Apoptosis is thought to be essential for epithelial turnover in intestinal mucosa and tissue homoeostasis in intestinal epithelium. Abnormal apoptosis would result in intestinal mucosal barrier damage and gastrointestinal disorders(1). In swine production, weaning is a critical process for piglets due to dramatic changes in diets and environment. The obvious intestinal morphological changes induced by weaning stress are villous shedding, villous shortening and crypt hyperplasia(Reference Cera, Mahan and Cross2). Indeed, reactive oxygen species (ROS)-induced apoptosis has been widely studied, which is also an important mechanism for many chemotherapeutic agents. Oxidative stress results from the overproduction of ROS, which are also known as mediators of damage to cell structures, including lipids and membranes, proteins and DNA(Reference Poli, Leonarduzzi and Biasi3). The effect of ROS is balanced by the antioxidant action of antioxidant enzymes as well as non-enzymatic antioxidants. The most efficient enzymatic antioxidants include superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase(Reference Mates, Perez-Gomez and Nunez de Castro4). Non-enzymatic antioxidants are represented by vitamin C, vitamin E, glutathione (GSH) and so on(Reference Valko, Rhodes and Moncol5). Reducing oxidative stress by supplementation of non-enzymatic antioxidants, such as l-arginine(Reference Tan, Yin and Kong6), GSH(Reference Circu and Aw7) and vitamin C(Reference Schoultz, Mckay and Graepel8), could potentially reduce ROS-induced damage and inhibit apoptosis.
We have demonstrated previously that weaning increases the concentrations of NO and H2O2 in serum in post-weaning piglets and that feeding an antioxidant-containing diet could prevent free radical-induced damage and suppress oxidative stress(Reference Zhu, Zhao and Chen9). However, the relationships among weaning-induced free radical generation, apoptosis and intestinal mucosal damage are still unclear in pigs. Therefore, elucidation of the mechanisms of weaning-induced apoptosis might help in understanding the physiological regulation of the apoptotic process in pig intestinal mucosa.
GSH, responsible for the detoxification of ROS, is the primary metabolic precursor for intestinal epithelial cells(Reference Oehler and Roth10). Intracellular GSH depletion induces chromosomal DNA fragmentation, which is associated with apoptosis and necrosis(Reference Higuchi11). Glutamine administration can protect against gut injury, and one of the possible ways of action is related to the preservation of GSH content(Reference Mondello, Galuppo and Mazzon12, Reference Owari, Wasa and Oue13). N-acetyl cysteine (NAC), a well-known thiol antioxidant and GSH precursor, has been demonstrated to be beneficial for preventing ROS-induced damages(Reference Quadrilatero and Hoffman-Goetz14, Reference Denning, Takaishi and Crowe15). Recently, it has been proved that NAC prevents exercise-induced intestinal lymphocyte apoptosis by maintaining intracellular GSH contents and reducing mitochondrial membrane depolarisation(Reference Quadrilatero and Hoffman-Goetz14). Administration of NAC before ischaemia in experiments was effective in the prevention of bacterial translocation and oxidative stress(Reference Ocal, Avlan and Cinel16). Moreover, NAC is a clinically proven safe dietary supplement that may be useful in clinical therapy for ROS-mediated diseases(Reference Reliene, Pollard and Sobol17). These beneficial properties of NAC suggest that it may be considered as a useful additive to diets for the prevention of weaning stress.
In the present study, we attempted to characterise the relationship between apoptosis and intestinal mucosal damage induced by weaning in piglets. We also aimed to investigate whether NAC can effectively prevent weaning-induced intestinal damage in piglets. Particular attention was afforded to changes in serum antioxidant activities, intestinal morphology, and enterocyte apoptosis and functions in an attempt to identify any intracellular signalling changes regulated by weaning and NAC treatment. To our knowledge, this is the first study in piglets to simultaneously evaluate weaning-induced apoptosis and the potential wound-healing function of NAC through the regulation of cell apoptosis in response to weaning stress.
Materials and methods
Animals and experiment design
The experiment was approved by the Shanghai Jiaotong University Institutional Animal Care and Use Committee. A total of 150 14-d-old piglets (Duroc × Landrace) from fifteen litters were randomly divided by litter into control, weaning and NAC groups with five litters per group. The piglets were kept with the sow in conventional farrowing pens and suckled until 21 d of age. From 14 to 25 d of age, piglets in the control and weaning groups had ad libitum access to the basal diet and the NAC-treated piglets were fed the basal diet supplemented with 500 mg/kg of NAC. The dosage of supplemental NAC was chosen based on the study by Hou et al. (Reference Hou, Wang and Zhang18) with 35-d-old piglets. Dietary ingredients, analysed energy and nutrient contents of the basal diets (as-fed basis) with 14·48 MJ/kg digestible energy and 20·50 % crude protein (N × 6·25) have been described previously(Reference Zhu, Zhao and Chen9). At 21 d of age, piglets of the weaning and NAC groups were weaned and moved from the farrowing pens to nursery pens without mixing any litters. The control piglets were retained in the farrowing pens to suckle until the 25 d experiment. Temperature in the farrowing and nursery pens was maintained at about 30°C.
Sample collection
At 25 d of age, three piglets from each litter, resulting in a total of fifteen piglets per treatment, were selected and blood samples were collected from the anterior vena cava. The selected piglets had a body weight that was close to the average for the litter. Serum was separated by centrifugation at 3500 g for 15 min at 4°C and stored at − 20°C until analysis for cortisol and antioxidative physiological measures. One piglet was randomly chosen from each litter, resulting in a total of five piglets per treatment. The piglets were anaesthetised by intramuscular injection of sodium pentobarbital (50 mg/kg body weight) and then euthanised, and gut samples were collected. The entire small intestine was carefully removed and placed on ice. Some pieces, approximately 2 cm in length, were resected from the middle portion of the jejunum and fixed in 4 % neutral buffered formalin for the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) assay. In addition, some specimens of jejunum sections were fixed with 2·5 % glutaraldehyde for transmission electron microscopy examination. Other segments of the jejunum were rinsed thoroughly with physiological saline and opened longitudinally to expose the intestinal epithelium. The mucosal layer was harvested by gentle scraping of the epithelium with a glass slide, as described by Noda et al. (Reference Noda, Iwakiri and Fujimoto19). Then, the mucosal specimens were frozen in liquid N2 and stored at − 70°C. Half of the specimens were used for the evaluation of apoptotic proteins with ELISA and the remaining half were used for quantitative RT-PCR analyses.
Serum cortisol content
Serum concentrations of cortisol were quantified using a Coat-A-Count Cortisol kit (Diagnostic Product Corporation) according to the manufacturer's protocol. Unknown concentrations were compared against the cortisol kit standard and expressed as μg/l of cortisol. This value was then converted and expressed as ng/ml of cortisol. The optical density at 450 nm was measured after colouration. All assays were performed in duplicate. The minimum detection limit was 7 ng/ml, and the use of the cortisol kits for porcine samples has been validated(Reference Daniel, Keisler and Sterle20).
Antioxidative physiological analyses
The activities of SOD and GSH-Px and the content of malondialdehyde (MDA) in serum were determined to evaluate the antioxidant ability and lipid peroxidation in pigs, using assay kits according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute). In brief, the activity of total SOD was determined by monitoring the inhibition of nitro blue tetrazolium reduction. One unit of SOD activity was defined as the amount that reduced the absorbance at 550 nm by 50 % in 1 ml serum, and data were expressed as units/ml serum. The activity of GSH-Px was determined by measuring the reduction of GSH per min on the basis of its catalysis. The final result was expressed as a decrease of 1 μmol/l of GSH per 5 min for 0·1 ml serum at 37°C after the subtraction of the non-enzymatic reaction, and data were expressed as units/ml serum and then converted to units/μl. MDA content was analysed with 2-thiobarbituric acid, and data were expressed as nmol/ml serum. All absorbance levels were measured using a UV–visible spectrophotometer (Tongfang, Inc.).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling staining
Jejunum samples, fixed in 4 % neutral buffered formalin, were embedded in paraffin and sectioned. The specimens were stained with haematoxylin and eosin for histological examination. Mucosal length (villus height plus crypt depth) was analysed with a light microscope (Nikon). Fragmented DNA was stained using the TUNEL assay method with some modifications using a FragEL™ DNA Fragmentation Detection Kit (Merck). In brief, the specimens were de-waxed and incubated with 20 μg/ml proteinase K for 20 min at room temperature and then reacted with the terminal deoxynucleotidyl transferase enzyme for 60 min at 37°C. The specimens were then incubated with anti-digoxigenin peroxidase at room temperature for 30 min followed by a thorough washing with PBS. Finally, the specimens were incubated with diaminobenzidine solution and counterstained with methyl green. A minimum of twenty crypts were randomly selected for apoptotic index analysis, and the number of apoptotic cells was calculated. The apoptotic index was determined by dividing the number of apoptotic cells by the total number of cells in the crypt column and multiplying by 100.
Specimen preparation for electron microscopy
Mucosal specimens of jejunum sections were fixed with 2·5 % glutaraldehyde in 0·1 mol/l cacodylate buffer (pH 7·2) for 2 h at 4°C. These specimens were then washed three times with the same buffer and post-fixed with osmium tetroxide for 2.5 h at 4°C. They were later washed with 0·1 % sodium acetate, stained with 1 % uranyl acetate, dehydrated with ethanol and embedded in Spurr's low-viscosity resin. Representative areas were sectioned and stained with toluidine blue. Ultra-thin sections were prepared and double stained with uranium and lead. A Hitachi H-600 transmission electron microscope (Hitachi) was used to visualise the ultrastructure of the enterocytes and the distribution of epithelial cell microvilli.
Concentrations of caspase in the jejunum
Relative amounts of caspase-3, caspase-8 and caspase-9 in the homogenates of the jejunum of the selected piglets were determined using specific ELISA kits (R&D Systems, Inc.) according to the manufacturer's instructions. Each individual sample was analysed in triplicate. In brief, individual jejunum samples were homogenised in ten volumes of PBS (pH 7·0) on ice and centrifuged at 3000 g for 15 min. The supernatant was decanted and stored. Protein concentrations in the supernatant were determined using a BCA protein assay kit (Pierce). Caspase concentrations were determined using a spectrophotometer (450 nm) on a microtitre plate reader.
Quantitative RT-PCR
Total RNA was extracted from jejunal tissues using TRIzol reagent (Invitrogen) following the manufacturer's instructions and purified using DNase I (Invitrogen) for 15 min. The yield and quality of RNA were assessed using a spectrophotometer (Bio-Rad Laboratories, GmbH), considering the ideal absorbance ratio (1·8 ≤ A260/280 ≤ 2·0). The integrity of RNA was checked by electrophoresis on a 1·5 % agarose gel. A volume equivalent to 1 μg of total RNA was reverse- transcribed using random primers according to the manufacturer's protocol (TaKaRa). The resulting complementary DNA was diluted and used as a PCR template to evaluate gene expression. Quantitative RT-PCR was conducted on an Eppendorf Mastercycler ep Realplex Real-Time Quantitative PCR System (Eppendorf) using SYBR Premix Ex Taq™ kits (TaKaRa) under the following conditions: pre-denaturation at 95°C for 30 s and forty cycles of 95°C for 5 s, 60°C for 30 s and 72°C for 15 s. A dissociation curve was constructed at the end of the reaction to ensure that only one amplicon was formed. Primers for the genes of interest were designed based on the pig (Sus scrofa) sequence (Table 1) using primer design software (Primer Premier 5.0; PREMIER Biosoft International). Amplification efficiencies were calculated based on the slope of the line (E= 10( − 1/slope)− 1), considering an ideal value range (0·95 ≤ E≤ 1·05). All experiments were repeated in triplicate. The geometric means of the relative abundances were normalised against porcine β-actin, glyceraldehyde 3-phosphate dehydrogenase and β-2-microglobulin genes using the ![]() $$2^{ - \Delta \Delta C _{t}} $$ method(Reference Livak and Schmittgen21). Relative gene expressions in the weaned and NAC-treated piglets represented the comparison v. suckling piglets and were reported as a fold change from the value of the suckling piglets.
$$2^{ - \Delta \Delta C _{t}} $$ method(Reference Livak and Schmittgen21). Relative gene expressions in the weaned and NAC-treated piglets represented the comparison v. suckling piglets and were reported as a fold change from the value of the suckling piglets.
Table 1 Primer sequences used for quantitative RT-PCR

ACTB, β-actin; F, forward primer; R, reverse primer; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; B2M, β-2-microglobulin; Bax, B-cell lymphoma-2-associated X protein; Bcl-2, B-cell lymphoma-2; αvβ6, integrin αvβ6.
Statistical analyses
All data are presented as means with their standard errors. Differences among the examined groups were determined using one-way ANOVA followed by Tukey's test. Values with P< 0·05 were considered as statistically significant. Trends were reported when 0·10 < P< 0·05.
Results
Serum cortisol content
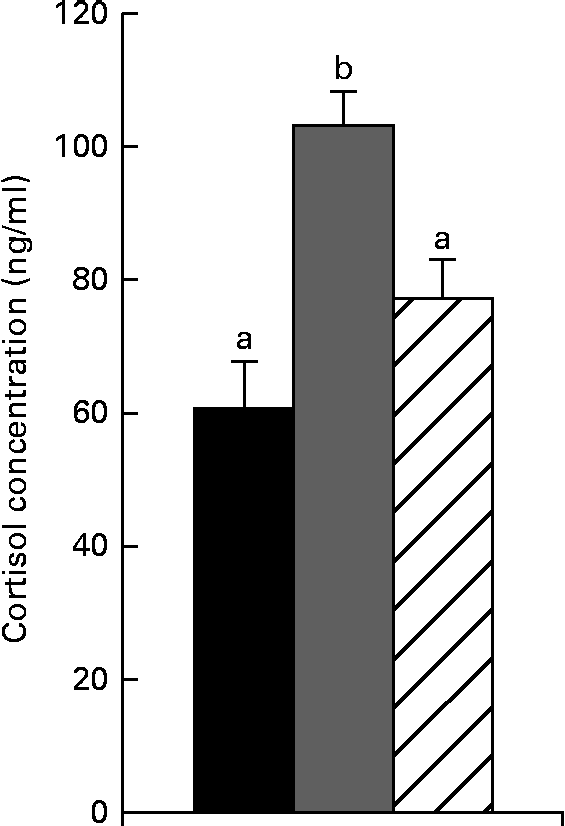
As shown in Fig. 1, cortisol concentrations were increased (P< 0·05) after weaning compared with the control piglets. Feeding the NAC-containing diet decreased cortisol concentrations (P< 0·05) compared with the weaned piglets. No significant differences in cortisol concentrations were found between the control and NAC-treated piglets (P>0·10).

Fig. 1 Serum cortisol concentrations in piglets of the control, weaning and N-acetyl cysteine (NAC) groups at 25 d of age. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). Control group (■), piglets suckled from 14 to 25 d of age. Weaning group (![]() ), piglets weaned at 21 d of age and fed the basal diet. NAC group (
), piglets weaned at 21 d of age and fed the basal diet. NAC group (![]() ), piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC.
), piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC.
Oxidative stress induced by weaning
The activities of SOD and GSH-Px and the content of MDA in serum are presented in Table 2. The activities of GSH-Px in serum were significantly lower (P< 0·05) in the weaned piglets than in the control piglets. The activities of SOD also showed a trend to be lower (P= 0·055) in the weaning group compared with the control group. The content of MDA, the product of lipid peroxidation, was significantly increased (P< 0·05) in the piglets of the weaning group compared with those of the control group. Supplementing the diet with NAC increased the activities of SOD and GSH-Px (P< 0·05) compared with the weaning group and lessened (P< 0·05) the MDA content to levels that were not different from those observed in the control piglets.
Table 2 Serum superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities and malondialdehyde (MDA) content in piglets of the control, weaning and N-acetyl cysteine (NAC) groups at 25 d of age (Mean values with their pooled standard errors, n 5)

a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Piglets suckling from 14 to 25 d of age.
† Piglets weaned at 21 d of age and fed the basal diet.
‡ Piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC.
§ One unit of SOD activity was defined as the amount required to inhibit the reduction in nitro blue tetrazolium by 50 % of maximum inhibition in 1 ml serum.
∥ One unit of GSH-Px activity was defined as a decrease of 1 μmol/l of GSH per 5 min for 0·1 ml serum at 37°C after subtraction of the non-enzymatic reaction.
Light microscopy findings
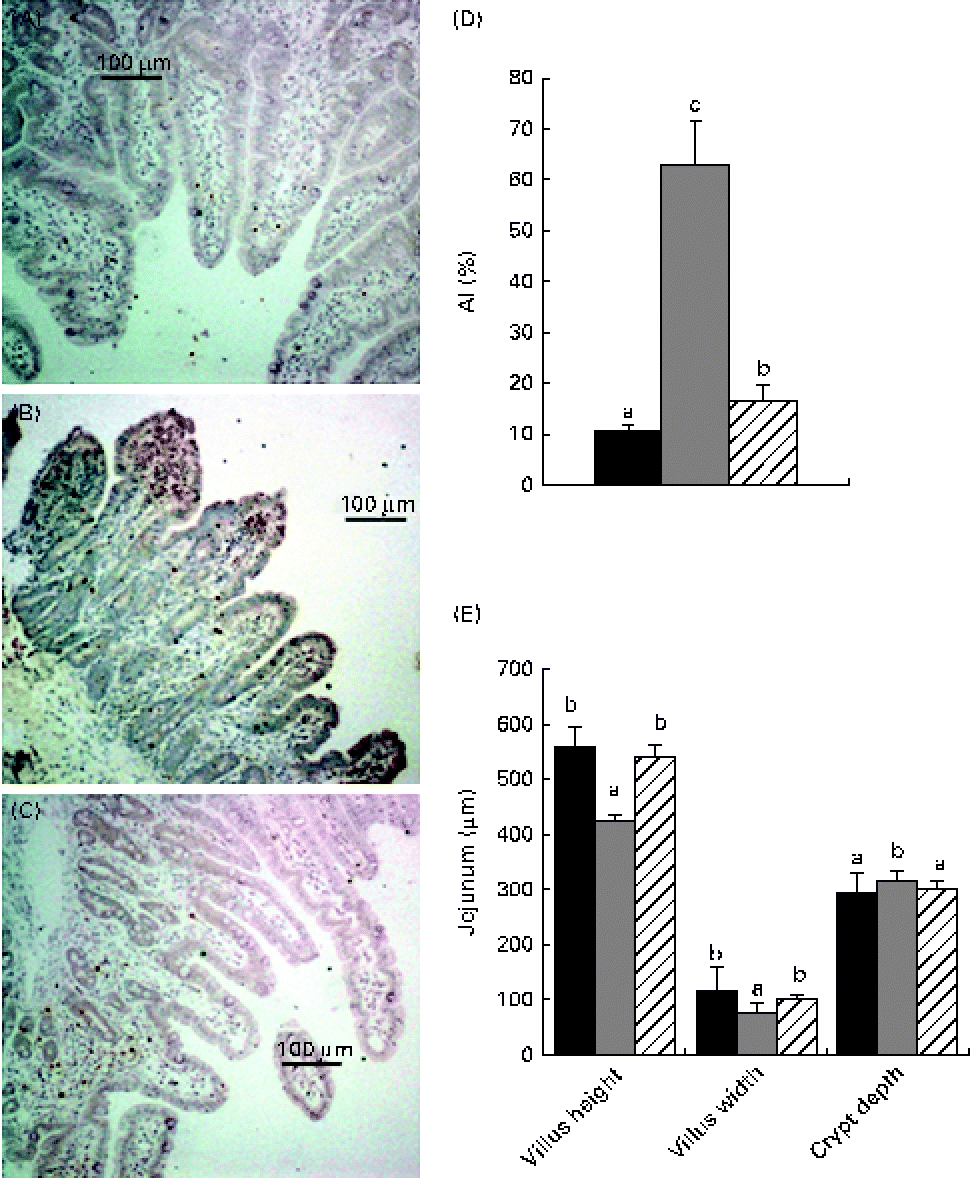
Representative histological graphs of haematoxylin and eosin-stained intestine samples of the control, weaning and NAC groups are shown in Fig. 2(E). The control specimens showed normal and typical leaf-like villus crypts. In contrast, villus height in the jejunum was significantly decreased (P< 0·05) in the weaning group, whereas villus width showed a small decrease (P< 0·05) in the weaned piglets relative to the control piglets. In addition, the weaned piglets showed larger crypt depths (P< 0·05) than the control piglets. Feeding the NAC-containing diet increased (P< 0·05) villus height and villus width (P< 0·05) and decreased the crypt depths (P< 0·05) in the jejunum compared with the weaned piglets. The increased number of apoptotic cells (apoptotic nuclei are stained dark brown) with significant localisation of apoptotic cells in the upper tip of the intestinal villi (Fig. 2(A)–(C)) was revealed by the TUNEL assay in the weaned piglets. Moreover, the apoptotic index was higher in the weaned piglets (P< 0·05) than in the control piglets (Fig. 2(D)). A few apoptotic cells were present at the villus tips in the jejunum of the NAC group, and the apoptotic index (P< 0·05) in the piglets fed the NAC diet was decreased compared with those of the weaning group.

Fig. 2 Light micrographs of the jejunum of the (A) control, (B) weaning and (C) N-acetyl cysteine (NAC) groups stained using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling method. Magnifications: × 100. Apoptotic nuclei are stained dark brown. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). (D) Apoptotic index (AI). (E) Histological evaluation of the jejunum of the control, weaning and NAC groups stained with haematoxylin and eosin. Control group (■), piglets suckled from 14 to 25 d of age. Weaning group (![]() ), piglets weaned at 21 d of age and fed the basal diet. NAC group (
), piglets weaned at 21 d of age and fed the basal diet. NAC group (![]() ), piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC.
), piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC.
Electron microscopy findings
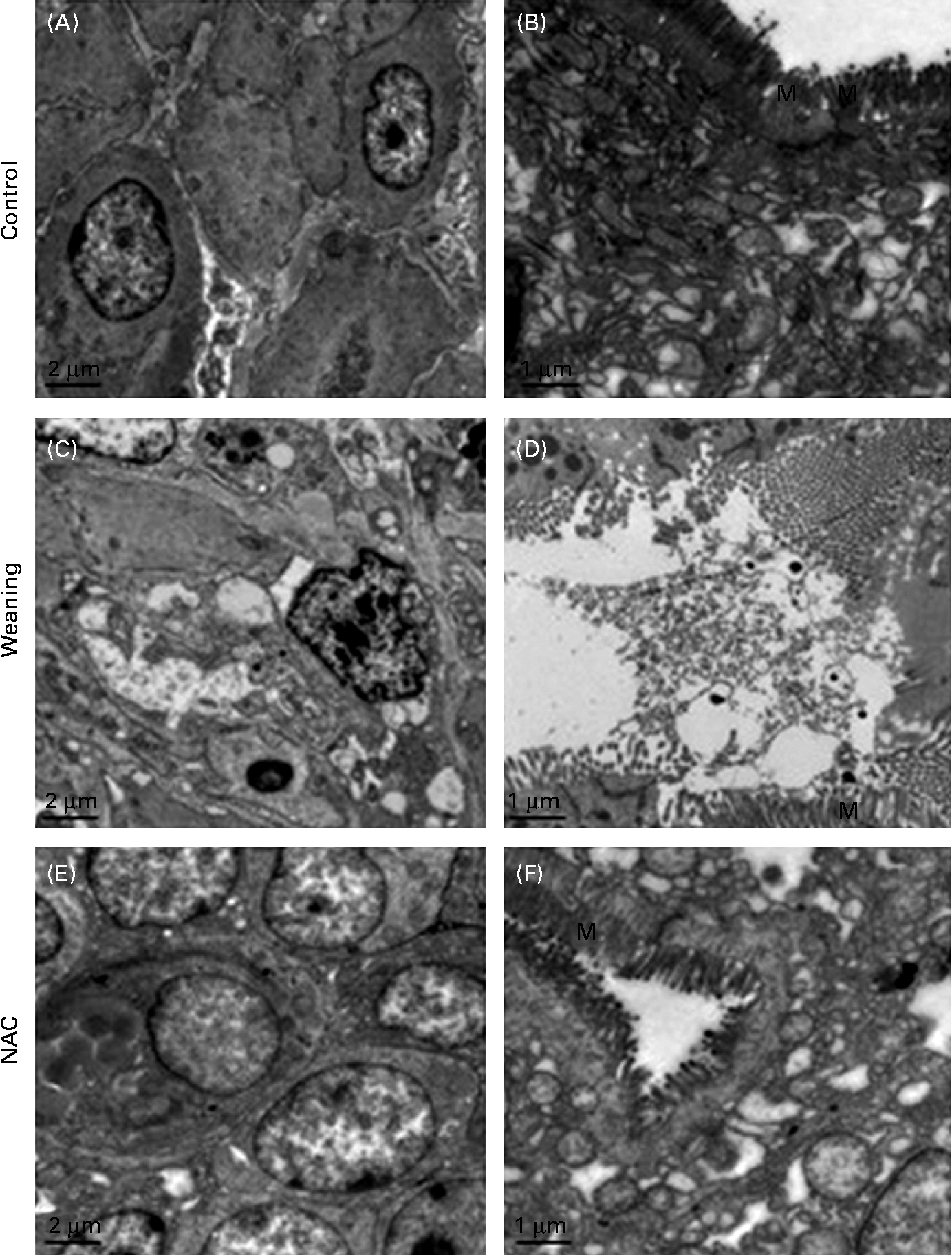
Electron microscopic examination of the jejunal mucosa is shown in Fig. 3. In the weaning group, some enterocytes showed necrotic injuries characterised by serious damage to the plasma membrane, complete loss of junction specialisations between adjacent cells and swollen mitochondria without crests. Some other detached cells had characteristic features of apoptosis with vacuolated cytoplasm, cell compaction, condensed chromatin and fragmented mitochondria. No apoptotic features were found in the intestinal epithelia of the control group except severe chromatin agglutination and mitochondrial ridge damage. The microvilli were well developed in the control piglets. In the NAC group, the microvilli were well developed, and the enterocyte ultrastructure was similar to that of the control group.

Fig. 3 Examination of jejunal mucosal injury by transmission electron microscopy. Control, piglets suckled from 14 to 25 d of age. Weaning, piglets weaned at 21 d of age and fed the basal diet. NAC, N-acetyl cysteine group, piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC. (A) Most of the enterocytes were well connected with intact adherence, while several cells showed chromatin agglutination and mitochondrial ridge damage. (B) Microvilli were well developed. (C) Some epithelial cells showed compaction and segregation of chromatin against the nuclear envelope, disrupted basement membrane, autophagosomes and swollen mitochondria. Some enterocytes showed vacuolated cytoplasm, degenerated cell organelles (mitochondria and endoplasmic reticulum) and autophagosomes. (D) Microvilli loss. (E) Cell linkage was tight, but several enterocytes showed chromatin agglutination and presented signs of blebbing. (F) The microvilli were well developed and similar to those of the control piglets. Original magnification: × 5000 (A, C, E); × 10 000 (B, D, F). M, microvilli.
ELISA for caspase content
As shown in Table 3, the concentrations of caspase-3, caspase-8 and caspase-9 were strongly increased (P< 0·05) in the jejunum of the weaned piglets when compared with the control piglets, showing an increased apoptosis due to the weaning treatment. The concentrations of caspase-3 and caspase-9 tended to decrease (P= 0·092 and P= 0·075) in the NAC-treated group compared with the weaning group. Compared with the control piglets, concentrations of caspase-3, caspase-8 and caspase-9 were still strongly increased (P< 0·05) after the NAC-containing diet treatment.
Table 3 Jejunal tissue caspase concentrations in piglets of the control, weaning and N-acetyl cysteine (NAC) groups at 25 d of age (Mean values with their pooled standard errors, n 5)

a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Piglets suckled from 14 to 25 d of age.
† Piglets weaned at 21 d of age and fed the basal diet.
‡ Piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC.
Quantitative RT-PCR analysis of apoptosis genes
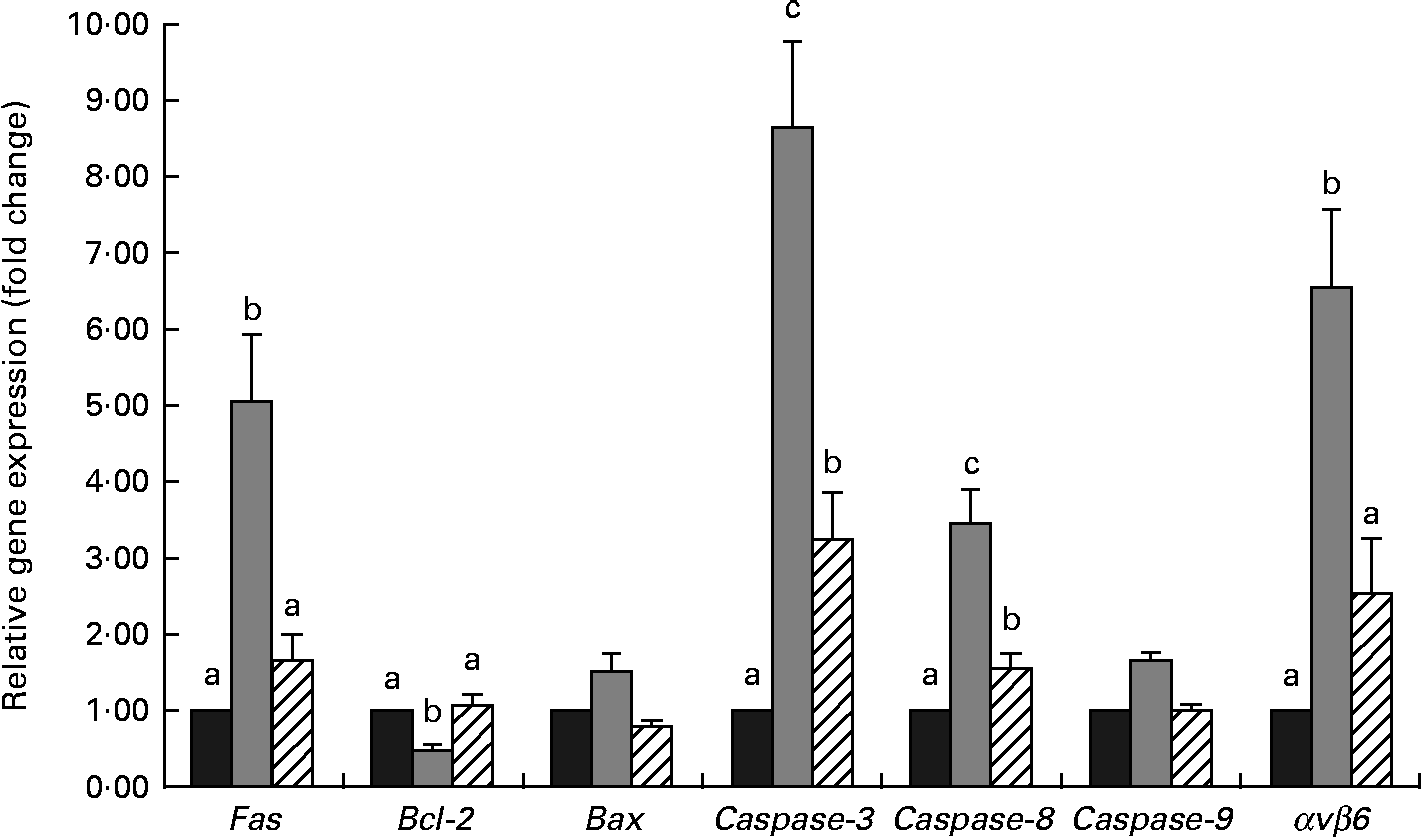
Results for quantitative RT-PCR analysis are shown in Fig. 4. A significant increase (P< 0·05) in caspase-3, caspase-8, Fas and integrin αvβ6 (αvβ6) expressions was observed in the weaned piglets compared with the control piglets. No significant differences in the pro-apoptotic factor B-cell lymphoma-2 (Bcl-2)-associated X protein and caspase-9 expressions (P>0·10) were observed among the three groups, while weaning decreased (P< 0·05) the expression of the anti-apoptotic factor Bcl-2 in the weaned piglets compared with the control piglets. The NAC-containing diet decreased (P< 0·05) the expressions of caspase-3, caspase-8, αvβ6 and Fas compared with the weaning group. Compared with the weaning group, the expression of Bcl-2 was increased (P< 0·05) in the NAC group.

Fig. 4 Relative expression of apoptosis-related genes in piglets of the control, weaning and N-acetyl cysteine (NAC) groups at 25 d of age. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). mRNA levels in the weaning and NAC groups are presented as the multiples of gene expression level in the control group, which was set as 1·0. Control group (![]() ), piglets suckled from 14 to 25 d of age. Weaning group (
), piglets suckled from 14 to 25 d of age. Weaning group (![]() ), piglets weaned at 21 d of age and fed the basal diet. NAC group (
), piglets weaned at 21 d of age and fed the basal diet. NAC group (![]() ), piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein; αvβ6, integrin αvβ6.
), piglets weaned at 21 d of age and fed the basal diet supplemented with 500 mg/kg of NAC. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein; αvβ6, integrin αvβ6.
Discussion
Cortisol is the primary glucocorticoid released during stress, which is normally increased for at least the first 24 h post-weaning in pigs, revealing the occurrence of a stress response(Reference Koopmans, Guzik and Van der Meulen22). Antioxidant supplementation is able to decrease cortisol concentrations under stress(Reference Koopmans, Guzik and Van der Meulen22, Reference Ming, Xie and Xu23). In the present study, the increased cortisol concentrations after weaning suggest that the piglets experienced stress at weaning. NAC, a well-known antioxidant(Reference Quadrilatero and Hoffman-Goetz14), tended to decrease cortisol concentrations after weaning, indicating that it has the potential to counteract weaning stress.
Stress, especially oxidant stress, that is caused by reactive nitrogen species and/or ROS is known to play a major role in the pathogenesis of tissue injury in many diseases, and the removal of free radicals may offer a treatment choice. SOD enzymes, as the major cellular antioxidants, can directly scavenge the excessive free radicals such as OH−, H2O2 and O2 − by working in conjunction with other H2O2-removing enzymes, such as GSH-Px(Reference Rodriguez, Shinyashiki and Froines24). GSH, a potent endogenous antioxidant, is a first line of defence against free radicals and is often depleted as a consequence of the increased status of oxidative stress and inflammation(Reference Higuchi11). Lipid peroxidation, a free radical-related process, is a chain reaction leading to the oxidation of fatty acids, disrupting membrane structure and producing toxic metabolites such as MDA(Reference Romero, Bosch-Morell and Romero25). In the present study, we observed higher lipid peroxidation and reduced SOD and GSH-Px activities in the weaned piglets compared with the control piglets, which is in agreement with previous findings in intestinal ischaemia–reperfusion injury in rats(Reference Zhao, Zhang and Shen26). We also demonstrated that weaning induced free radical generation, including NO and H2O2, and decreased O2 concentrations in serum(Reference Zhu, Zhao and Chen9). Hence, in response to the increased production of free radicals after weaning, antioxidant enzymes (SOD and GSH-Px) may be broken down by free radicals. In addition, studies have reported that NAC has protective effects on the occurrence of oxidative stress resulting from ischaemia–reperfusion by preventing MDA, SOD and GSH-Px changes in animal models(Reference Ozyurt, Iraz and Koca27, Reference Koca, Yurttas and Cayci28). As a potent antioxidant, NAC directly scavenges H2O2, OH− and hypochloric acid in vitro (Reference Aruoma, Halliwell and Hoey29) and exerts antioxidative effects by facilitating the generation of intracellular antioxidants, such as GSH(Reference Song, Kellum and Kaldas30). In the present study, administration of the NAC-containing diet to the weaned piglets significantly reduced lipid peroxidation and restored the SOD and GSH-Px activities to near-control levels; this is probably because of its free radical-scavenging activity or endogenous synthesis of GSH by NAC(Reference Aruoma, Halliwell and Hoey29, Reference Song, Kellum and Kaldas30). The high SOD activities in the NAC-treated piglets than in the control piglets might be due to the restoration of GSH by NAC and the early NAC supplementation for 12 d (from 14 to 25 d of age). It could be that the increased SOD and GSH-Px activities in serum may reflect the up-regulation of the antioxidant defence system in response to weaning stress in the NAC- treated piglets. These results suggest that NAC may have a protective effect on oxidative stress resulting from weaning in the piglet models.
As apoptosis plays a crucial role in free radical-induced gastrointestinal damage(Reference Souza, Tortori and Castelo-Branco31, Reference Siggers and Hackam32), we examined enterocyte apoptosis and histopathological changes in the intestine in response to weaning stress and determined whether NAC supplementation could improve the integrity of intestinal mucosa in weaned piglets. Confirmation of apoptosis was based on the findings obtained from both morphological and biochemical measurements. The TUNEL assay and ELISA procedure confirmed an increased apoptosis in the intestinal mucosa of the weaned piglets. However, cell death occurs by two distinctly different pathways: programmed cell death (apoptosis) or necrosis(Reference Sun, Wang and Wallen33). In the present study, transmission electron microscopy was used to distinguish apoptosis and necrosis, since necrosis is associated with histological signs of inflammation, whereas apoptosis is not. In the present study, concurrent necrosis and apoptosis were observed in the jejunum of the weaned piglets. These data provide an explanation for the clinical observation that piglets that are weaned tend to develop intestinal inflammation easily(Reference McCracken, Spurlock and Roos34). This would also explain why there is an increase of pro-inflammatory cytokines in the intestine of the weaned piglets(Reference Pie, Lalles and Blazy35). These findings obtained from the TUNEL assay were confirmed by the noted increase in caspase-3, caspase-8 and caspase-9 concentrations through the ELISA method. Besides, we also found that the mucosal histology of piglets in the NAC group was preserved better than that of the piglets in the weaning group. Here, it seems that the potential wound-healing function of the NAC-containing diet is related to the preserved morphological integrity of intestinal mucosa.
There are two major apoptotic pathways: the intrinsic (mitochondria-dependent apoptosis) and the extrinsic (Fas-dependent apoptosis) pathways. The intrinsic pathway is mitochondria mediated and mainly characterised by the activation of caspase-9(Reference Wang36), whereas the extrinsic pathway involves caspase-8, which is activated through the activation of membrane death receptors, such as Fas(Reference Budihardjo, Oliver and Lutter37). Both these pathways converge to a common execution phase of apoptosis that requires proteolytic activation of caspase-3, triggering a cascade of caspase activation(Reference Riedl and Shi38). In the present study, the increased caspase-3, caspase-8 and Fas expressions and the decreased anti-apoptotic Bcl-2 expressions in piglets of the weaning group suggest that both mitochondria-dependent apoptosis and Fas-dependent apoptosis are induced in epithelial cell apoptosis following the weaning treatment. The expressions of caspase-3, caspase-8, Bcl-2 and Fas were reversed by the NAC-containing diet treatment. Thus, it appears that the caspase-3, caspase-8, Bcl-2 and Fas genes may be regulated via redox-sensitive mechanisms in intestinal epithelial cells. However, no significant effect was observed on the expressions of Bcl-2 associated X protein and caspase-9 in the NAC-treated piglets. This likely reflects the prolonged kinetics of apoptosis in epithelial cells. In addition, the intrinsic pathway is essential for the ROS-mediated apoptosis(Reference Takahashi, Masuda and Sun39), but is not exclusive to this death pathway. In fact, ROS-mediated apoptosis is shared between mitochondria-dependent apoptosis and Fas-dependent apoptosis(Reference Denning, Takaishi and Crowe15, Reference Takahashi, Masuda and Sun39). Evidence has proved that ROS scavengers, such as GSH, NAC and SOD, can cause the inhibition of Fas-induced apoptosis in different cell types(Reference Denning, Takaishi and Crowe15, Reference Malassagne, Ferret and Hammond40, Reference Chiba, Takahashi and Sato41). In the present study, we observed that an increase in Fas, caspase-8 and caspase-3 expressions coincided with decreases in the GSH-Px and SOD activities in the weaned piglets, and these were reversed by the NAC-containing diet. These results suggest that the ability of NAC to inhibit cell death in the weaned piglets might be more inclined to decrease apoptosis via Fas-dependent apoptosis.
Integrin αvβ6 is a well-known cell surface receptor and is not expressed constitutively by healthy adult epithelial cells, but is rapidly enhanced upon stimulation such as cell injury, cancer and inflammation, and its expression is maintained until wound closure is complete(Reference Blanco-Mezquita, Hutcheon and Stepp42). High expression of αvβ6 in colon cancer is associated with a more aggressive disease outcome, leading to the spread and development of cancer(Reference Bates43). In the present study, we showed a dramatic increase in the expression of αvβ6 in the weaned piglets, which is consistent with the previous studies of gut challenged with nematodes(Reference Knight, Wright and Brown44). The high expression of αvβ6 in the weaned piglets might provide an explanation for the clinical observation that weaned piglets with small-intestinal inflammation tend to have worse outcomes than diarrhoea(Reference Cera, Mahan and Cross45). Furthermore, on blocking the function of αvβ6 in colon cancer cells, apoptosis is enhanced and signalling involves the mitochondrial pathways(Reference Zhao-Yang, Ke-Sen and Qing-Si46). Enhanced mitochondrial ROS production is classically reported during cell apoptosis. Mitochondrial dysfunction promotes cancer cell migration through the induction of integrin αvβ5 via an increase in mitochondria-generated ROS(Reference Hung, Huang and Wu47). In the case of weaned piglets, damage of the intestinal epithelium has been observed due to weaning stress via enhanced free radical generation(Reference Zhu, Zhao and Chen9). Hence, we think that the increased apoptosis in the weaned piglets is associated with the expression of αvβ6. Additionally, αvβ6 is up-regulated during tissue remodelling, which occurs in epithelial repair as well as in cancer, but the timing and topology of the expression of αvβ6 are important for normal repair(Reference Wang, Dolinski and Kikuchi48). Partial inhibition of αvβ6 may prevent pulmonary fibrosis without exacerbating inflammation(Reference Horan, Wood and Ona49). Here, the down-regulated expression of αvβ6 in the NAC-treated piglets might indicate that the potential wound-healing function stimulated by NAC might be associated with the regulation of αvβ6. However, the expression of αvβ6 was still higher than that in the control piglets, as well as high caspase-3, caspase-8 and caspase-9 concentrations were observed using the ELISA method. These data may reflect that further studies should be conducted to more completely determine the optimal dosage and time of NAC.
In conclusion, weaning could increase enterocyte apoptosis by activating both the intrinsic and extrinsic apoptotic pathways in post-weaning piglets. The NAC-containing diet used in the present study could preserve the morphological integrity of intestinal mucosa and inhibit cell death by reducing apoptosis via Fas-dependent apoptosis and αvβ6 expression in weaned piglets. The signalling mechanisms still remain to be elucidated.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513001608
Acknowledgements
The present study was financially supported by the National Natural Science Foundation of China (grant no. 30972103). The authors thank J. Gu, C. C. Xu and S. F. Yang for their assistance in sample collection and raising of the piglets. The contributions of each author are as follows: X. Cai and Q. G. participated in the experiments together with L. Z., who also performed the data analysis and wrote the manuscript; J. X., X. Chen and S. Z. designed and supervised the study. All authors read and approved the final manuscript. The authors declare no competing financial interests.