrient digestibility. Faecal samples were dried at 60°C for 48 h, mixed and ground to pass through a 1 mm screen for further nutrient analysis. Samples were analysed for CP (22), ash (4 h at 550°C), ether extract (22) and neutral-detergent fibre using an ANKOM200 fibre analyser (Ankom Technology) according to the procedures described in Van Soest et al. (Reference Van Soest, Robertson and Lewis23). Acid-insoluble ash was used as an internal marker to estimate the apparent total tract digestibility of DM, organic matter, CP, neutral-detergent fibre and ether extract(Reference Van Keulen and Young27).
Feeding (standing, lying, eating and drinking) and non-nutritive oral behaviours were recorded by direct monitoring of all the calves for 24 h every 5 min on two consecutive days during two consecutive weeks (50–64 d)(Reference Castells, Bach and Araujo28).
Blood samples were collected from the jugular vein of the calves into 10 ml pre-evacuated tubes on days 30 and 60 three hours after the morning feeding meal. Two separate tubes were used for each calf to obtain serum (no-additive tubes) and plasma (heparinised tubes with sodium fluoride and potassium oxalate to prevent glycolysis) samples. The blood samples were clotted on ice and immediately centrifuged (3000 rpm for 15 min at 4°C) for serum separation. The heparinised tubes were immediately centrifuged (3000 rpm for 15 min at 4°C) and plasma samples were collected. The serum and plasma samples were stored at –20°C until subsequent analysis. The plasma samples were analysed to determine the glucose, TAG, cholesterol, blood urea nitrogen, creatinine, albumin, total protein and liver enzymes, including aspartate aminotransferase, alanine aminotransferase and lactate dehydrogenase levels with a spectrophotometric autoanalyser (UNICCO, 2100; Zistchemi Co.), using the commercially available clinical investigation kits (Pars-Azmun Co. Ltd) according to the manufacturers’ instructions. Serum samples were used to measure the bovine serum amyloid A (Bioassay), TNF-α, IFN-γ and IL-6 (Karmania Pars Gene, Elisa kits) and haptoglobin (immunoturbidimetric assay; Biorex-Fars) levels. ELISA measurements were done according to the manufacturer’s instructions by an ELISA reader (DANA 3200; Garny). Malondialdehyde (MDA, Nalondi™-Lipid Peroxidation Assay Kit-MDA; Navand-salamat) was also measured in the serum samples using a plate reader (DANA 3200; Garny) at 550 nm.
The FA profile of the plasma samples was determined on day 60 of the experiment. For this purpose, lipid extraction was carried out three times with chloroform/methanol (C/M, 2/1, v/v) to a final volume of 100 ml administered under an argon gas blanket (Folch et al.)(Reference Folch, Lees and Sloane-Stanley29). The flasks were centrifuged (1800 g for 10 min), and the organic fraction was separated and treated with anhydrous sodium sulphate and then vaporised using a rotary evaporator (Büchi) at 40°C under vacuum conditions. Using mild methanolysis/methylation via methanolic hydrochloride acid (HCl/MeOH), fatty acid methyl esters were prepared by a method explained in Ichihara and Fukubayashi(Reference Ichihara and Fukubayashi30). Hexane was utilised as a solvent for extraction, and nonadecanoic acid was utilised as an internal standard. For FA analysis, Agilent 6890 gas chromatograph equipped with an autoinjector (Agilent 7683 series) and Flame Ionization Detector (FID) detector was used. Samples (1 µl) were injected in split mode, 50:1, into a RESTEK column for fatty acid methyl esters (Rtx®-2330, 105 m × 250 µm × 0. 2 µm; Cat#10 729; Serial#1 525 353, Restek Corporation). The detector and injector temperatures were set at 250°C. N2, with a constant flow of 1 ml/min, was chosen as the carrier gas. Based on the method described by Lee et al. (Reference Lee, Tweed and Moloney31), the oven temperature was set at the gradient temperature rise with some modifications, starting at 70°C for 1 min, and then it was increased by 5°C/min to 100°C and was kept there for 2 min. Then, the column temperature was increased by 10°C/min to 175°C and was maintained for 35 min. Eventually, the temperature was increased by 4°C/min to 225°C and was kept there for 35 min. Based on a fatty acid methyl esters standard mix (GLC 463; Nu-Chek Prep Inc.; reference mixture 47 885, Supelco Inc., Bellefonte PAGLC 463 reference mixture, http://www.nu-chekprep.com/10 11 catalog.pdf), individual peaks were specified.
Statistical analysis
Collected data were analysed using the ‘lme’ procedure of R (Version 0.99.483, 2009–2015 Rstudio, Inc.)(Reference Mohtashami and Hashemi32). Feed intake, ADG and FE data were considered as the repeated measurements over time and analysed in three separate intervals (days 3–30; days 31–65 and days 3–65). They were divided into three periods in the ‘Results’ section as the early stage of growth (days 3–30), close to the weaning period (days 31–65) and the entire experimental period (days 3–65). The following model was used: Yijk = μ + Fati + Tj + (Fat × T)ij + β(Xi − X̄) + ϵijk, where Yijk is the dependent variable; µ is the overall mean; Fati is the effect of FA source supplementation (SBO, FO or their MIX); Tj is the effect of sampling time; (Fat × T)ij is the interaction between the supplemental FA source and sampling time; β(Xi−X̄) is the covariate variable and ϵij is the overall error term. The apparent nutrient digestibility, growth indices, feeding behaviours and blood parameters were analysed with the same mixed model described above but without the effect of the time. Faecal and health score data were analysed using a multivariable logistic mixed model. For BW and skeletal growth, the initial values were considered to be covariates. The differences between means were separated using the Tukey test. The threshold of significance was set at P ≤ 0·05; trends were declared at 0·05 < P ≤ 0·10.
Results
Intake of starter and fatty acids
The results for calf starter intake, total DMI (milk + starter) and calculated intake of essential FA are reported in Table 4. The starter intake was the highest for the SBO diet compared with the other treatments during days 30–65; however, results showed that the supplementation of FO significantly decreased starter intake compared with the CON (P = 0·01). Because the milk feeding value was constant among the experimental treatments, the differences in total DMI (P = 0·04) were attributed to the different starter intake among the experimental diets.
Table 4. Effects of different fat sources (soyabean oil, fish oil or their mix) on intake of starter and fatty acid in Holstein calves during heat stress
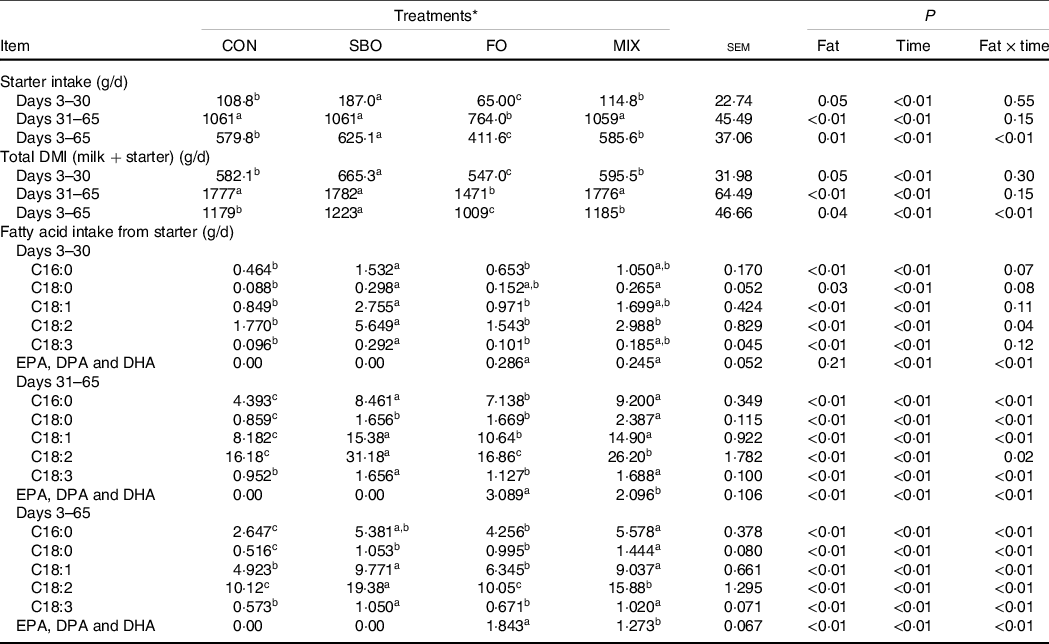
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
The intake of EFA was affected by the different fat supplements. The intake of linoleic acid (C18:2) was increased in the SBO and MIX groups but decreased in the FO group compared with the CON group (P < 0·05). Even though the daily starter intake was reduced in the FO diet, the intake of n-3 FA increased compared with the CON diet. The intake of EPA, DPA and DHA increased compared with the other treatments when the calves received the FO supplement in the starter diet (P < 0·01).
Growth indices, feed efficiency and nutrient digestibility
The BW, ADG, FE and nutrient digestibility results are reported in Table 5. The greatest BW at the weaning date was observed in the SBO diet (P = 0·001). However, the FO supplementation reduced the ADG during the entire study period compared with the other groups; thus, the calves fed the FO supplemented starter diet had a lower BW compared with the other experimental treatments. The calves which received FO showed the highest FE among the experimental diets during all the experimental periods (P < 0·05).
Table 5. Effects of different fat sources (soyabean oil, fish oil or their mix) on average daily gain, body weight, feed efficiency and nutrient digestibility in Holstein calves during heat stress
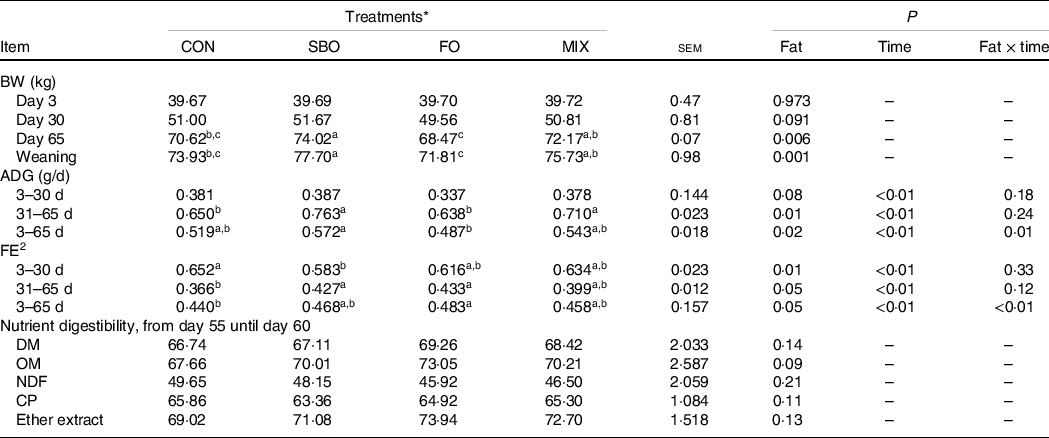
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
2 Feed efficiency = kg of ADG to kg of total DMI.
Regarding the time effect, the results show that the starter intake and different FA intake were increased in the different fat-supplemented calves compared with the CON diet as calves aged. Moreover, the ADG was increased during days 31–65 compared with days 3–30 for the calves receiving the SBO and MIX diets compared with the CON and FO diets.
Other than a tendency for organic matter improvement in fat in the supplemented diets compared with the CON group (P = 0·09), none of the other nutrients’ digestibility has been influenced by the experimental treatments in the present study.
Fat supplementation did not impact wither height, body barrel, hip width, pin width and hip to pin dimensions (Table 6). However, the SBO supplementation showed improved body length compared with the FO and CON groups (P = 0·05).
Table 6. Effects of different fat sources (soyabean oil, fish oil or their mix) on skeletal growth indices in Holstein calves during heat stress
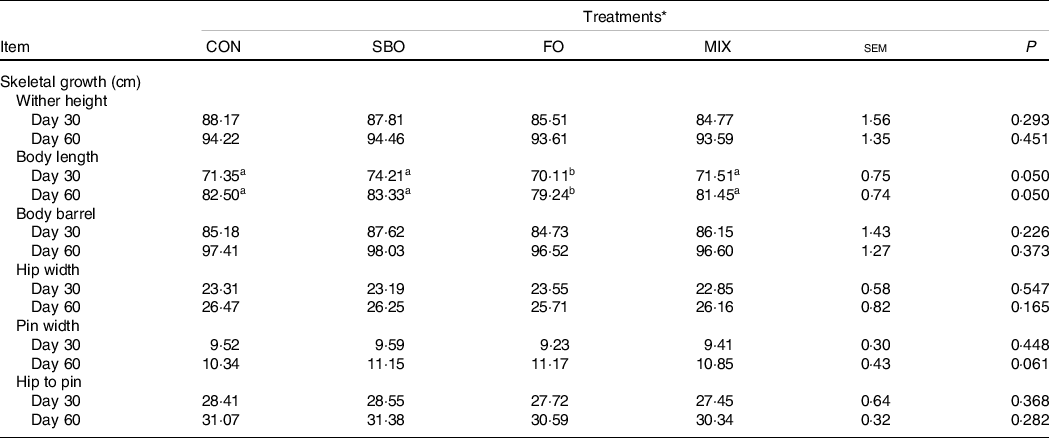
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
Health indices and microbial counts in faeces
The health indices, including faecal scores, days with diarrhoea and calf’s appearance scores, are presented in Table 7. Our results show that the SBO supplemented calves had fewer days with diarrhoea in comparison with the other groups (P > 0·05). There was no significant difference in nasal, ear and eye scores among the treatments (P > 0·05).
Table 7. Effects of different fat sources (soyabean oil, fish oil or their mix) on faecal scores, days with diarrhoea, general appearance indices, body temperature and respirator rate in Holstein calves during heat stress
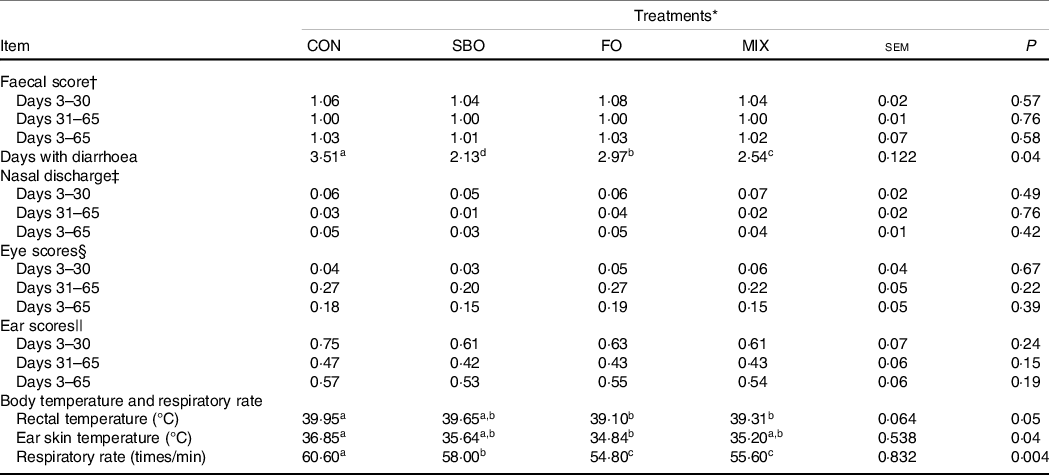
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
† Faeces score scale: 0 = firm faeces, no diarrhoea; 1 = soft faeces, no diarrhoea, 2 = mild diarrhoea and 3 = watery, severe diarrhoea.
‡ Nasal discharge (0 = normal serous discharge; 1 = small amount of unilateral cloudy discharge; 2 = bilateral, cloudy or excessive mucus discharge and 3 = copious bilateral mucopurulent discharge).
§ Eye scores (0 = normal; 1 = small amount of ocular discharge; 2 = moderate amount of bilateral discharge and 3 = heavy ocular discharge).
|| Ear scores (0 = normal; 1 = ear flick or head shake; 2 = slight unilateral droop and 3 = head tilt or bilateral droop).
Results regarding the body response to heat, including rectal and ear skin temperatures and respiratory rate, are presented in Table 7. Fat supplementation significantly reduced rectal temperature compared with the CON group. Accordingly, FO supplemented calves had the lowest rectal temperature (P = 0·05) and ear skin temperature (P = 0·04) among the dietary treatments. In addition, fat supplementation decreased the respiratory rate compared with the CON diet (P < 0·01), with no differences being recorded between different fat sources.
Regarding the microbial population evaluated in the faeces, the results report the counts of Lactobacillus species did not differ among treatments (P = 0·45; Table 8). However, FO supplementation reduced the E. coli population compared with the CON (P = 0·05). Notably, salmonella was not detected in any faecal samples.
Table 8. Effect of different fat sources (soyabean oil, fish oil or their mix) on faecal viable microbial counts in Holstein calves during heat stress
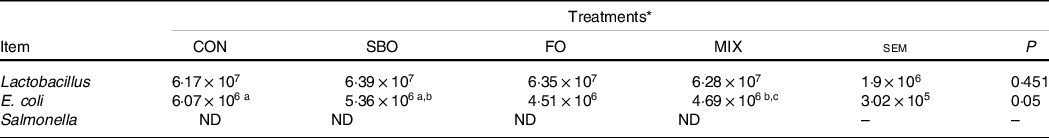
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
Feeding behaviours
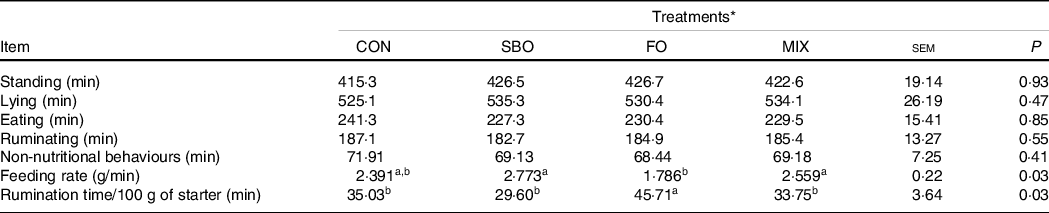
Total eating time, ruminating, lying, standing times and non-nutritional behaviours were the same across the experimental treatments (P > 0·05; Table 9). Supplementation of the diets with FO significantly decreased the feeding rate and increased the time spent for rumination per kg of starter DM (P = 0·03).
Table 9. Effect of different fat sources (soyabean oil, fish oil or their mix) on feeding behaviours in Holstein calves during heat stress
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
Blood metabolites, inflammatory markers and fatty acid profile
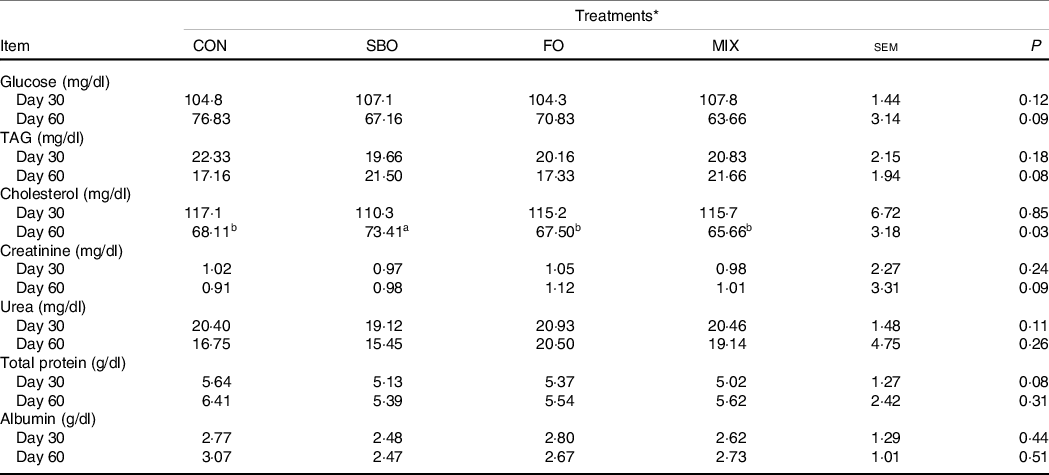
Fat supplementation did not affect blood urea nitrogen and albumin concentrations (Table 10). Calves receiving the supplemental fat sources tended to have lower blood glucose concentration (day 60; P = 0·09) and total protein concentrations (day 30; P = 0·08) but tended to have higher creatinine concentration (day 60; P = 0·09) compared with the other experimental treatments. The calves fed SBO had higher cholesterol levels compared with the CON diet (day 60; P = 0·03).
Table 10. Effect of different fat sources (soyabean oil, fish oil or their mix) on blood metabolites in Holstein calves during heat stress
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
Fat supplementation did not affect alanine aminotransferase and lactate dehydrogenase concentrations, but the aspartate aminotransferase concentration was significantly reduced in the SBO supplemented calves (day 60; P = 0·01) (Table 11). The serum levels of IL-6 were not affected by the dietary treatments; however, the serum levels of haptoglobin, serum amyloid A, TNF-α, and IFN-γ were higher in the CON compared with the fat supplemented diets (P < 0·05). Results showed that FO supplemented calves showed the lowest levels of pro-inflammatory cytokines and acute-phase proteins. The levels of MDA as the lipid peroxide marker were reduced in the plasma of calves receiving FO supplement in the starter diet (P < 0·05).
Table 11. Effect of different fat sources (soyabean oil, fish oil or their mix) on liver enzymes, oxidative markers and inflammatory cytokines in Holstein calves during heat stress

AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; IFN-γ, interferon-gamma; SAA, serum amyloid A; MDA, malondialdehyde.
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
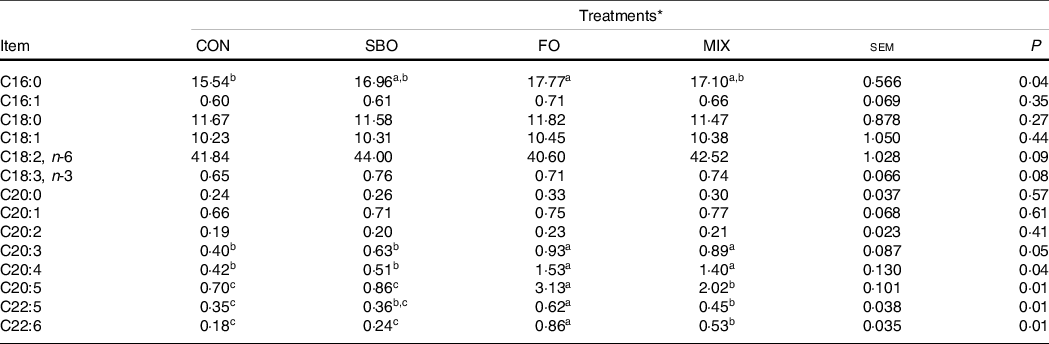
Table 12 summarises the results of the FA profiles in the plasma of the experimental calves. Supplemented fat sources increased C16:0 compared with the CON group (P = 0·04). Feeding FO to the dairy calves increased the plasma concentrations of C20:3, C20:4; C20:5; C20:6 compared with the other experimental treatments (P < 0·05). Feeding SBO resulted in slightly higher plasma levels of linoleic (P = 0·09) and linolenic acid (P = 0·08).
Table 12. Effect of different fat sources (soyabean oil, fish oil or their mix) on plasma concentration of selected fatty acid methyl esters in Holstein calves during heat stress
a,b,cMean values within a row with unlike superscript letters were significantly different (P < 0.05).
* Treatments were: (1) no supplemented fat in starter diet (CON), (2) the starter diet supplemented with soyabean oil rich in n-6 FA (SBO), (3) the starter diet supplemented with fish oil rich in n-3 FA (FO) and (4) the starter diet supplemented with an equal amount of soyabean oil and fish oil (MIX).
Discussion
Nowadays, heat stress is one of the environmental conditions that negatively affect a calf’s intake, growth performance, welfare and well-being. As indicated in Table 1, the THI in our experimental period was above the normal range of a thermo-neutral zone during the rearing period, such that it could be considered putting the animals under heat-stress conditions(Reference Folch, Lees and Sloane-Stanley29,Reference Pejman and Aghdam Shahryar33) .
Intake of starter and individual fatty acids
Because the quantity and schedule of the milk feeding were constant across the experimental treatments, no difference was observed in the DM provided by the milk. However, the starter intake and subsequent total DM intake (milk DM + starter DM) were greater in SBO-fed calves compared with those fed the CON diet but were lower in FO-fed calves compared with those fed the CON diet. Comparing the effect of different FA sources on intake, previous studies have stated that UFA has the potential to reduce DMI, whereas SFA has little effect on it(Reference Palmquist34). Doppenberg and Palmquist(Reference Doppenberg and Palmquist35) have shown that fat supplementation in liquid feed reduces calf starter intake and, consequently, reduces the ADG in fat-supplemented calves. Similar to our results, Kadkhoday et al. (Reference Kadkhoday, Riasia and Alikhani36) have reported that calf starter intake increases when palm FA or a combination of palm and flaxseed oil is given to the calves near weaning time (42–56 d of age). Our results clarify that, in addition to the starter fat content, the FA profile can also influence starter intake in dairy calves during heat stress. Generally, it has been verified that lipids exert lower metabolic heat production than carbohydrates and proteins and that they can be incorporated into the diet with little heat production(Reference Wang, Liu and Yang37). Discrepancies regarding the starter intake responses to supplemented fat sources may be related to differences in milk feeding amount and offering regimens, calf starter ingredients, the level of supplemented fat and climate. In addition, Hill et al. (Reference Hill, Bateman and Aldrich7) found that FA composition and the ratio of individual FA in supplemented fat sources can affect the starter intake and animal growth performance. Ghasemi et al. (Reference Ghasemi, Azad-Shahraki and Khorvash38) have also reported higher starter intake in cold stressed calves fed SBO diets compared with the CON diets, but supplementation of FO in that study caused a reduction in the starter intake. McDonnell et al. (Reference McDonnell, John and Doherty39) have noted a reduction in starter intake in calves that received FO supplemented through milk replacer. With respect to the individual FA, our results show an increase in dietary consumption of specific FA in experimental animals compared with the control group (linoleic and linolenic acid in the SBO diet and EPA and DHA in the FO diet). Kadkhoday et al. (Reference Kadkhoday, Riasia and Alikhani36) and Hill et al. (Reference Hill, Bateman and Aldrich40) have written of an increased linolenic acid intake in flaxseed supplemented calves. Increased individual FA consumption was expected because of the supplemented diets with various fat sources. It is also notable here that starter intake and the individual FA intakes were increased by the calves ageing, which was related to the greater nutrient requirements of elder calves during days 31–65 compared with younger calves during days 3–30 of the current experiment.
Growth performance, feed efficiency and total tract digestibility
The supplementation with SBO caused calves to achieve the greatest ADG during days 31–65 compared with other calves, which can be partly related to higher starter intake. This resulted in the higher BW for calves receiving SBO compared with the other experimental groups on day 65 of the experiment. Garcia et al. (Reference Garcia, Shin and Schlaefli41) have reported that total BW gain by female calves during the first 30 d tended to increase linearly with the increased intake of essential FA. Kazemi-Bonchenari et al. (Reference Kazemi-Bonchenari, Dehghan-Banadaky and Fattahnia8) showed that the final BW was greater in calves fed on the LSO rich in n-3 FA compared with the un-supplemented calves. Ghasemi et al. (Reference Ghasemi, Azad-Shahraki and Khorvash38) also noted higher than average ADG during the pre-weaning period and subsequent higher BW at weaning for SBO diets compared with control treatment under cold stress. Kadkhoday et al. (Reference Kadkhoday, Riasia and Alikhani36) and Hill et al. (Reference Hill, Bateman and Aldrich40) measured higher ADG and higher weaning weight for flaxseed oil supplemented calves and attributed it to the beneficial health and immunity improvement effects of the UFA. Kazemi-Bonchenari et al. (Reference Kazemi-Bonchenari, Dehghan-Banadaky and Fattahnia8) reported ADG was increased with LSO supplementation that is rich in C18:3 FA content. However, supplementation of FO reduced the ADG and BW of calves at weaning, which is in line with total DMI reduction in these calves. Agreeing with our results, Ghasemi et al. (Reference Ghasemi, Azad-Shahraki and Khorvash38) stated that the ADG was lower when the FO was incorporated into the starter diet of dairy calves. McDonnell et al. (Reference McDonnell, John and Doherty39) found a tremendous reduction in weaning weight in calves fed FO supplemented through milk replacer. It seems that the lower ADG in FO-fed calves is mostly related to lower starter intake, as observed in the present study or the above-mentioned studies. However, it is notable that environmental conditions (heat stress, cold stress and thermo-neutral conditions) and method of oil delivery (mixed with milk replacer or mixed with a starter such as Ca salt) can also influence the obtained results(Reference Ghorbani, Kazemi-Bonchenari and Hossein Yazdi5,Reference Yousefinejad, Fatahnia and Kazemi-Bonchenari6) .
In the present study, supplementation of FO increased FE compared with the CON diet. Because the calves supplemented with FS had a lower starter intake and the amount of starter consumed is an important factor in calculating the FE, the increased FE in FO supplemented calves would seem to be partly related to a lower starter intake. In addition, from the individual FA supplementation point of view, it could have been related to the beneficial effects of the essential FA found in the FO on health and immune function in heat stress. Ghasemi et al. (Reference Ghasemi, Azad-Shahraki and Khorvash38) reported that supplementation with different oil sources did not affect the FE. However, some other studies have noted higher FE in calves fed with C18:3 FA compared with the un-supplemented calves(Reference Kazemi-Bonchenari, Dehghan-Banadaky and Fattahnia8,Reference Kadkhoday, Riasia and Alikhani36,Reference Hill, Bateman and Aldrich40) , although some previous studies used a similar number of animals while evaluating fat sources in dairy calves(Reference Ghasemi, Azad-Shahraki and Khorvash38,Reference Hill, Bateman and Aldrich40,Reference Kazemi-Bonchenari, Falahati and Poorhamdollah49) . Further research is warranted using a greater number of experimental animals to evaluate the influence of various FA sources on the weight gain and FE of dairy calves in different environmental conditions.
Body length was increased by supplementation of SBO in the present study. Kadkhoday et al. (Reference Kadkhoday, Riasia and Alikhani36) reported that calves treated with flaxseed oil had higher hip height compared with the control animals. Having said this, positive(Reference Hill, Aldrich and Schlotterbeck42), negative(Reference Fokkink, Hill and Bateman43) or no effects(Reference Ghasemi, Azad-Shahraki and Khorvash38) have previously been noted when considering the growth indices following the supplementation of different fat sources in dairy calves. Kazemi-Bonchenari et al. (Reference Kazemi-Bonchenari, Dehghan-Banadaky and Fattahnia8) have described how calves supplemented with LSO had greater wither and hip heights compared with the un-supplemented calves. Maggio et al. (Reference Maggio, Artoni and Lauretani44), in a comprehensive review, supported an idea about the beneficial effect of PUFA, such as linoleic, linolenic acid and CLA, on bone metabolism and their regulatory effect on osteoblastogenesis and osteoclast activity. Although, according to this perspective, FA supplied through SBO supplementation increased body length in dairy calves, FA supplied through FO was not effective in bone growth. This is probably related to a lower starter intake and, hence, fewer nutrients being available for growth in the FO calves rather than the SBO supplemented calves.
Health indices and microbial faecal counts
Regardless of the FA source supplemented in the present study, the CON group recorded the highest rectal temperature among the experimental treatments. Higher rectal temperature and increased respiratory rate were reported as a routine sign of heat stress that was reduced in fat supplemented treatments in the present study. The lower rectal temperature found in the fat supplemented groups can be related to the alleviation of nutrient metabolism effects on heat increment value. Fat sources have lower heat increment than carbohydrates and proteins and lower metabolic heat load in the body(Reference Wang, Liu and Yang37).
The beneficial effects of essential FA supplementation, especially linolenic acid, on the immune system function and health indices of dairy calves have been noted in previous studies(Reference Kadkhoday, Riasia and Alikhani36,Reference Hill, Aldrich and Schlotterbeck42) . In the present study, rectal temperature and respiratory rate were reduced while calves received FO or a mixed diet containing both SBO and FO. Previous work on dairy calves has demonstrated that supplementation of a C18:3 FA source (LSO) reduced rectal temperature compared with an un-supplemented group(Reference Kazemi-Bonchenari, Dehghan-Banadaky and Fattahnia8). Hill et al. (Reference Hill, Bateman and Aldrich7) stated that the inclusion of a mixture of FA sources (butyrate, medium-chain FA and α-linolenic acid) in the starter diet of dairy calves reduced the inflammatory effects of vaccination. In addition, McDonnell et al. (Reference McDonnell, John and Doherty39) reported lower rectal temperatures for FO supplemented calves compared with the non-supplemented calves.
Supplementing the starter diets with FO rich in n-3 FA resulted in the lowest E. coli counts in the faeces compared with other treatments. Previous studies have indicated that faecal microbial counts can be used as a proper index for a lower gut microbial population(Reference Alimirzaei, Alijoo and Dehghan-Banadaky45). The E. coli population, which is an important pathogen in the gut of dairy calves, is responsible for some infectious diseases and lowering the efficiency in calf production(Reference Alimirzaei, Alijoo and Dehghan-Banadaky45). It is notable here that FO supplementation has been used for treating the canine chronic enteropathies due to the lucrative effects of the FA content of FO(Reference Ontsouka, Burgener and Luckschander-Zeller46). The present study results clarify that, in addition to the fat level in the starter diet that can lessen the heat load and E. coli population during heat stress, the individual FA profile can also be an important issue in driving a healthier condition in calves during this kind of environmental stress.
Feeding behaviours
In our experiment, the different fat supplements did not affect the feeding behaviour and the total time spent standing or lying. However, considering the feeding rate (total minutes spent to consume 1 kg of starter diet), the results indicate that FO supplemented calves eat more slowly than the SBO supplemented and CON groups. In addition, FO supplemented calves had shown to expend more rumination time per kg of starter DM intake. Kadkhoday et al. (Reference Kadkhoday, Riasia and Alikhani36) reported that the supplementation of different FA sources did not affect the feeding behaviour of milk-fed calves. However, concurring with our results, some others have reported that feeding a UFA source reduced the feeding rate in dairy cows(Reference Harvatine and Allen47). More research is required to evaluate fat feeding levels and the sources of feeding behaviour in dairy calves.
Blood metabolites, fatty acid profiles and inflammatory markers
Supplemental fat sources in the present study tended to reduce the plasma glucose concentrations on day 60 compared with the CON group. Kadkhoday et al. (Reference Kadkhoday, Riasia and Alikhani36) detailed how supplementing the starters with different FA sources did not influence the blood glucose concentration. Moreover, Garcia et al. (Reference Garcia, Shin and Schlaefli41) reported that linoleic and α-linolenic acid supplementation did not exert any differences in plasma concentrations of glucose, insulin and IGF-1 in dairy calves. However, some studies have noted that FO supplementation or feeding microalgal DHA in the starter diet or milk replacer reduced serum glucose and insulin levels in calves(Reference Ghasemi, Azad-Shahraki and Khorvash38,Reference McDonnell, John and Doherty39) . It can be postulated that because starch is the main influencer of blood glucose concentration in dairy calves(Reference Makizadeh, Kazemi-Bonchenari and Mansoori-Yarahmadi48), the replacement of fat sources with a starch source (maize grain, in the present study) had the potential to reduce blood glucose concentrations, thanks to the reduced ruminal propionate concentration(Reference Ghorbani, Kazemi-Bonchenari and Hossein Yazdi5,Reference Makizadeh, Kazemi-Bonchenari and Mansoori-Yarahmadi48) .
The measured blood metabolites were in the normal ranges found in the dairy calves(Reference Kazemi-Bonchenari, Dehghan-Banadaky and Fattahnia8,Reference Makizadeh, Kazemi-Bonchenari and Mansoori-Yarahmadi48,Reference Kazemi-Bonchenari, Falahati and Poorhamdollah49) . The blood concentration of cholesterol was affected by the supplementation of fat. The supplementation of SBO increased the blood cholesterol concentration, which is consistent with previous studies in dairy calves(Reference Ghorbani, Kazemi-Bonchenari and Hossein Yazdi5). High-fat diets, in pre-ruminant diets, have been reported to stimulate the secretion of TAG-rich lipoproteins (such as chylomicrons) as well as HDL(Reference Piot, Hocquette and Herpin50). Our results indicate that feeding a starter diet supplemented with an equal amount of SBO and FO reduced the blood concentration of cholesterol but not TAG. Kadkhoday et al. (Reference Kadkhoday, Riasia and Alikhani36) have reported that feeding SBO and tallow increased the concentrations of blood lipids, such as cholesterol, TAG and HDL, and palm fat increased blood concentrations of cholesterol and HDL at the post-weaning period. Our results indicate that, regardless of the fat level in the starter diet, the FA source can play a pivotal role in fat-derived metabolite concentrations in the blood of dairy calves which, consequently, may impact the animal whole-body metabolism during heat-stress conditions.
Considering the results for liver enzyme activity in the present study, no effect was found for alanine aminotransferase and lactate dehydrogenase but the highest concentration for blood aspartate aminotransferase was found in the CON group. Hepatotoxicity, or liver damage, has been reported to occur in heat-stressed rats(Reference Zhang, Chen and Tang51), chickens(Reference Huang, Yang and Rehman52) or ruminants(Reference Kim, Lee and Jeon53). Our results show that regardless of the FA sources supplemented in the present study, an increased fat level in heat stress has the potential to reduce the aspartate aminotransferase concentration, indicating a more favourable function of the liver in the heat-stressed dairy calves supplemented with fat compared with those on an un-supplemented diet. Previous research has indicated lower hepatic enzyme activity in plasma for animals supplemented with fats compared with animals who do not receive fat in their diet(Reference Bilal, Patel and Burkitt54). This may be partly related to the bio-conversion of eighteen-carbon FA, both of linoleic and linolenic acid, to long-chain PUFA, such as EPA and DHA(Reference Domenichielloa, Kitsona and Chuck55). Higher EPA and DHA levels in plasma that were found in the present study are in line with this postulation. More work is needed to evaluate the fat level and source supplementation on the liver function of dairy calves in different rearing conditions.
High ambient temperature has been reported as a potent inducer of oxidative stress and, consequently, inflammation in animals(Reference Kaldur, Kals and Ööpik56). Different stressors, such as nutritional and environmental stresses, lead to the overproduction of free radicals, a disturbance of the redox balance, and the subsequent effects of oxidative stress(Reference Abuelo, Hernández and Benedito57). In the present study, the levels of MDA as a lipid peroxides marker and total antioxidant capacity were higher in the CON group and were not affected by the dietary supplementation of SBO. However, FO supplementation reduced MDA in the present study. Kargar et al. (Reference Kargar, Ghorbani and Fievez58) claimed that in heat-stressed dairy cows, FO supplementation did not influence the plasma levels of MDA and total antioxidants compared with an SBO diet. From the biological studies perspective, Li et al. (Reference Li, Zhang and Wang59) have reported that FO is involved in the maintenance of epithelial tight junction integrity and the recovery of gut microbiota in humans. Krizak et al. (Reference Krizak, Frimmel and Bernatova60) have also stated that FO can protect endothelial barrier function during lipopolysaccharide-induced inflammation and exert an anti-inflammatory effect and reduced oxidative response compared with the non-supplemented LPL-infused rats. Liu et al. (Reference Liu, Chen and Odle61) have indicated that FO alleviates E. coli LPS-induced intestinal injury and enhances intestinal integrity in weaned piglets. In another study, Zhang et al. (Reference Zhang, Chen and Tang51) claimed that FO can reduce inflammation and gut permeability in severe cases of immune system failure. In addition, Beguin et al. (Reference Beguin, Errachid and Larondelle62) have noted that DHA supplementation limited the effect of inflammatory stimulus tight junctions and barrier function of intestinal cells. Despite the many available studies regarding the FO effects in different biological experiments that are mentioned above, little knowledge exists regarding the effects of PUFA supplementation and heat-stressed pre-weaned calves.
Serum levels of haptoglobin, serum amyloid A, TNF-α and IFN-γ were higher in the CON diet and reduced with fat supplementation in the starter diet. The effects of heat stress on inflammatory cytokines have been reported in previous studies(Reference Min, Zheng and Zhao63). Min et al. (Reference Min, Zheng and Zhao63) concluded that long-term moderate heat stress caused an inflammatory response in dairy cows, and that plasma levels of TNF-α and IL-6 were increased. Increased adrenocortical function has also been positively associated with circulating haptoglobin concentrations in cattle(Reference Cooke, Bohnert and Cappellozza64). Accordingly, Kim et al. (Reference Kim, Lee and Jeon53) stated that plasma haptoglobin concentrations would increase in heat-stressed calves. From a practical perspective in dairy farms, most of the starter diets offered for dairy calves are formulated based on maize grain and soyabean meal. Hence, the deficiency of n-3 FA is clear in dairy calved fed diets with a high ratio of n-6:n-3 FA at 15:1. As was noted in Hill et al. (Reference Hill, Bateman and Aldrich40), this high ratio can be more critical during heat-stress conditions. Therefore, our results indicate that supplementing the starter diets with FO rich in n-3 FA can improve calf immunity status in heat stress. Greater EPA and DHA in FO supplemented calves show that elevated n-3 FA in the blood can support an improvement in the immune function. In line with our results, McDonnell et al. (Reference McDonnell, John and Doherty39) have reported a significant increase in plasma levels of EPA and DHA in calves fed FO supplemented milk replacer compared with a control group. Lower inflammatory signs in FO supplemented calves can be related to higher long-chain PUFA contents of the plasma(Reference McDonnell, John and Doherty39,Reference Garcia, Shin and Schlaefli41) .
Conclusions
The findings of the present study suggest that supplementing SBO, rich in n-6 FA, in a starter diet (3 %, DM basis) during heat-stress conditions increased starter intake, daily gain, weaning weight and some growth indicators compared with the FO supplemented or un-supplemented starter feeds. However, supplemented FO (3 %, DM basis), rich in n-3 FA, increased the immune function of calves during heat stress and increased FE, which was partly due to the reduced starter intake. To conclude, the results show that a mixture of n-6 and n-3 FA sources (1·5 % SBO and 1·5 % FO, DM basis) may be recommended for milk-fed calves which are being reared under heat-stress conditions to avoid the reduction in starter intake and improve immune system performance.
Acknowledgements
The authors are grateful to the staff of the Moghan Agriculture and Livestock Production Company for their assistance in this experiment.
This work was financially supported mainly by Urmia University. Authors appreciate for partial financial supports done by Kimiya Danesh Alvand Co. 646 (Tehran, Iran).
H. K.-B., M. D.-B. and R. P. designed the study. B. M. conducted the research. B. M. and H. K.-B. analysed the data and wrote the main part of the manuscript. M. K.-B. and E. D. assisted in manuscript preparation. B. M., H. K.-B., M. K.-B. and M. H. G. prepared the revised final manuscript. All authors interpreted the findings and critically reviewed the manuscript. All authors read and approved the final manuscript.
The authors declare that no conflicts of interest exist.















