Head-and-neck cancer comprises tumours in the paranasal sinuses, salivary glands, nasal cavity, oral cavity, pharynx and larynx. In 2018, over 800 000 new cases and over 400 000 head-and-neck cancer deaths were estimated worldwide(Reference Bray, Ferlay and Soerjomataram1). Treatment of this condition may include surgery, radiotherapy and chemotherapy, which may be associated with side-effects that could influence nutritional intake. Moreover, other factors may further contribute to unintentional weight loss and malnutrition, including cancer cachexia, anorexia, increase in nutritional needs, psychological factors, besides a mechanical obstruction caused by tumour location(Reference Bressan, Stevanin and Bianchi2). Malnutrition and weight loss in head-and-neck cancer patients undergoing treatment is associated with worse prognosis, decreased treatment tolerance and deterioration in the quality of life(Reference van Bokhorst-de van der, van Leeuwen and Kuik3–Reference Gellrich, Handschel and Holtmann6).
Nutritional counselling is already established as first-line nutritional intervention in cancer patients(Reference Arends, Bachmann and Baracos7,Reference Arends, Baracos and Bertz8) , which includes advice on how to manage symptoms and to improve nutritional intake. Oral nutritional supplements may also be used to deliver mainly energy and protein to patients who face difficulties in reaching their nutritional needs only by eating an enriched diet. Previous research has indeed shown that oral supplements might increase nutritional intake(Reference Baldwin, Spiro and Ahern9); however, there is still uncertainty as to whether this intervention affects patient-important outcomes such as mortality, treatment tolerance and quality of life. The UK National Multidisciplinary Guidelines provide a comprehensive guidance on the nutritional management of head-and-neck cancer patients(Reference Talwar, Donnelly and Skelly10) and reaffirm the importance of considering quality of life and treatment tolerance among the aims of nutritional interventions. However, during its development, only limited evidence was available to inform their recommendations on oral nutritional interventions.
Previous literature reviews took varied approaches to investigate nutritional interventions in this patient group. In a systematic review published in 2013(Reference Langius, Zandbergen and Eerenstein11), the authors examined the impact of several nutritional interventions on nutritional status, quality of life and mortality. Assessing the relevant randomised controlled trials revealed inconsistent or lack of evidence for the effect of oral nutritional supplements, preventing the authors from conducting a meta-analysis. Moreover, the author of a narrative review published in 2015(Reference Bossola12) relied on both randomised and non-randomised designs to study the impact of several nutritional interventions, including nutritional counselling and oral nutritional supplements, on treatment-related toxicities, survival and on the prevention and treatment of malnutrition. Even though promising results were found in the primary literature, no systematic approach to assessing bias or the overall certainty of evidence was employed, precluding any firm conclusions. Nevertheless, this work validly signalised the limited availability of data on treatment tolerance outcomes, favouring the planning of future trials.
In light of these findings, this study provides an opportunity to advance our understanding of the effects of oral nutritional supplements and to address the gaps and limitations in the current body of evidence. Therefore, this study aims to systematically review the evidence of the effects of oral nutritional supplements in head-and-neck cancer patients receiving radiotherapy or chemoradiotherapy on their mortality, treatment tolerance and quality of life, besides additional outcomes such as functional status, body weight and adverse effects relating to the intervention.
Materials and methods
This review followed the guidance provided in the sixth revision of the Cochrane Handbook for Systematic Reviews of Interventions (Reference Higgins, Thomas and Chandler13). We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines(Reference Liberati, Altman and Tetzlaff14).
Protocol and registration
This systematic review is registered in PROSPERO (CRD42018118972).
Eligibility criteria
We included randomised controlled trials on head-and-neck cancer patients aged >18 years, receiving any type of chemotherapy, radiotherapy or concurrent chemoradiotherapy, regardless of surgery status. We considered studies on oral nutritional supplements, defined as calorie-protein-rich enteral food supplements used to manage disease-related malnutrition. Oral nutritional supplements containing nutrients such as n-3 fatty acids, arginine, glutamine and micronutrients were also considered eligible. The oral nutritional supplement intervention could additionally contain any form of dietary prescription, counselling, general advice or usual care. Studies on alternative routes of enteral nutrition (e.g. tube feeding, gastrostomy feeding) and parenteral nutrition were not included. Comparator groups that were not assigned to receive oral nutritional supplements were eligible for inclusion. There were no restrictions on language, publication time or status. Even though we have predefined outcomes of interest, all studies fulfilling the population, intervention and comparator eligibility criteria were considered eligible.
Outcomes of interest
Outcomes of interest were mortality, tolerance to treatment (suspension of treatment, interruption of treatment, dose reduction, non-haematological toxicities), quality of life, functional status, body weight and adverse effects relating to the supplements. We defined the timing of outcome assessment to be at the end of anti-cancer treatment, and at the longest follow-up after the end of treatment for mortality and long-term non-haematological toxicities.
Search methods for the identification of studies
We searched the following databases from inception until 27 July 2019: MEDLINE via PubMed (1946 onwards); Cochrane Central Register of Controlled Trials (CENTRAL; 2019, issue 7) and Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library; Embase (1974 onwards); CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1980 onwards); ISI Web of Science: Core Collection (1945 onwards); and LILACS via Virtual Health Library (VHL) (Latin American and Caribbean Health Science Information database; 1982 onwards). We also searched the following trial registers on 27 July 2019: ClinicalTrials.gov, www.clinicaltrials.gov/; and the International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp/en/. Attempts of searching the grey literature included the following sources: OpenGrey, www.opengrey.eu/; Google Scholar, www.scholar.google.com/ (the first 200 results); and ProQUEST Dissertations & Theses Global (1637 onwards). Other resources included reviewing reference lists of all included studies, and attempts to contact the authors of relevant studies to clarify published information and to seek unpublished results.
One author (A. T. M.) of this review developed the search strategies, which were peer-reviewed by two information specialists from the university library at the Federal University of Santa Catarina, Brazil. We included broad concepts relating to or describing the population and the intervention to maximise sensitivity, alongside database-specific filters for controlled trials of interventions where appropriate and if available and which had been previously tested. The search strategies contained both controlled vocabulary terms (e.g. MeSH, Emtree) and free-text terms considering spelling variants, synonyms, acronyms and the use of truncation and proximity operators. We applied no language or time limits. All strategies used are provided in online Supplementary Table S1.
Selection of studies
One author (A. T. M.) of this review imported all references identified by the search process into the software (Mendeley) and removed duplicates. Two authors (A. T. M. and J. P.) independently used the online software Rayyan(Reference Ouzzani, Hammady and Fedorowicz15) to screen the titles and abstracts of all retrieved references, and to identify potentially eligible records. We obtained the full text of agreed references and collated multiple reports of the same study. We assessed eligibility through a standardised form created in Google Forms, and resolved disagreements through consensus or by involving a third author (E. B. S. M. T.).
Data collection
At least two authors (A. T. M., L. P. d. L., D. S. B. and P. V. K.) extracted data from each included study independently and in duplicate. We used a pre-piloted electronic data extraction form created in Google Forms. We collected data relating to the methods (design, unit of allocation and analysis, recruitment method and period, handling of missing data, statistical methods, random sequence generation, allocation concealment, blinding), participants (setting, region and country, inclusion and exclusion criteria, baseline characteristics of interest), interventions (description, number randomised to each group, duration of intervention and follow-up, co-interventions, integrity of delivery, compliance assessment and result), outcomes (domain, measurement tool, direction of effect for scales, timing of measurements, outcome assessor, metric used, method of aggregation), results (number of participants included in the analysis, number of participants who withdrew, were lost to follow-up or were excluded and reasons, summary data, between-group estimates when available, other results) and other general data (funding sources, declaration of interest and notes on any other information judged to be of importance). We extracted data that were only available in Chinese using Google Translate.
Assessment of risk of bias
Two review authors (A. T. M. and D. S. B.) assessed the risk of bias in included studies independently and in duplicate using the revised Cochrane risk-of-bias tool for randomised trials (RoB 2)(Reference Higgins, Sterne and Savović16). The domains of the tool include bias arising from randomisation, bias due to deviation from intended interventions, bias due to missing outcome data, bias in outcome measurement and bias in the selection of reported results. In accordance with the tool guidance, we performed assessments for each result of each time-point of each outcome. We piloted the tool on two results of two studies to improve the reliability of assessments. We resolved disagreements through consensus or by involving a third author (E. B. S. M. T.). Two authors (A. T. M. and P. V. K.) performed and tabulated the assessment of risk of bias due to missing results and of conflicts of interest using previously extracted information from included studies.
Assessment of the certainty of evidence
Two authors (A. T. M. and D. S. B.) assessed the certainty of evidence independently and in duplicate in accordance with the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach(Reference Schünemann, Brożek and Guyatt17). For each comparison, we judged the evidence for each outcome from the summary of findings as ‘high’, ‘moderate’, ‘low’ and ‘very low’, reflecting our assessment of the following domains: risk of bias, inconsistency, indirectness, imprecision and publication bias. This classification was incorporated in the presentation and interpretation of review findings. We used GRADEpro(18) to generate a summary of findings.
Synthesis of results
One author (A. T. M.) summarised the characteristics of included studies based on previously extracted data and itemised the characteristics of interventions in a standardised way across studies. In order to identify possible comparisons and to group studies within each comparison, we prepared a matrix including the main PICO (Population, Intervention, Comparison, and Outcome) elements of interest. At this stage, we determined what data were available for synthesis and whether statistical synthesis or structured reporting of the effects was appropriate. Structured reporting included the calculation of effect estimates for all outcomes using a common metric to aid in the interpretation and presentation of a forest plot or in a tabular format. For the interpretation of results, we considered the direction of effect, size of effect and the certainty of evidence.
Statistical analysis
We performed statistical analysis when the studies contributing to each synthesis were similar in terms of their PICO elements and the data available were sufficient. We restricted the analysis to the results of studies at a low risk of bias or at some concerns of bias, when possible, and performed a sensitivity analysis including all available results. For the meta-analysis, we combined the risk ratios (RR) for dichotomous data and mean differences for continuous data using random-effects models, because we assumed that the intervention effects were not expected to be truly identical. We used the Hartung–Knapp correction in all analyses. We assessed statistical heterogeneity using I 2 and χ 2 statistical tests. To interpret I 2, we considered the following definitions: 0–40 % might not be important; 30–60 % may represent moderate heterogeneity; 50–90 % may represent substantial heterogeneity; and 75–100 % represents considerable heterogeneity(Reference Deeks, Higgins and Altman19). In the review protocol, we planned a subgroup analysis to explore possible explanations for substantial and considerable heterogeneity. When this was not feasible (e.g. very few studies in the analysis), we did not perform a meta-analysis but conducted a structured reporting of the effects. To interpret the χ 2 test, P ≤ 0·05 indicated evidence of heterogeneity. When data were not presented in a way appropriate for inclusion in a meta-analysis, we performed conversions (e.g. from CI to standard deviation) in accordance with recommended methods(Reference Higgins, Li and Deeks20). We inputted means and standard deviations from medians, ranges and/or interquartile ranges, where applicable, for the results of quality of life in one trial(Reference Jiang, Ding and Li21), and for the results of body weight in one trial(Reference Chitapanarux, Pisprasert and Tharavichitkul22), as proposed by Wan et al.(Reference Wan, Wang and Liu23) We approximated mean and standard error from figures for the results of body weight in one trial, using WebPlotDigitizer(Reference Rohatgi24). For all the analyses, we used R(25) and the package meta(Reference Schwarzer26). A report of full analysis is included as Supporting Information, presenting all forest plots, risk-of-bias assessments for each analysis, besides the full analysis code. We considered P < 0·05 as indicating statistical significance.
Differences between protocol and review
Changes that occurred during the review process are reported in the order of increasing transparency. We did not use Scopus as, although we planned to use it in an attempt to compensate for Embase, the latter eventually became available to us. Google Scholar was additionally searched to improve our search of grey literature. We removed the exclusion criteria ‘no dietary intervention’ for comparator groups, as it conflicted with the inclusion criteria ‘ad libitum diet without oral nutritional supplements’. The outcomes of interest do not fully correspond to our protocol, since we redefined them a posteriori to better suit our goal of emphasising patient-important outcomes, and to keep the review in a manageable size. We resolved this by discussion, considering evidence about head-and-neck cancer patients’ preferences(Reference Blanchard, Volk and Ringash27) and our clinical experience. All steps were also planned to be performed by three review authors, but this was not possible because one of the authors left the project.
Results
Literature search
The search retrieved thirty references for fifteen eligible trials(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Arnold and Richter28–Reference Caccialanza, Pedrazzoli and Cereda54) , of which five(35,36,38–40) were study registries of ongoing or unpublished studies (online Supplementary Table S2). The remaining ten studies randomised a total of 695 participants, contributing to four comparisons:
-
(1) Comparison one: nutritional counselling plus oral nutritional supplements v. nutritional counselling alone (five studies(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Arnold and Richter28,Reference Cereda, Cappello and Colombo32,Reference Nayel, El-Ghoneimy and El-Haddad41) , 399 participants);
-
(2) Comparison two: nutritional counselling plus oral nutritional supplements v. ad libitum diet (two studies(Reference Moriarty, Moloney and Mulgrew29,Reference Ding, Dou and Wang33) , 115 participants);
-
(3) Comparison three: oral nutritional supplements v. ad libitum diet (three studies(Reference Ravasco, Monteiro-Grillo and Marques Vidal30,Reference Calaguas31,Reference Harada, Minami and Ferdous34) , 156 participants); and
-
(4) Comparison four: oral nutritional supplements v. nutritional counselling (one study(Reference Ravasco, Monteiro-Grillo and Marques Vidal30), fifty participants).
One study(Reference Ravasco, Monteiro-Grillo and Marques Vidal30) contributed to more than one comparison. We were unable to obtain the full text of two potentially eligible references(Reference Han, Yang and Zhao55,Reference Melzner, Grabenbauer and Schwab56) and to assess the eligibility of two studies reported in conference abstracts(Reference Huang, Cao and Piao57,Reference Zhang, Xie and Huang58) and five clinical trial registries(35,36,38–40) due to insufficiently reported information, so these citations are awaiting classification. We attempted to contact the study authors but have not received any response. The search process is represented in Fig. 1.
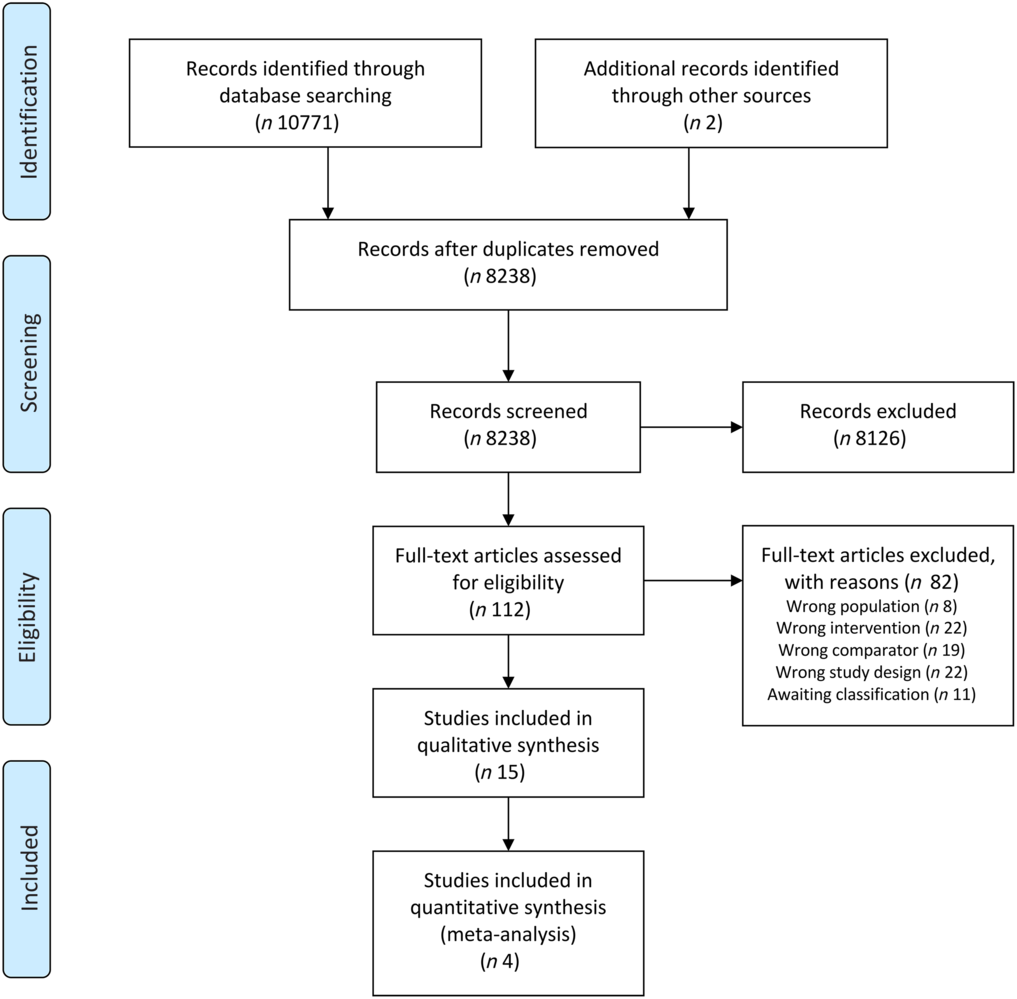
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Representation of information flow in the systematic review.
Included studies
The characteristics of included studies are presented in Table 1. All studies included patients with head-and-neck cancer, but some of them focused on specific tumour sites, namely nasopharyngeal cancer(Reference Jiang, Ding and Li21,Reference Ding, Dou and Wang33) and oral cancer(Reference Harada, Minami and Ferdous34). One study(Reference Calaguas31) presented a more heterogeneous population comprising cancers of head and neck, chest and mediastinum, and abdomen and pelvis, although it did not contribute to outcome data. Participants’ mean or median age ranged from 46·7 to 70·8 years (median 55·2 years). In all studies, most participants were male.
Table 1. Characteristics of included studies*
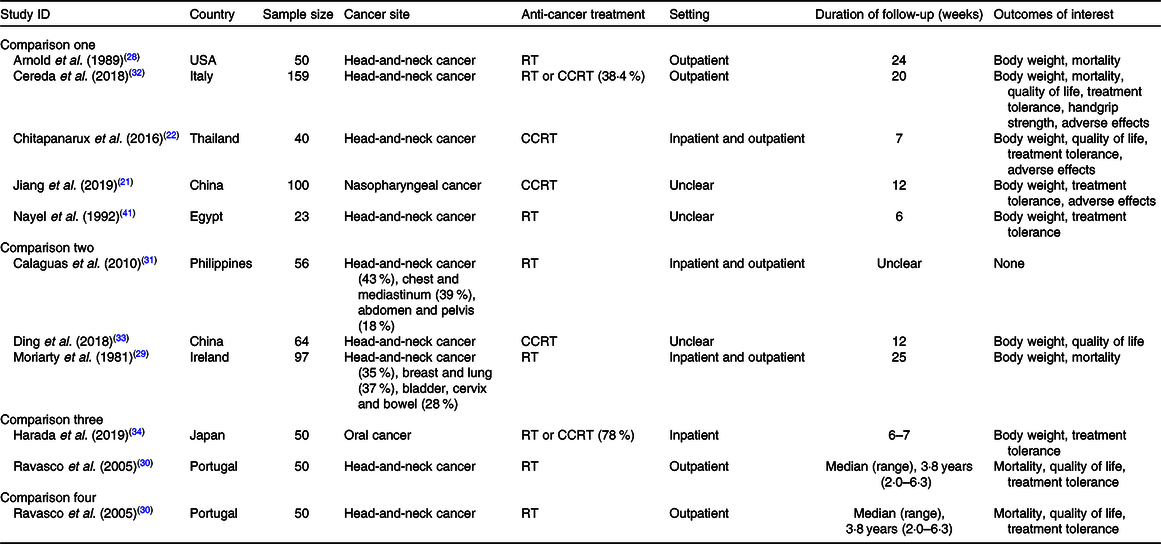
RT, radiotherapy; CCRT, concurrent chemoradiotherapy.
* Information collated from multiple reports of included studies, including full-text articles, conference abstracts and personal communication with study authors.
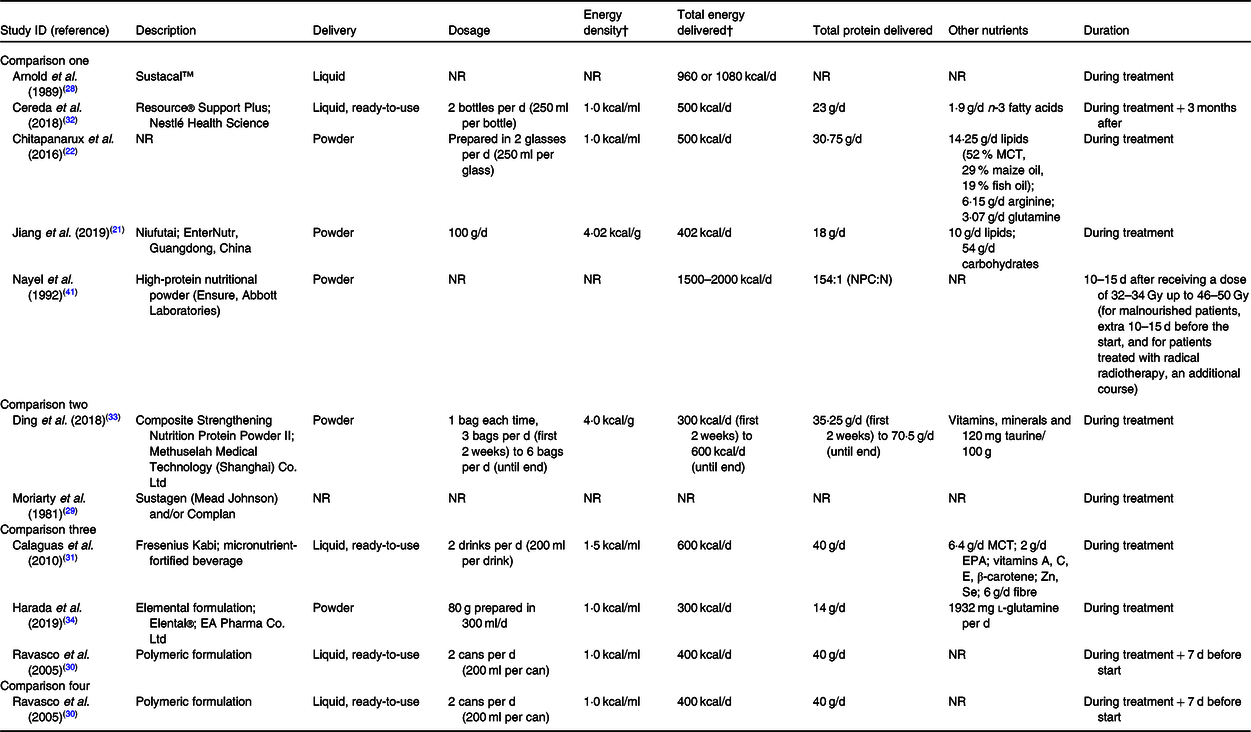
The characteristics of oral nutritional supplements studied are presented in Table 2. They varied in terms of format, dosage, energy and protein content, besides overall composition. The nutritional counselling component of the interventions was described in many different ways, and seemed to vary in intensity and content, from general dietary advice to intensive nutritional counselling. Moreover, it was not sufficiently described in most studies. The characteristics of nutritional counselling are presented in online Supplementary Table S3.
Table 2. Characteristics of oral nutritional supplements in the included studies*

NR, not reported; MCT, medium-chain TAG; NPC:N, non-protein calorie-to-nitrogen ratio.
* Information collated from multiple reports of included studies, including full-text articles, conference abstracts and personal communication with study authors.
† To convert kcal to kJ, multiply by 4·184.
Of the outcomes considered in this review, body weight was most commonly reported in seven studies(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Arnold and Richter28,Reference Cereda, Cappello and Colombo32-Reference Harada, Minami and Ferdous34,Reference Nayel, El-Ghoneimy and El-Haddad41) . By contrast, in comparisons two and three, only one study(Reference Ding, Dou and Wang33,Reference Harada, Minami and Ferdous34) reported this outcome, and in comparison four, none did. The assessment of treatment tolerance varied across studies, including outcomes such as complete suspension of treatment, interruption of treatment, dose reduction, besides many treatment-related toxicities. One study(Reference Calaguas31) in comparison three presented data on Hb, total leucocytes, neutrophils, lymphocytes and platelet counts (haematological toxicities), which are not considered in this review. The assessment of overall completeness of the evidence regarding the outcomes of interest of this review is presented in online Supplementary Table S3.
Risk of bias in included studies
A large proportion of the assessed results were at a high risk of bias overall (Fig. 2). Detailed explanations for each judgement and responses to each signalling question are available in online Supplementary Table S4. In comparison one, we judged only seven results to be at low risk of bias overall: body weight (at both time-points), functional status (at both time-points), temporary interruption of radiotherapy for ≥5 d, radiotherapy dose reduction and chemotherapy dose reduction. In comparisons two, three and four, the body of evidence consisted only of results at a high risk of bias overall. Results were most often at a high risk of bias in the measurement of outcomes, and this was related to the subjectivity of the assessments of quality of life and non-haematological toxicities in the context of an unblinded trial of a nutritional intervention that may be expected to confer some benefit. In addition, most results were at concerns of bias in the selection of reported results, because a study protocol was often unavailable or lacked important information.

Fig. 2. Assessments of risk of bias for each comparison using the revised Cochrane risk-of-bias tool for randomised trials (RoB 2). Bar charts representing the proportions of results at a low risk of bias, some concerns and a high risk of bias for all the domains assessed in each comparison. ![]() , Low risk of bias;
, Low risk of bias; ![]() , some concerns;
, some concerns; ![]() , high risk of bias.
, high risk of bias.
Risk of bias at the synthesis level
Regarding bias due to missing results, we found one result for body weight in comparison two that was not available because it was statistically non-significant. We could not rule out that most results were unavailable because of the nature of the findings, as it was unclear whether they were assessed (online Supplementary Table S5). Not all study authors responded to our attempts of get clarified this information, and some study authors could not be reached, namely those of studies reported before 1992. Regarding conflicts of interest, our assessments were made more challenging by the absence of declarations of interest in many of the included studies. One study(Reference Cereda, Cappello and Colombo32) was judged to be at notable concern about the conflict of interest. Even though sponsors only contributed the provision of oral nutritional supplements, the main authors had noteworthy relationships with companies interested in such products (online Supplementary Table S6).
Summary of findings
Summary of findings for comparisons one, two, three and four are presented in Tables 3, 4, 5 and 6 following GRADE guidelines.
Table 3. Summary of findings for comparison one: nutritional counselling plus oral nutritional supplements compared with nutritional counselling alone
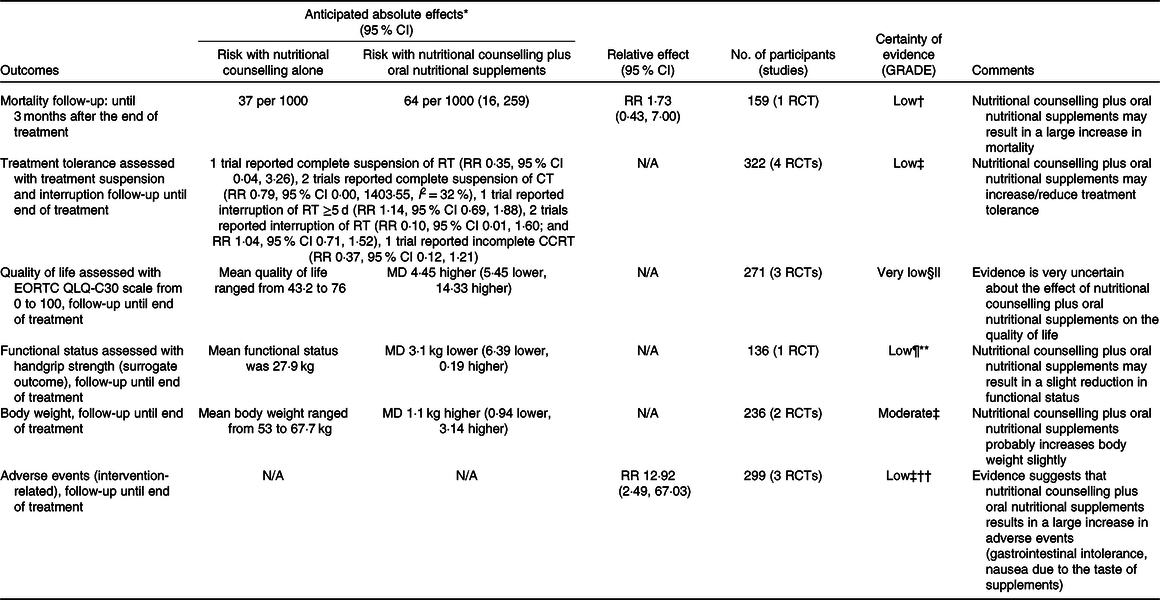
GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio; RCT, randomised controlled trial; RT, radiotherapy; N/A, not applicable; CT, chemotherapy; CCRT, concurrent chemoradiotherapy; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire; MD, mean difference.
* Risk in the intervention group (and its 95 % CI) is based on the assumed risk in the comparison group and the relative effect of intervention (and its 95 % CI).
† Downgraded two levels because there were very few events, optimal information size criterion was not met and the CI included both appreciable benefit and appreciable harm.
‡ Downgraded two levels because for most outcomes there were few events, optimal information size criterion was not met and the CI included both appreciable benefit and appreciable harm.
§ Downgraded two levels because of three studies with an overall high risk of bias, including a high risk of bias due to missing outcomes and outcome measurement (subjective, unblinded and possible expectations of benefits from oral nutritional supplements).
|| Optimal information size criterion was met, the CI did not exclude no effect and included appreciable benefit (5·4 points for improvement and −6·5 points for deterioration).
¶ Downgraded because handgrip strength is a surrogate outcome for an actual outcome of interest.
** Downgraded because even though the optimal information size criterion was met, the CI did not exclude no effect and included appreciable harm (5–6·5 kg).
†† Downgraded because of an overall high risk of bias, due to missing outcome data and outcome measurement (subjective and unblinded).
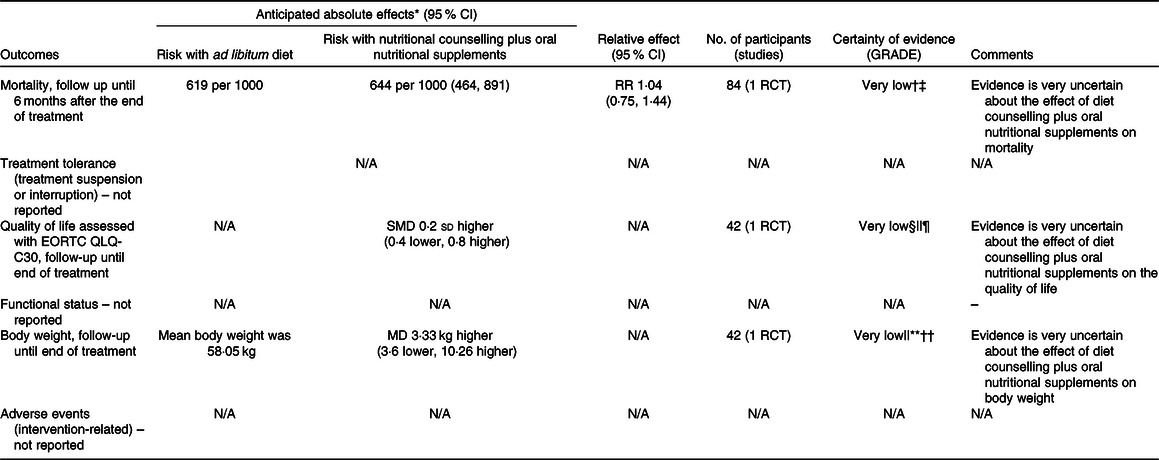
Table 4. Summary of findings for comparison two: nutritional counselling plus oral nutritional supplements compared with ad libitum diet
GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio; RCT, randomised controlled trial; N/A, not applicable; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire; SMD, standard mean difference; MD, mean difference.
* Risk in the intervention group (and its 95 % CI) is based on the assumed risk in the comparison group and the relative effect of intervention (and its 95 % CI).
† Downgraded because of results with an overall high risk of bias (due to randomisation, some concern on deviations from intended interventions, and the selection of reported results).
‡ Downgraded two levels because there were very few events, optimal information size criterion was not met and the CI included both appreciable benefit and appreciable harm.
§ Downgraded because of results with an overall high risk of bias (due to missing outcome data and outcome measurement – subjective and unblinded study, and some concerns on randomisation and the selection of reported results).
|| Downgraded because the study population only comprised nasopharyngeal cancer patients.
¶ Optimal information size criterion was met, but the CI did not exclude no effect, and included appreciable benefit or appreciable harm.
** Downgraded because of results with an overall high risk of bias (due to missing outcome data and some concerns on randomisation and the selection of reported results).
†† Downgraded because the optimal information size criterion was not met.
Table 5. Summary of findings for comparison three: oral nutritional supplements compared with ad libitum diet in head-and-neck cancer patients undergoing chemoradiotherapy

GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio; RCT, randomised controlled trial; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire; N/A, not applicable; MD, mean difference.
* Risk in the intervention group (and its 95 % CI) is based on the assumed risk in the comparison group and the relative effect of intervention (and its 95 % CI).
† Downgraded because of results with an overall high risk of bias (due to deviations from intended interventions and missing outcome data, and some concerns on the selection of reported results).
‡ Downgraded two levels because there were very few events, optimal information size criterion was not met and the CI included both appreciable benefit and appreciable harm.
§ Downgraded because of results with some concerns of bias due to randomisation and the selection of reported results.
|| Downgraded because the study population only comprised oral cancer patients.
¶ Downgraded because of results with an overall high risk of bias (due to outcome measurement – subjective and unblinded study, and some concerns of bias due to the selection of reported results).
** Downgraded because the optimal information size criterion was not met.
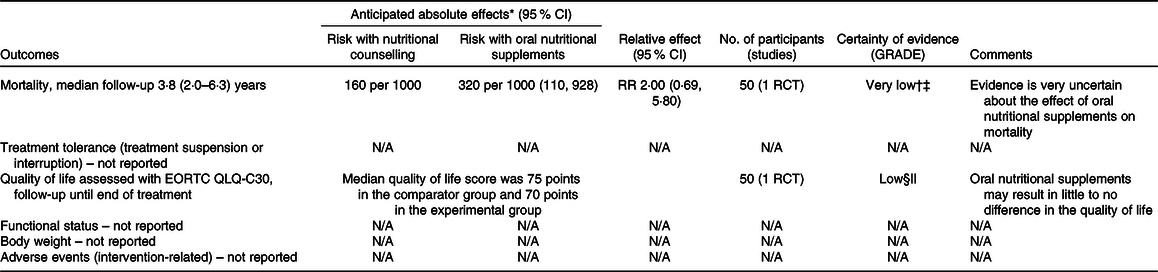
Table 6. Summary of findings for comparison four: oral nutritional supplements compared with nutritional counselling
GRADE, Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio; RCT, randomised controlled trial; N/A, not applicable; EORTC QLQ-C30, European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire.
* Risk in the intervention group (and its 95 % CI) is based on the assumed risk in the comparison group and the relative effect of intervention (and its 95 % CI).
† Downgraded because of results with an overall high risk of bias (due to deviations from intended interventions and missing outcome data, and some concerns on the selection of reported results).
‡ Downgraded two levels because there were very few events, optimal information size criterion was not met and the CI included both appreciable benefit and appreciable harm.
§ Downgraded because of results with an high risk of bias (due to outcome measurement – subjective and unblinded study, and some concerns of bias due to the selection of reported results).
|| Downgraded because the optimal information size criterion was not met.
Effect of interventions on mortality
Four studies reported data on mortality in comparison one, but one study(Reference Jiang, Ding and Li21) contributed no events. In the remaining comparisons, one study in each comparison reported data on this outcome. Considering only the study with results attracting concerns of risk of bias(Reference Cereda, Cappello and Colombo32), we found less certain evidence that nutritional counselling plus oral nutritional supplements compared with nutritional counselling alone may result in a large increase in mortality (RR 1·73, 95 % CI 0·43, 7·00; one study, 159 participants). Sensitivity analysis involving results at a high risk of bias overall had little effect on the point estimate, and produced an even more imprecise estimate of the effect (RR 1·77, 95 % CI 0·11, 29·48, I 2 = 7 %; three studies, 249 participants). The inclusion of results at a high risk of bias may have also warranted a downgrade in the certainty of evidence. In the remaining comparisons, we cannot tell from the results whether any of the interventions compared with any of the comparator groups had an important effect on mortality (very low certainty evidence). Fig. 3 presents the main results for this outcome.
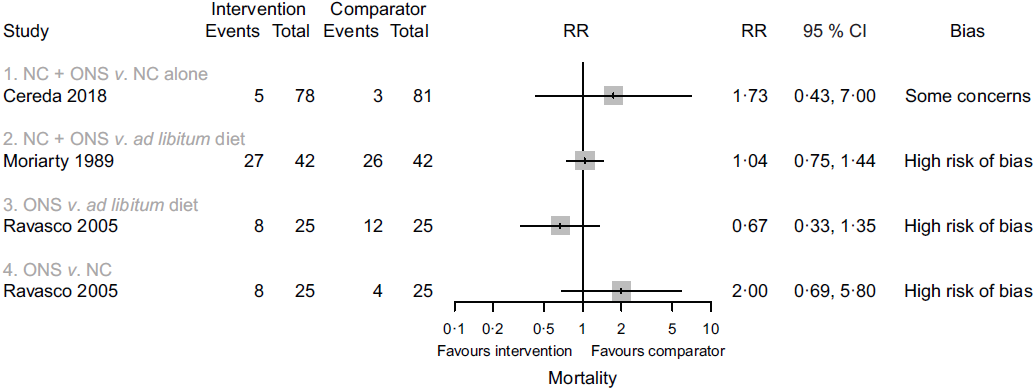
Fig. 3. Forest plot of structured reporting of the effects of interventions on mortality. RR, risk ratio; NC, nutritional counselling; ONS, oral nutritional supplements.
Effect of interventions on treatment tolerance
In comparison one, four studies(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Cereda, Cappello and Colombo32,Reference Nayel, El-Ghoneimy and El-Haddad41) reported outcomes relating to anti-cancer treatment tolerance. We found low certainty evidence that nutritional counselling plus oral nutritional supplements compared with nutritional counselling alone may result in a large reduction in complete suspension of radiotherapy (RR 0·35, 95 % CI 0·04, 3·26; one study, 159 participants), a reduction in complete suspension of chemotherapy (RR 0·79, 95 % CI 0·00, 1403·55, I 2 = 32 %; two studies, 161 participants), and a large reduction in incomplete chemoradiotherapy (RR 0·37, 95 % CI 0·12, 1·21; one study, forty participants). We also found low certainty evidence of little to no difference in the interruption of radiotherapy ≥5 d (RR 1·14, 95 % CI 0·69, 1·88; one study, 159 participants) and of both a large reduction and little to no difference in any interruption of radiotherapy (RR 0·10, 95 % CI 0·01, 1·60; one study, twenty-three participants; and RR 1·04, 95 % CI 0·71, 1·52; one study, 159 participants). Sensitivity analysis involving the results at a high risk of bias overall had a considerable effect on our findings, because the included study found a large increase in any interruption of radiotherapy (RR 9·00, 95 % CI 0·50, 162·89; one study, 100 participants), although it would have decreased our certainty in the evidence. Moreover, we found low certainty evidence of a large reduction in radiotherapy dose (RR 0·17, 95 % CI 0·02, 1·40; one study, 159 participants) and of a large reduction in chemotherapy dose (RR 0·47, 95 % CI 0·21, 1·07; one study, sixty-one participants). In comparison three, one study reported an interruption of chemoradiotherapy, but the evidence was of very low certainty, and we cannot tell whether oral nutritional supplements compared with ad libitum diet had an important effect. In comparisons two and four, there was no available evidence for outcomes relating to the success of anti-cancer treatment. Fig. 4 presents the main results for these outcomes.
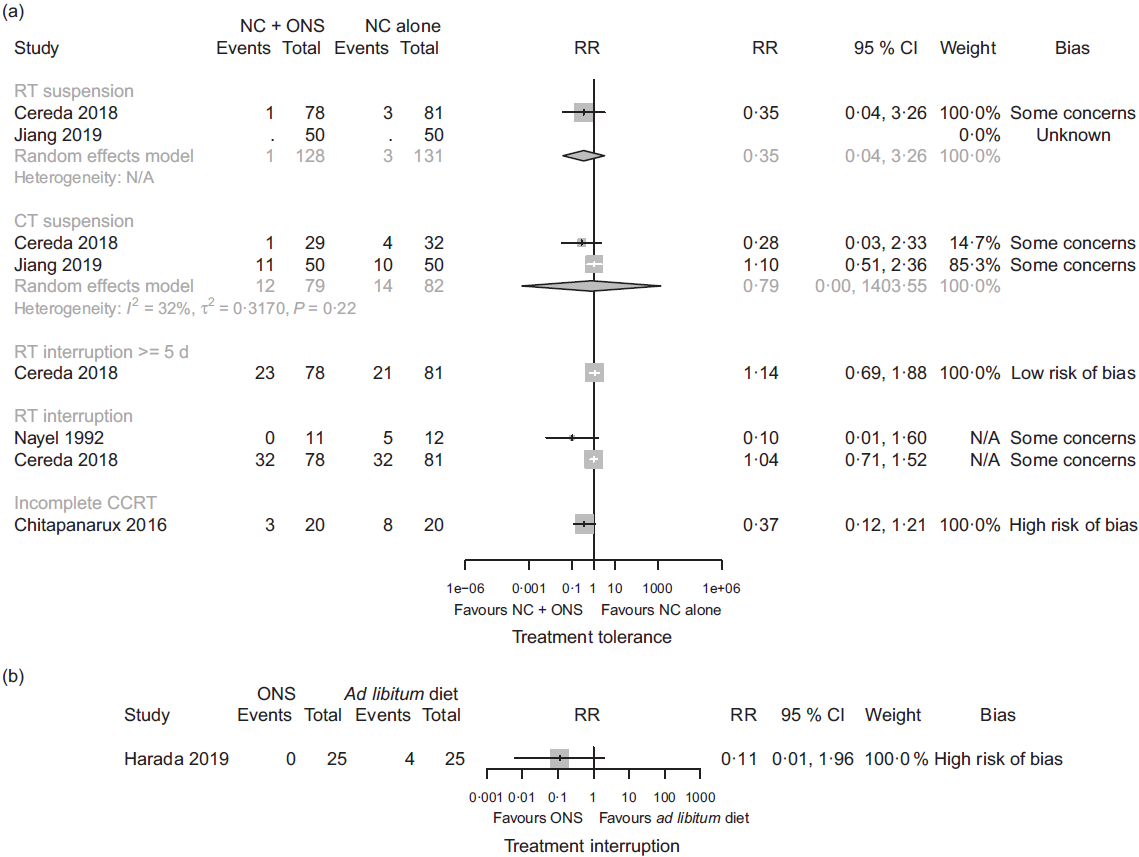
Fig. 4. Forest plots of (a) random-effects meta-analysis and structured reporting of the effects of nutritional counselling (NC) plus oral nutritional supplements (ONS) compared with NC alone (comparison one) on treatment tolerance outcomes included in the summary of findings and (b) structured reporting of the effects of ONS compared with ad libitum diet (comparison three) on treatment interruption. RR, risk ratio; RT, radiotherapy; N/A, not applicable; CT, chemotherapy; CCRT, chemoradiotherapy.
Non-haematological toxicity outcomes were often reported and are presented in detail in online Supplementary Table S7. In comparison one, the included studies reported results for mucositis(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Cereda, Cappello and Colombo32,Reference Nayel, El-Ghoneimy and El-Haddad41) , radiation dermatitis(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22) , nausea(Reference Jiang, Ding and Li21), dry mouth(Reference Jiang, Ding and Li21,Reference Nayel, El-Ghoneimy and El-Haddad41) , swallowing difficulty and taste and appetite changes(Reference Nayel, El-Ghoneimy and El-Haddad41). In comparison two, we found no evidence for these outcomes. In comparisons three and four, we found evidence for anorexia, dysgeusia, nausea/vomiting, odynophagia/dysphagia, xerostomia and permanent xerostomia and/or taste alterations(Reference Ravasco, Monteiro-Grillo and Marques Vidal30). One study(Reference Harada, Minami and Ferdous34) in comparison three also provided outcome data for mucositis. We found evidence of a reduction and an increase in some of these outcomes, but the estimates were often imprecise and/or the results were at a high risk of bias.
Effect of interventions on the quality of life
In comparison one, three studies(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Cereda, Cappello and Colombo32) reported data on the global quality of life, as assessed by the European Organization for the Research and Treatment of Cancer Quality-of-Life Questionnaire (EORTC QLQ-C30), but the evidence was of very low certainty. Sensitivity analysis excluding the results derived from inputted means and standard deviations had some effect on the estimate (data presented in the analysis report), although it did not change the overall certainty of this evidence. In comparison two, one study(Reference Ding, Dou and Wang33) contributed with data, and the evidence was also very uncertain. Fig. 5 presents the main results for this outcome in comparisons one and two. In comparisons three and four, one study(Reference Ravasco, Monteiro-Grillo and Marques Vidal30) reported data only in medians. The median quality of life score in the group that received oral nutritional supplements was 70 points, while the median score in the group that consumed an ad libitum diet and in the group that received nutritional counselling alone was 30 and 75 points, respectively (low certainty evidence).
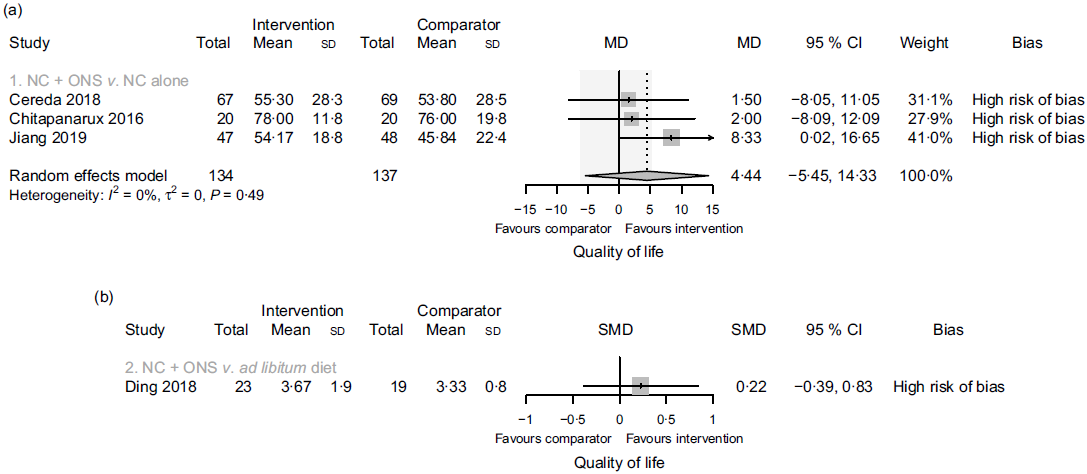
Fig. 5. Forest plots of (a) random-effects meta-analysis of the effects of nutritional counselling (NC) plus oral nutritional supplements (ONS) compared with NC alone (comparison one) on the quality of life and (b) structured reporting of the effects of NC plus ONS compared with ad libitum diet (comparison two) on the quality of life. Shaded area indicates minimal important difference for the global health status/quality of life scale for improvement (deterioration): 5·4 (−6·5). MD, mean difference; SMD, standardised mean difference.
Subscales of the EORTC QLQ-C30 questionnaire were seldom originally reported. Nonetheless, authors of two studies included in comparison one(Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Cereda, Cappello and Colombo32) provided data for these outcomes through personal communication, and they are presented in detail in online Supplementary Table S8. In comparison two, data from five subscales out of fourteen were available in the study(Reference Ding, Dou and Wang33) that assessed the quality of life. In comparisons three and four, outcome data for all subscales were available. Overall, we found limited evidence from the results at a high risk of bias, often very imprecise, and mostly displaying substantial or considerable heterogeneity. In comparison two, we found evidence of a large increase in the nausea subscale (standard mean difference (SMD) 1·2, 95 % CI 0·5, 1·9; one study, forty-two participants, high risk of bias) and a large decrease in the pain subscale (SMD −1·0, 95 % CI −1·7, −0·4; one study, forty-two participants, high risk of bias). In comparisons three and four, there were apparent differences between intervention groups; however, the absence of dispersion measures alongside the reported medians hindered adequate interpretation of study results.
Effect of interventions on functional status
Outcome data for functional status were only available in one study(Reference Cereda, Cappello and Colombo32) in comparison one, using the surrogate outcome handgrip strength. Nutritional counselling plus oral nutritional supplements compared with nutritional counselling alone may result in a slight reduction in functional status (Fig. 6).

Fig. 6. Forest plot of structured reporting of the effects of nutritional counselling (NC) plus oral nutritional supplements (ONS) compared with NC alone (comparison one) on functional status. MD, mean difference; N/A, not applicable.
Effect of interventions on body weight
In comparison one, five studies(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Arnold and Richter28,Reference Cereda, Cappello and Colombo32,Reference Nayel, El-Ghoneimy and El-Haddad41) reported data on body weight. There is moderate certainty evidence that nutritional counselling plus oral nutritional supplements compared with nutritional counselling alone probably results in a slight increase in body weight (mean difference + 1·10 kg, 95 % CI −0·94, 3·14, I 2 = 0 %; two studies, 236 participants). Sensitivity analysis involving the results at a high risk of bias had little effect on the estimate (mean difference + 1·08 kg, 95 % CI −0·45, 2·61, I 2 = 24 %; four studies, 344 participants), and in this subset, a further exclusion of the results derived from inputted means and standard deviations produced a slightly more precise estimate (mean difference + 1·00 kg, 95 % CI −0·06, 2·07, I 2 = 0 %; three studies, 304 participants). In this analysis, one of the studies(Reference Nayel, El-Ghoneimy and El-Haddad41) could not be pooled because the outcome was presented as percentage change, which had variable definitions. In comparison two, two studies(Reference Moriarty, Moloney and Mulgrew29,Reference Ding, Dou and Wang33) provided outcome data, but one(Reference Moriarty, Moloney and Mulgrew29) of them only stated that the difference between groups was not statistically significant. Overall, the evidence was very uncertain. In comparison three, one study(Reference Harada, Minami and Ferdous34) reported results for this outcome, but the evidence was also very uncertain. In comparison four, we found no evidence for this outcome. Fig. 7 presents the main results.

Fig. 7. Forest plot of random-effects meta-analysis and structured reporting of the effects of interventions on body weight at the end of treatment. Int., intervention; Com., comparator; MD, mean difference; NC, nutritional counselling; ONS, oral nutritional supplements; N/A, not applicable.
Effect of interventions on intervention-related adverse effects
Evidence on the adverse effects of interventions was available in four studies(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Cereda, Cappello and Colombo32,Reference Nayel, El-Ghoneimy and El-Haddad41) included in comparison one. In one study(Reference Nayel, El-Ghoneimy and El-Haddad41), patients recorded any side-effects that may have been attributed to oral nutritional supplements, but none was reported, so it did not contribute to the outcome data. There is low certainty evidence that nutritional counselling plus oral nutritional supplements compared with nutritional counselling alone results in a large increase in adverse effects relating to supplement use (Fig. 8). One study(Reference Cereda, Cappello and Colombo32) reported gastrointestinal intolerance to supplements, described as feeling of fullness in nine participants, resulting in three participants discontinuing their use. A second study(Reference Chitapanarux, Pisprasert and Tharavichitkul22) found nausea due to the taste of supplements in seven participants, who also left the study for that reason. The third study(Reference Jiang, Ding and Li21) also found nausea caused by the flavour and smell of supplements, leading to vomiting after consumption in three participants. These participants discontinued supplementation and dropped out of the study.

Fig. 8. Forest plots of random-effects meta-analysis and structured reporting of the effects of nutritional counselling (NC) plus oral nutritional supplements (ONS) compared with NC alone (comparison one) on adverse effects relating to the intervention. RR, risk ratio.
Discussion
This review found low certainty evidence that nutritional counselling plus oral nutritional supplements, compared with nutritional counselling alone, may result in a large increase in mortality. We also found that they may increase treatment tolerance, as assessed by the suspension and interruption of anti-cancer treatment. Regarding the quality of life, evidence was very uncertain when nutritional counselling plus oral nutritional supplements was compared with nutritional counselling alone or to ad libitum diet. Moreover, oral nutritional supplements may result in a large increase in the quality of life compared with ad libitum diet, but they may result in little to no difference in the quality of life compared with nutritional counselling. Regarding functional status, we found nutritional counselling plus oral nutritional supplements, compared with nutritional counselling alone, may reduce handgrip strength slightly. When compared with nutritional counselling alone, nutritional counselling plus oral nutritional supplements probably slightly increases body weight, but we are uncertain about the clinical significance of this finding. In the other comparisons, evidence for this outcome was very uncertain or missing. Although the evidence was of low certainty, nutritional counselling plus oral nutritional supplements compared with nutritional counselling alone appeared to largely increase adverse effects relating to the supplement use such as nausea, vomiting and feeling of fullness.
When assessing the whole body of evidence, we found a paucity of studies investigating our review question. Moreover, different comparisons arose, and for most of them, there was limited evidence for the outcomes considered to be of most importance to patients in this review. Comparison one was the most complete, comparing nutritional counselling plus oral nutritional supplements to nutritional counselling alone. This was expected since nutritional counselling forms the basis of the management of cancer patients undergoing treatment and is widely recommended(Reference Arends, Bodoky and Bozzetti59). One of the included studies(Reference Cereda, Cappello and Colombo32) intended to have an extra arm in the trial, namely ‘usual care’, without any nutritional counselling or supplements, but this was not approved by the local ethics committee. In contrast, two studies(Reference Ding, Dou and Wang33,Reference Harada, Minami and Ferdous34) that are contemporary to the one mentioned are still investigating interventions compared with ad libitum diet, suggesting this may not be a closed issue, and more studies with such comparisons might be available in the future.
The certainty of evidence across comparisons ranged from very low to moderate. Serious imprecision was a major source of uncertainty in our estimates, because confidence intervals often crossed no effect and included appreciable benefits and appreciable harms. Publication bias could not be formally investigated because of the low number of available studies. We did, however, perform a comprehensive search to identify unpublished studies, conference abstracts and original data through personal communication. Regarding unpublished studies, we assumed their results were unavailable not because of publication bias but because the trials had only been recently completed.
In respect of the risk of bias at study level, most results were at a high risk of bias overall. Judgement of bias arising from randomisation was hindered by an incomplete reporting of studies. This feature was not exclusive to older studies, but also in two studies published in 2016 and 2019, when reporting guidelines such as the CONSORT (Consolidated Standards of Reporting Trials) were already available. Deviations from intended interventions, if occurred, were generally not related to the experimental context. Some trials offered nutritional supplements or other interventions such as parenteral nutrition to comparator groups because of ethical reasons, and this was judged to reflect usual practice justified by the worsening of participant’s condition. Missing outcome data often related to the acceptability of supplements and to the deterioration of participant’s health condition. This may affect the applicability of findings because participants who remained in the trial may be different from the ones who dropped out. For instance, worsening of health may be related to clinical differences that may affect outcomes. Furthermore, participants included in the final analysis might correspond to a group of people who tolerated the supplements well, and may not reflect a general population that can tolerate the supplements or not. In one study(Reference Cereda, Cappello and Colombo32), the authors considered missingness to be at random because they found no differences between dropouts and participants who remained in the study. Because the data used to reach those judgements were not shown in the article, we requested additional information from the authors but without success. We decided to trust the information provided, but acknowledge that some readers might be sceptical about it. This should be taken into account when interpreting the risk of bias of this study’s results. A risk of bias in outcome measurement was common for subjective outcomes. Because of the nature of interventions, this can be difficult to counteract, especially for patient-reported outcomes. However, we also found that outcome assessors not reported by patients were seldom blinded, missing an opportunity of achieving results with a lower risk of bias. Assessing the risk of bias in the reported results was challenging, because few available protocols were incomplete or contained unexplained differences, leading to at least some concerns in this domain. We judged three results of one study(Reference Cereda, Cappello and Colombo32) to be at a high risk of bias because they appeared to have been based on results from multiple outcome definitions and composite outcomes that were not pre-registered in the protocol. However, by contacting study authors, we obtained data for individual outcomes from composite outcomes and considered only these results.
To the best of our knowledge, this is the first systematic review on the subject to have incorporated the GRADE approach into the interpretation and communication of findings. One systematic review(Reference Langius, Zandbergen and Eerenstein11) published in 2013 sought evidence for a range of nutritional interventions in head-and-neck cancer patients receiving radiotherapy or chemoradiotherapy. The authors found three studies(Reference Arnold and Richter28,Reference Ravasco, Monteiro-Grillo and Marques Vidal30,Reference Nayel, El-Ghoneimy and El-Haddad41) on oral nutritional supplements, which are also included in the present review. This study only considered oral nutritional supplements v. no supplements, which included comparisons of oral nutritional supplements plus nutritional counselling v. nutritional counselling alone (comparison one), and of oral nutritional supplements v. ad libitum diet (comparison three) as defined in our review. In comparisons one and three, our review adds new data from three studies(Reference Jiang, Ding and Li21,Reference Chitapanarux, Pisprasert and Tharavichitkul22,Reference Cereda, Cappello and Colombo32) and one study(Reference Harada, Minami and Ferdous34), respectively. In the previous review, results were described as positive, beneficial or related to statistical significance, but not much in terms of effect size and overall certainty of evidence. Findings were reported as inconsistent and sometimes limited to one study, but included increases in body weight and other anthropometric parameters and improvement in quality-of-life scores in patients receiving supplements compared with ad libitum diet. When compared with nutritional counselling alone, there was no clear benefit of nutritional counselling plus supplements. Even though much uncertainty still remains, in the present review we identified important changes in the body of evidence comparing nutritional counselling plus oral nutritional supplements to nutritional counselling alone, namely the outcomes of most importance to patients such as mortality and tolerance to anti-cancer treatment. Data for quality of life were available from three trials, although the evidence was of very low certainty. In addition, we found a slight increase in body weight to be of moderate certainty. Our findings were also balanced, considering the impact of oral nutritional supplements on adverse effects relating to the intervention.
We found some limitations in the present review, which should be considered when interpreting our findings. Even though our search was designed to be comprehensive and we attempted to search grey literature, some citations could not be accessed for full reading, and it is unclear how much this could have affected the estimates presented in this review. In addition, we chose to present only those outcomes of most importance to patients. This could have introduced reporting bias, since many outcomes were not considered in the final analysis, and the order of importance of those outcomes is expected to vary among other authors, clinicians, patients and decision-makers. Lastly, because of the different treatment comparisons considered in this review, we divided the included studies into four sets for pair-wise meta-analyses. In this situation, a network meta-analysis could have potentially improved our estimates by increasing precision, assuming necessary assumptions would have been met and adequate methods would have been employed. However, this approach could have widened our research question, including making additional comparisons of no relevance to this review. Additionally, given the overall very low to low certainty evidence available, the application of this method would probably not substantially change our conclusions. Nevertheless, we encourage the planning of a network meta-analysis in future evidence synthesis featuring a wider review question on this subject.
Our assessments of risk of bias revealed many limitations that future trials should attempt to mitigate. Reasons for dropouts were often related to patients’ tolerance to supplements, so special attention must be conceded to the selection of a product with high tolerability. A pre-registration of the study protocol containing specific information on outcomes intended to be assessed, timing of assessment and what analyses are intended to be conducted may reduce the risk of bias in the selection of reported results. Moreover, we found that outcome assessors were seldom blinded, even when this was feasible (e.g. non-haematological toxicity outcomes). We found several gaps in the evidence base, which could be addressed by future trials. For instance, studies with a longer follow-up might be able to better study the effect of interventions on mortality. Functional status was also seldom assessed or reported, and this could provide a better insight into the clinical significance of the impact of interventions on body weight.
In conclusion, we emphasise the uncertainty surrounding much of the evidence relating to the outcomes of most importance to patients, most often ranging from very low to low certainty. Moreover, the current state of evidence could be substantially improved via a widespread implementation of higher standards in planning, conduction and reporting of future trials. Possible increases in mortality, treatment tolerance, and quality of life, besides a possible decrease in functional status, are worthy of further investigation. Nutritional counselling plus oral nutritional supplements probably increases body weight slightly, compared with nutritional counselling alone. Possible adverse effects of oral nutritional supplements should not be overlooked.
Acknowledgements
We thank the Undergraduate Programme in Nutrition and the Postgraduation Programme in Nutrition at the Federal University of Santa Catarina, and the Coordination of Improvement of Higher Education Personnel (CAPES)/Fellowship Program Social Demand for the scholarship awarded to L. P. d. L. and J. P. We thank Karyn Lehmkuhl and Sirlene Pintro, librarians at the Federal University of Santa Catarina, for their advice on the construction of search strategy. We thank the authors who provided original data and/or details about their studies through personal communication: Dr Emanuele Cereda, Dr Imjai Chitapanarux and Dr Koji Harada.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
A. T. M. drafted the manuscript. A. T. M., D. S. B., L. P. d. L. and J. P. contributed to the conception of work, data acquisition, analysis, interpretation of data and critical review of the manuscript. P. V. K. contributed to data acquisition, analysis, interpretation of data and critical review of the manuscript. E. B. S. M. T. contributed to the conception of work and critical review of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520002329