Fermented milk, such as yogurt and cultured soured milk, has been considered as a functional food and widely consumed over the world for centuries. Fermented milk is a product obtained from the fermentation of milk by the action of suitable micro-organisms and the resultant reduction of pH with or without coagulation (isoelectric precipitation). The commonly used micro-organisms for the fermentation process include Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus and Lactobacillus kefiri. Today, other micro-organisms are increasingly being used to produce fermented milk. Since this fermented milk has been shown to have a number of pleiotropic health benefits, it may be referred to as probiotic fermented milk( Reference Ebringer, Ferencík and Krajcovic 1 ). Evidence from large prospective cohort studies has suggested that probiotic fermented milk may exert protective effects against many chronic diseases, including type 2 diabetes( Reference Tong, Dong and Wu 2 ), CVD( Reference Sonestedt, Wirfalt and Wallstrom 3 ) and stroke( Reference Dalmeijer, Struijk and van der Schouw 4 ). Also, a recent meta-analysis of clinical trials has reported that consumption of probiotic yogurt significantly lowers total cholesterol and LDL-cholesterol concentrations( Reference Guo, Liu and Zhang 5 ). However, a number of clinical trials investigating the effect of probiotic fermented milk on blood pressure (BP) have yielded mixed results( Reference Hata, Yamamoto and Ohni 6 – Reference Usinger, Ibsen and Linneberg 18 ). These inconsistent findings may result from differences in sample size, study intervention, study population or study quality, and therefore a clear view on the overall impact of probiotic fermented milk on BP is difficult to obtain. Thus, in the present study, we aimed to systematically examine the effect of probiotic fermented milk on BP by conducting a meta-analysis of randomised controlled trials.
Experimental methods
Literature search
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in the report of this meta-analysis( Reference Moher, Liberati and Tetzlaff 19 ). We conducted an electronic search of PubMed, Cochrane library and the ClinicalTrials.gov databases up to March 2012 for relevant studies. Search terms included ‘yogurt’, ‘yoghurt’, ‘sour milk’, ‘fermented milk’, ‘probiotic’, ‘blood pressure’ and ‘hypertension’. Reference lists in the retrieved articles obtained from the electronic search were also manually scanned.
Study selection
Studies were included if they (1) were randomised, placebo-controlled trials, (2) used probiotic fermented milk as the intervention product and (3) reported the net changes in BP and the associated standard deviations (or data to calculate them). Because we aimed to examine the effect of probiotic fermented milk, studies using enzymatically hydrolysed milk as the intervention product were excluded. We also excluded studies that had a co-intervention of other supplementations or were duplicate reports from the same trial.
Data extraction and quality assessment
The following characteristics of each study were recorded: first author's last name, publication year and country of origin; design details, including whether parallel or cross-over and open label, single blind or double blind; treatment duration; daily dose of probiotic fermented milk. Participant characteristics including age, sex, baseline BP and antihypertensive medication use were also recorded. If more than one time point for follow-up was reported, we used the data from the longest follow-up time period. Similarly, we used the data from the highest dose when there was more than one single dose for the intervention. One study included two independent strata (hypertensive and normotensive) and was subsequently treated as two trials( Reference Agerholm-Larsen, Raben and Haulrik 7 ).
Study quality was assessed in terms of randomisation, allocation concealment, blinding, description of withdrawals and availability of intention-to-treat analysis.
Statistical analysis
In parallel trials, the net effect of probiotic fermented milk on BP was calculated as the difference in mean systolic BP and diastolic BP change between the intervention and control groups. In cross-over trials, the effect of probiotic fermented milk was calculated as the difference in BP between the intervention and control periods. Studies that did not report standard deviation values had these values imputed from standard errors, CI or P values using a standard formula( 20 ).
The between-study heterogeneity was tested using Cochran's Q test at the P< 0·10 level of significance. The I 2 statistic, a quantitative measure of inconsistency across studies, was also calculated( Reference Higgins and Thompson 21 ). Either a fixed-effects or, in the presence of heterogeneity, a random-effects model was used to calculate the pooled effect size.
To explore the possible influences of study designs and participant characteristics on combined effect sizes, we further conducted pre-specified subgroup analyses stratified by hypertension status, study location and duration of intervention. To test the robustness of the results, we performed sensitivity analyses limited to parallel trials, double-blind trials and trials in which participants did not use antihypertensive medications. In addition, we repeated the analyses by omitting one trial in each turn to investigate the influence of a single trial on the overall effect estimate.
Potential publication bias was assessed using Begg's funnel plots and Egger's regression test at the P< 0·10 level of significance( Reference Egger, Davey Smith and Schneider 22 ). In case of publication bias, a non-parametric ‘trim and fill’ method was used to adjust for this bias( Reference Duval and Tweedie 23 ). Alternatively, we performed a sensitivity analysis in which smaller studies that reported more extreme effect sizes were excluded. All analyses were performed using STATA version 11.0 (StataCorp). P values < 0·05 were considered statistically significant, except where otherwise specified.
Results
Literature search
Fig. 1 shows the flow of the literature search. An initial search of the three electronic databases identified 235 records, of which the majority were excluded based on title and abstract scan, leaving twenty-seven articles for full-text review. Of these articles, fourteen were excluded because they used enzymatically hydrolysed milk or other fermented milk products rather than probiotic fermented milk as the intervention product, had a co-intervention of other supplementations, measured acute effect only or were duplicate reports from the same trial (detailed reasons for the exclusion of these fourteen articles are given in Table S1, available online). Finally, thirteen articles (fourteen trials) were selected for the final analysis.
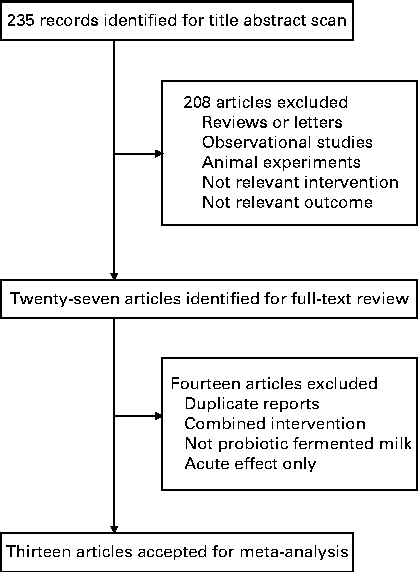
Fig. 1 Flow chart of the study selection.
Study characteristics
The characteristics of studies and participants for the fourteen trials are given in Table 1. The trials were published between 1996 and 2010. Of the fourteen trials, eight were conducted in European countries and the remaining in Japan. The sample size varied from 20 to 108, with a sum of 702 and a median of 40. The treatment was double blind in eleven trials, single blind in two trials and open label in the remaining one trial. All trials but one had a parallel design. The duration of intervention was from 4 to 24 weeks, with a median of 8 weeks. The dose of probiotic fermented milk varied from 100 to 450 g/d, and most of the control groups received a milk-based placebo product (e.g. artificially acidified milk). In eight trials, all participants were hypertensive patients; in three trials, patients used antihypertensive medications.
Table 1 Characteristics of clinical trials examining the effect of probiotic fermented milk on blood pressure (BP)
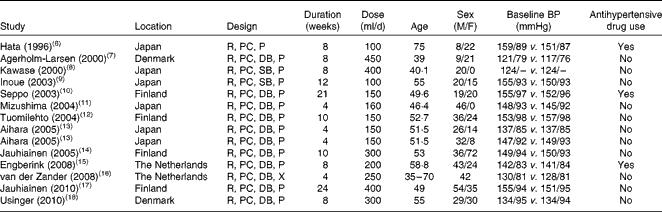
M, male; F, female; R, randomised; PC, placebo controlled; P, parallel; DB, double blind; SB, single blind; –, no information available; X, cross-over.
Study quality differed across the trials (Table SII, available online). All trials reported random allocation, but few of them reported details of sequence generation and allocation concealment. In nine trials, dropout reasons along with numbers were mentioned. Another three trials analysed data according to an intention-to-treat principle.
Main analysis
Of the fourteen trials, thirteen reported a reduction in systolic BP after probiotic fermented milk intervention, with mean net changes ranging from − 1·5 to − 12·4 mmHg. Compared with the control, the pooled effect size was − 3·10 mmHg (95 % CI − 4·64, − 1·56) for systolic BP (Fig. 2), with no evidence of heterogeneity between the trials (P= 0·19 and I 2= 24·1 %).
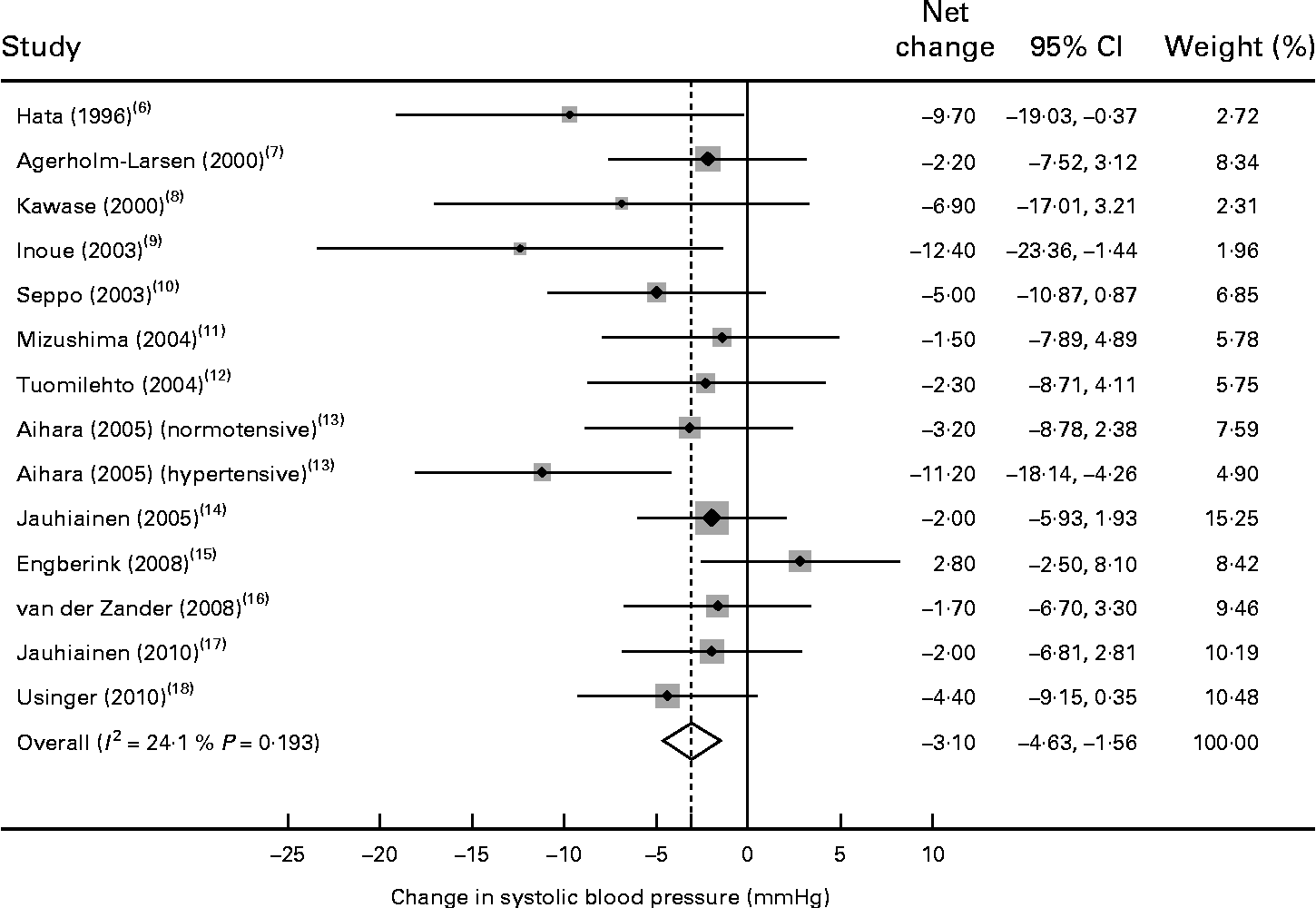
Fig. 2 Meta-analysis of the effect of probiotic fermented milk on systolic blood pressure when compared with placebo.
Of the thirteen trials, one trial( Reference Agerholm-Larsen, Raben and Haulrik 7 ) reported data for systolic BP only and eleven trials reported a reduction in diastolic BP after probiotic fermented milk consumption, with mean net changes ranging from − 0·3 to − 6·5 mmHg. The pooled effect size was − 1·09 mmHg (95 % CI − 2·11, − 0·06) for diastolic BP (Fig. 3), without an indication of heterogeneity (P= 0·15 and I 2= 29 %).
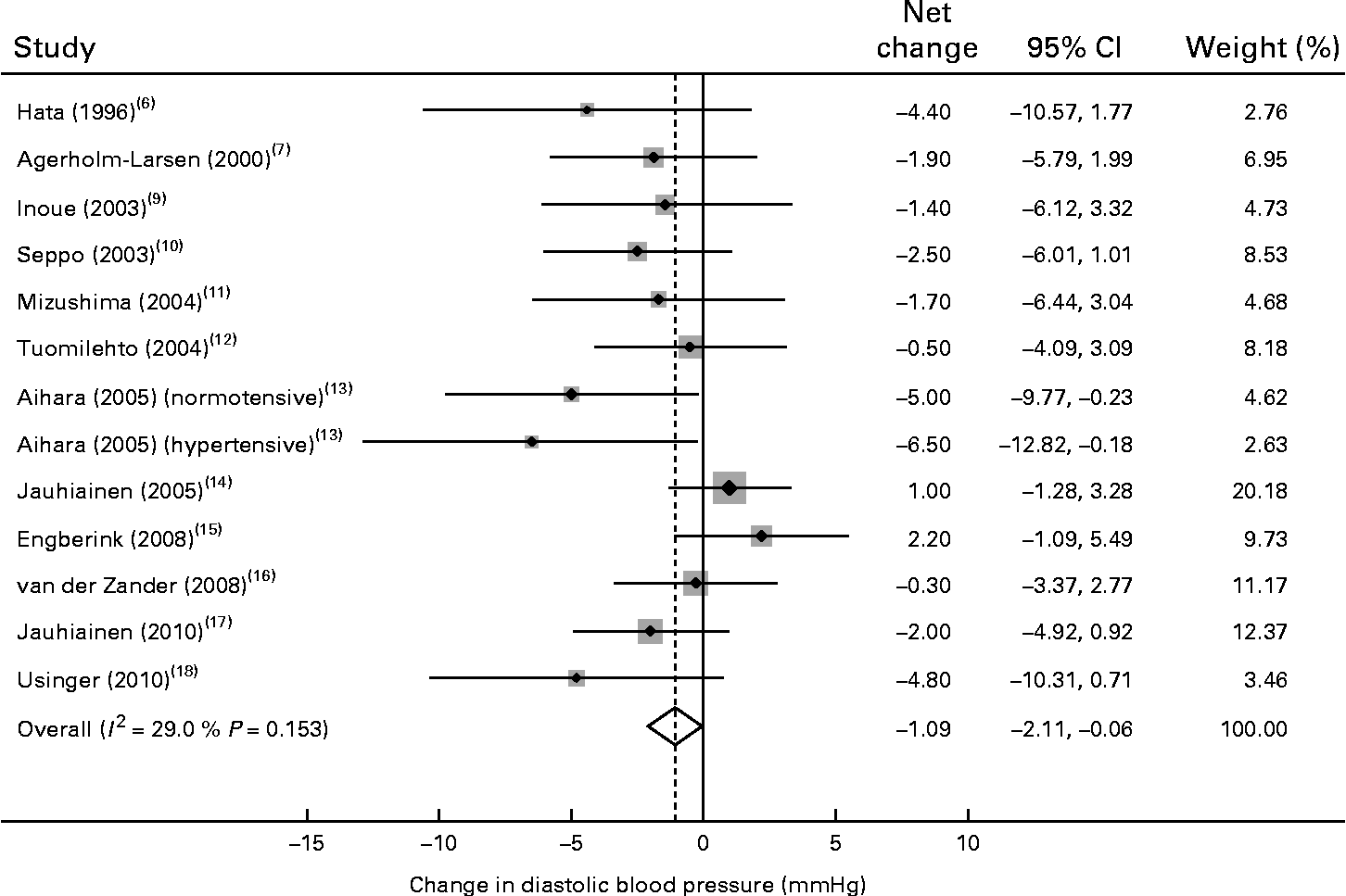
Fig. 3 Meta-analysis of the effect of probiotic fermented milk on diastolic blood pressure when compared with placebo.
Subgroup and sensitivity analyses
Table 2 presents the results of subgroup analyses according to hypertension status, study location and duration of intervention. Hypertensive participants, compared with normotensive ones, experienced a greater reduction in systolic BP ( − 3·98 v. − 2·09 mmHg). Trials conducted in Japan, compared with those carried out in European countries, showed a markedly greater reduction in both systolic BP ( − 6·12 v. − 2·08 mmHg) and diastolic BP ( − 3·45 v. − 0·52 mmHg).
Table 2 Subgroup analyses according to hypertension status, study location and duration of intervention
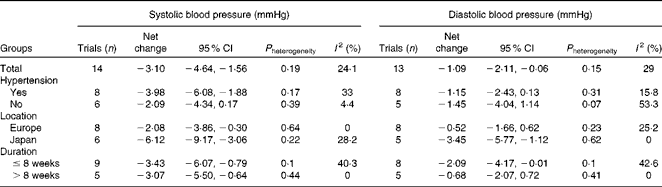
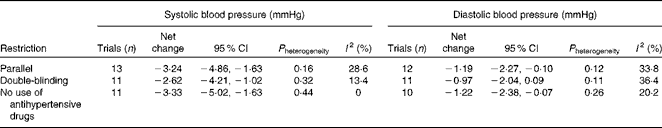
Table 3 presents the results of sensitivity analyses limited to parallel trials, double-blind trials and trials excluding participants using antihypertensive medications. In general, the results were similar with the overall combined effect sizes. Repeated analyses examining the impact of a single trial on the overall results by omitting one trial in each turn yielded a range from − 3·64 mmHg (95 % CI − 5·25, − 2·03) to − 2·68 mmHg (95 % CI − 4·26, − 1·11) for systolic BP and from − 0·90 mmHg (95 % CI − 1·95, 0·15) to − 1·64 mmHg (95 % CI − 2·76, − 0·47) for diastolic BP.
Table 3 Sensitivity analyses restricted to certain trials
Publication bias
Visual inspection of Begg's funnel plots showed some asymmetry, and Egger's test suggested evidence of publication bias (P= 0·02 for systolic BP and P= 0·002 for diastolic BP). However, in a sensitivity analysis using a non-parametric ‘trim and fill’ method, no study was removed and the overall effect size remained unchanged. When small trials showing more extreme results were excluded( Reference Hata, Yamamoto and Ohni 6 , Reference Inoue, Shirai and Ochiai 9 , Reference Aihara, Kajimoto and Hirata 13 ), the pooled effect size was slightly attenuated to − 2·26 mmHg (95 % CI − 3·88, − 0·64) for systolic BP and − 0·69 mmHg (95 % CI − 1·76, 0·39) for diastolic BP. As indicated by Egger's test, publication bias disappeared for systolic BP (P= 0·39), but it may remain for diastolic BP (P= 0·06).
Discussion
The present meta-analysis systematically examined the effect of probiotic fermented milk on BP. Findings from the present study showed that probiotic fermented milk, compared with placebo, produced a significant reduction of 3 mmHg in systolic BP and 1 mmHg in diastolic BP. The magnitudes of BP reductions reported herein are modest. However, on a population level, even a small reduction in BP could have important public health consequences; a 2 mmHg decrease in systolic BP has been shown to be associated with 10 % lower stroke mortality and 7 % lower CHD mortality( Reference Lewington, Clarke and Qizilbash 24 ).
We observed that probiotic fermented milk had a slightly greater effect on systolic BP in hypertensive participants than in normotensive ones ( − 3·98 v. − 2·09 mmHg). This highlights the potential role of probiotic fermented milk in the control and treatment of hypertension. The Dietary Approaches to Stop Hypertension trial demonstrated that a dietary pattern abundant in fruits, vegetables and low-fat dairy products can considerably reduce BP( Reference Appel, Moore and Obarzanek 25 ). Data from the present study provide further support for an important role of probiotic fermented milk in the Dietary Approaches to Stop Hypertension diet. Also, a recent finding based on the Framingham Heart Study Offspring Cohort has demonstrated that normotensive adults who had a high intake of yogurt (>2 % of total daily energy intake) were 31 % less likely to develop incident hypertension over 15 years( 26 ).
Another interesting finding of the present meta-analysis was that study location (Japan v. European countries) may modify the effect of probiotic fermented milk on BP. Analysis of trials conducted in Japan showed a greater reduction than those conducted in European countries for both systolic BP ( − 6·12 v. − 2·08 mmHg) and diastolic BP ( − 3·45 v. − 0·52 mmHg). The average intake of dairy foods in the Japanese population is lower than that in Western populations, and hence the intervention markedly enhanced their milk intake, especially fermented milk intake, which might explain the pronounced hypotensive effect observed in Japanese trials( Reference Usinger, Ibsen and Jensen 27 ). Alternatively, the weaker methodology used in some Japanese trials (e.g. insufficient blinding) might have overestimated the true effect of the treatment. A similar observation was also made for the BP-lowering effect of lactotripeptide consumption among Japanese, Finland and Netherlandish studies( Reference Qin, Xu and Dong 28 ), and ethnic difference in genetic disposition has been suggested to contribute to various degrees of hypertension( Reference Kato 29 ). However, due to a limited number of trials being performed to date, it is still inconclusive to confirm the role of genotype polymorphism in the underlying BP-lowering mechanism in the present study.
Several functional components in probiotic fermented milk may account for the BP-lowering effects. It is also possible that different fermented milk products act via different mechanisms or that a single product acts via multiple mechanisms. Two tripeptides, valine–proline–proline and isoleucine–proline–proline, have been extensively studied( Reference Xu, Qin and Wang 30 ), and, indeed, many products used in the trials included in the present meta-analysis contained them. These tripeptides exert angiotensin-converting enzyme inhibitory activity in vitro and are hypothesised to lower BP through this mechanism( Reference Ricci, Artacho and Olalla 31 ). In fact, two recent meta-analyses of clinical trials have documented mild BP-lowering effects of valine–proline–proline and isoleucine–proline–proline interventions( Reference Xu, Qin and Wang 30 , Reference Cicero, Gerocarni and Laghi 32 ), although the summary effect sizes were relatively weaker in the latter one( Reference Cicero, Gerocarni and Laghi 32 ). The fermented product used in the study carried out by Inoue et al. ( Reference Inoue, Shirai and Ochiai 9 ), however, exhibited no angiotensin-converting enzyme-inhibiting activity. Instead, the authors attributed the BP-lowering effect to the presence of γ-aminobutyric acid, where the test product contained 10–12 mg/ml, which has been shown to lower BP when administered orally to human subjects( 33 ).
Probiotic micro-organisms found in fermented milk, such as Lactobacillus helveticus and L. acidophilus, may influence gut microbiota composition, which is suggested to have an impact on the development of metabolic disorders( Reference Cani and Delzenne 34 ). It has also been suggested that bacterial cell wall fragments may have an antihypertensive effect( Reference Sawada, Furushiro and Hirai 35 , Reference Nakajima, Hata and Osono 36 ). Indeed, studies examined in the present meta-analysis used live micro-organisms in addition to the fermented base and, thus, the effect could have been at least partially attributable to them. However, in the Aihara et al. ( Reference Aihara, Kajimoto and Hirata 13 ) study, the test product displayed BP-lowering effects despite being depleted of bacterial fragments. In addition, no studies on the effect of pure probiotic biomass on BP in human subjects have been published. One study has investigated the effect of fermented rosehip drink on BP, but this product, similar to fermented milk, comprised the fermented matrix and the fermenting micro-organisms, both with potential functional contributions( Reference Naruszewicz, Johansson and Zapolska-Downar 37 ). Finally, some of the BP-lowering effects of fermented milk may derive from the mineral content of milk products, including K, Ca and P, proposed to exhibit an antihypertensive activity( Reference McGrane, Essery and Obbagy 38 ). However, the observed BP-lowering effects were probably not due to these minerals, because they were well balanced between the intervention and control groups in most trials.
To date, several prospective cohort studies have examined the association between fermented milk (mainly yogurt) consumption and the risk of developing hypertension( Reference Steffen, Kroenke and Yu 39 – Reference Engberink, Hendriksen and Schouten 42 ). All these studies have shown an inverse relationship with the relative risk, which ranged from 0·85 to 0·97, but none of them reached statistical significance. We hence combined these data and obtained a summary relative risk of 0·91 (95 % CI 0·84, 1·00; P= 0·04), indicating that higher intake of yogurt, compared with lower intake, was associated with a 9 % reduced risk of developing hypertension. Therefore, the findings of the present meta-analysis of randomised controlled trials are in line with those from cohort studies, both supporting a hypotensive effect of fermented milk.
The major strength of the present study is that all the included trials had a randomised, placebo-controlled design. Such a design minimises the risk of confounding bias, which is of great concern in observational studies. In addition, using a meta-analysis approach, we enlarged the sample size and hence increased the statistical power. However, potential limitations involved in either primary studies or the present meta-analysis should be considered while interpreting the findings. First, we included only published studies in the meta-analysis, raising the risk of publication bias. Funnel plots for both systolic BP and diastolic BP showed evidence of possible bias, favouring the publication of small trials with extreme effects. However, these trials had small weights in the present meta-analysis and excluding them from the analysis led to only a slight attenuation of the effect on systolic BP. Second, as with any meta-analysis, the validity depended upon the quality of primary studies. Although all trials were randomised and placebo controlled, the study quality varied; for example, the lack of double-blinding in several trials increased the risk of expectation bias( Reference Hata, Yamamoto and Ohni 6 , Reference Kawase, Hashimoto and Hosoda 8 , Reference Inoue, Shirai and Ochiai 9 ). Nevertheless, sensitivity analyses limited to double-blind trials yielded similar results. Third, most trials had a relatively small sample size (n < 60) and short treatment duration ( < 3 months). As has been mentioned previously, small studies with more extreme results may have increased the risk of publication bias, posing a potential threat to the validity of the present meta-analysis. Because of the short duration, whether the findings could translate into a long-term treatment effect is uncertain.
Conclusion
In summary, the present meta-analysis suggested that probiotic fermented milk has BP-lowering effects in pre-hypertensive and hypertensive subjects. However, while the role of fermented milk bioactives in BP lowering is well studied, the potential contribution to BP lowering by live micro-organisms exerting a probiotic effect remains to be elucidated in human subjects. Consequently, for the time being, the term ‘probiotic’, defined as ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’, is best avoided in connection with BP-lowering fermented milk. Well-designed, large-scale trials are required to confirm these findings.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513001712
Acknowledgements
The present study was funded by Nestec Limited (Nestlé R&D (China) Limited). L.-Q. Q. and Y. Z. designed the research. J.-Y. D. and L.-Q. Q. conducted the research. J.-Y. D. analysed the data. All authors wrote the paper. L.-Q. Q. and Y. Z. had the primary responsibility for the final content. None of the authors has any conflicts of interest with respect to the present study.








