The number of overweight children under the age of 5 years in 2013 was estimated to be over forty-two million; 75 % of these children are living in developing countries( 1 , Reference Ng, Fleming and Robinson 2 ). The relationship between body weight and insulin resistance in children is therefore an important research topic( Reference Henderson, Benedetti and Barnet 3 – Reference Masquio, Piano and Campos 5 ). A growing body of evidence suggests that excessive weight gain in infancy and in childhood is likely to be associated with insulin resistance in adults( Reference Barghava, Sachdev and Fall 6 – Reference Slining, Kuzawa and Mayer-Davis 8 ). The impact of early weight gain on insulin resistance in children has not been clearly identified, however. Insulin resistance in childhood is of great clinical importance as it may lead to diabetes type 2, hypertension, hepatic steatosis, endothelial dysfunction( Reference Ho, Garnett and Baur 9 – Reference Sartorio, Del Col and Agosti 11 ), CVD and the metabolic syndrome( Reference Ten and Maclaren 12 ) later in life. The prevalence of insulin resistance in childhood has shown a steady increase in recent years( Reference Chiarelli and Marcovecchio 13 , Reference Van Der Aa, Farsini and Knibbe 14 ), and this points to the need for appropriate and early preventive intervention strategies.
Systematic reviews suggest that educational interventions including breast-feeding and complementary feeding practices may be effective in improving the nutritional status of infants and young children( Reference Shi and Zhang 15 , Reference Imdad, Yakoob and Bhutta 16 ), and thereby also protect against future chronic diseases( Reference Lanigan and Singhal 17 ). We have shown in a field trial that maternal counselling for infant feeding at home can stimulate infant breast-feeding at 4, 6 and 12 months of age( Reference Vitolo, Bortolini and Feldens 18 ), which could provide some long-term protection against the development of insulin resistance( Reference Veena, Krishnaveni and Wills 19 , Reference Owen, Martin and Whincup 20 ). In the same field trial, maternal counselling during the 1st year of life was effective in reducing children’s energy-dense food consumption at 12 months( Reference Vitolo, Bortolini and Feldens 18 , Reference Vitolo, Bortolini and Campagnolo 21 ), in improving diet quality at 4 years( Reference Vitolo, Rauber and Campagnolo 22 ) and lipid profile in daughters at 8 years of age( Reference Louzada, Campagnolo and Rauber 23 ). We are not aware, however, of any studies of the potential benefits of maternal counselling on infant feeding in reducing insulin resistance in childhood, particularly in developing countries. We expect that efforts to prevent insulin resistance, especially if started early enough, could delay the progress of metabolic complications and optimise healthier outcomes( Reference Zheng, Xiao and Zhang 24 ). We therefore investigated whether the effects of our home-based infant nutrition interventions were still seen after the study population had reached the age of 8 years. For this study, our focus was on selected metabolic parameters related to insulin resistance, including serum glucose, insulin and the homoeostasis model assessment index of insulin resistance (HOMA-IR) measure. To further clarify the relationship between body size and insulin resistance in children, we also examined the relationship between the metabolic parameters and the weight changes over time in children of mothers who did and did not receive dietary counselling.
Methods
The randomised field trial was conducted at the maternity wards of a hospital in a low-income population setting in the city of São Leopoldo, Brazil. Mothers of healthy, singleton, full-term (>37 weeks) and normal birth weight (≥2500 g) babies were invited to participate. We excluded HIV-positive mothers, infants with congenital malformations or infants who were admitted to neonatal intensive care units, and individuals with breast-feeding impediments. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Universidade Federal de Ciências da Saúde de Porto Alegre. All parents provided written informed consent. We followed the Consolidated Standards for Reporting Trials guidelines to report on this randomised field study( Reference Schulz, Altman and Moher 25 ). The study has been registered at www.clinicaltrials.gov (identifier NCT00629629).
A total of 500 mother–child pairs were recruited by fieldworkers from maternity wards, representing 89·5 % of all invited mothers. To guarantee blinding of the intervention assignment, an investigator not involved in the recruitment conducted the randomisation procedure during the trial. Block randomisation was used to avoid imbalances during the randomisation process. Mothers who agreed to participate were sequentially listed on the basis of their time of delivery, grouped in blocks of five and their names separated in opaque, sealed envelopes. Two mothers from each block were randomly assigned to the intervention group, and the remaining three mothers were allocated to the control group. At the end of the randomisation, 200 children were allocated to the intervention group and 300 to the control group. We included more mother–child pairs in the control group as we expected greater losses to follow-up in controls because of a lower frequency of follow-up home visits.
For the original trial, we calculated that a sample of 363 infants would be required to detect a 65 % increase in the frequency of exclusive breast-feeding up to 4 months of age in the intervention group (with 80 % power and α=5 %), assuming a 21·5 % frequency of exclusive breast-feeding in the control group. For the current follow-up at age 8 years, 128 children would be required to detect a HOMA-IR change of 0·5 sd units, with 80 % power and α=5 %.
Intervention
The intervention consisted of dietary advice about breast-feeding and complementary feeding based on the ‘Ten steps for the healthy feeding for Brazilian children from birth to two years of age’( 26 ). It was carried out between October 2001 and June 2002 by home visits within 10 d of the child’s birth, on a monthly basis up to 6 months of age, and at 8, 10 and 12 months of age. The main purpose of the programme was to promote exclusive breast-feeding for 6 months followed by healthy complementary foods. During each home visit, mothers received dietary advice in accordance with the baby’s age. Mothers were advised against the addition of sugars (cane sugar, honey) to fruit, porridge, juices, milk or other liquids. They were encouraged to avoid fried food, soft drinks, sweets and salty snacks and to use salt in moderation. Advice on hygiene practices in food preparation and handling was provided. A simple coloured leaflet with food pictures comprising a healthy meal was used to guide the dietary advice and was given to the mother as a reminder. The writing material was simplified to take into consideration the mothers’ level of education. During each visit of about 40–60 min, the fieldworkers clarified and reinforced recommendations while respecting the mother’s level of cognition as well as cultural and economic background. The dietary intervention summary and the main counselling strategies applied during each home visit have been described elsewhere in detail( Reference Louzada, Campagnolo and Rauber 23 ). The counselling was carried out by paired undergraduate students in nutrition science. The fieldworkers who carried out the dietary advice received 8 h of theoretical training. During the intervention programme, quality control was ensured by weekly scheduled meetings with all fieldworkers and the coordinator of the programme to discuss all dietary advice provided to mothers. Mothers were encouraged to report any adverse events that occurred with children during the intervention.
Control group
Mothers in the control group received the recommended standard care. They were interviewed twice during the 1st year after childbirth (at 6 and 12 months of age) for data collection only. All mothers were encouraged to maintain normal paediatric visits for their babies during the study period. Nutritional diagnoses were provided to mothers, and they were advised to talk to the health professionals about the nutritional diagnosis that we provided to them. After the 1st year, children in intervention and control groups were followed-up during childhood, and new sets of data were collected at 4 and 8 years of age.
Data collection
Data for identification and data required for locating the families in the community were collected at the time of recruitment. Trained fieldworkers, not involved in the intervention and who were unaware of group allocation, conducted face-to-face structured interviews during home visits with the mothers at 1, 4 and 8 years following the infants’ birth. Every month, 10 % of the questionnaires were selected randomly and followed-up by telephone calls to the mothers to verify the authenticity of the collected data. Children’s sex, skin colour, birth weight and mode of delivery were obtained from hospital records. Pre-pregnancy weight and maternal weight at the end of pregnancy were self-reported, and mothers’ height was measured during home visits by fieldworkers when the children were 6 months old. Pre-pregnancy BMI was calculated as pre-pregnancy weight divided by the square of height (kg/m2). Gestational weight gain was calculated by subtracting pre-pregnancy weight from weight at the end of pregnancy. Household income and duration of exclusive breast-feeding data were collected during home visits. When the children were 1-year old, the mothers were asked whether their infants had received regular healthcare services in the 1st year after birth. At 8 years of age, for diet pattern analyses, two multiple-pass 24-h dietary recalls were collected for each child on two randomly selected and non-consecutive days upon home visits to the families. The mean nutritional composition of the two 24-h dietary recalls for each child was classified according to the Healthy Eating Index (HEI)( Reference Kennedy, Ohls and Carlson 27 ), which is an instrument that attributes scores to the diet quality of individuals; the details have been described elsewhere( Reference Rauber, Louzada and Feldens 28 , Reference Rauber, Louzada and Vitolo 29 ). In order to assess a sedentary lifestyle marker among 8-year-old children, mothers were asked to report the total (hours and minutes) night-time sleep duration and the total (hours and minutes) screen-time duration (including television, computer and video-game) on the preceding day of the interview.
Anthropometric measurements
At 1 year of age, all children were weighed without clothing on a portable digital scale (Techline), and their length was measured by using an infant stadiometer (Serwital Inc.). At ages 4 and 8 years, children were weighed to the nearest 0·1 kg in light clothing without shoes on a digital scale, and standing height was measured to the nearest 0·1 cm using a stadiometer (SECA). All measures were converted into z-scores of BMI-for-age on the basis of World Health Organization Growth Standards( 30 , Reference de Onis, Onyango and Borghi 31 ). Changes in growth measurements from birth to 8 years of age were analysed as BMI-for-age z-score variation, considering three periods: from birth to 1 year, from 1 to 4 years and from 4 to 8 years of age.
Glucose profile
At 8 years of age, venous blood samples were obtained from the right arm after an overnight fast to measure serum glucose and insulin concentrations and to calculate the HOMA-IR index. Analyses were performed at the laboratory of Cardiology Institute of Rio Grande do Sul by technicians who were unaware of study assignments. Glucose and insulin were estimated using an automatic analyser (Cobas Integra®; Roche). HOMA-IR was calculated as (insulin (μU/ml)×glucose (mmol/l))/22·5( Reference Matthews, HoskeR and Rudenski 32 ).
Statistical analyses
Analyses were performed by intention-to-treat and by sex. Non-normally distributed variables were log transformed for all statistical procedures. Untransformed values are presented in all tables for ease of clinical interpretation. Student’s t test was used to evaluate the effect of the intervention on independent continuous variables. Univariate and multivariate linear regressions were performed to examine the relation of intervention and selected anthropometric covariates of special interest on glucose and insulin concentration and HOMA-IR values at age 8 years, including pre-pregnancy BMI, gestational weight gain, child’s birth weight and BMI z-score from birth to 1, 4 and 8 years of age. In univariate models, the reported β-coefficients represent separate models for each listed variable, and in multivariate models β-coefficients are adjusted for all variables in the model. We also examined the effects of adjustment for baseline social, demographic and breast-feeding variables – child’s sex (male/female) and skin colour (white/not-white), maternal schooling, total household income, mode of delivery (normal/caesarean), exclusive breast-feeding (≥4 months) and breast-feeding – on our estimates of treatment effects. Finally, we further analysed the effect of adjustment for total HEI score, total sleep and screen-time duration at 8 years of age on our estimates of treatment effects. Collinearity was checked in all models. All statistical analyses were performed using SPSS 16.0 (SPSS IBM Inc.), and statistical significance was set at P<0·05 (two-sided).
Results
Among the 500 children initially recruited, 396 underwent anthropometric assessments at age 1 year, 345 at age 4 years and 309 at age 8 years (Fig. 1). A total of 305 children underwent glucose assessment and 303 children underwent insulin and HOMA-IR assessment at 8 years of age. No adverse events were reported during the intervention. The proportion of overweight children (BMI>1 sd) was 36·1 % (n 143) at age 1 year, 20·6 % (n 71) at age 4 years and 27·5 % (n 85) at age 8 years. There were no differences in overweight prevalence proportions between intervention and control groups for the three periods (1, 4 and 8 years), and this result persisted after analyses by sex. The median duration of exclusive breast-feeding was 3·5 months (95 % CI 0·5, 6·5) in the intervention group and 1·5 months (95 % CI 0·5, 6·5) in the control group; the median duration of breast-feeding was 12·5 months (95 % CI 0·5, 12·5) in the intervention group and 10·5 months (95 % CI 0·5, 12·5) in the control group.
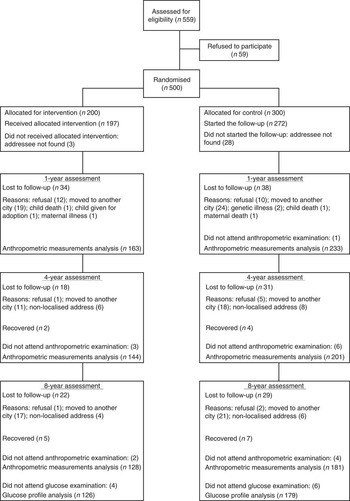
Fig. 1 Trial profile of participation in the randomised controlled trial from recruitment of mother–child pairs through the assessment at 8 years of age.
No differences were found between children who were lost to follow-up and those who remained at 8 years of age in terms of pre-pregnancy BMI (P=0·48), gestational weight gain (P=0·89), mode of delivery (P=0·88), weight at birth (P=0·55), maternal age at child’s birth (P=0·22) and maternal level of education (P=0·66). There were no differences between intervention and control groups on selected baseline characteristics (Table 1).
Table 1 Characteristics of children and their households at baseline according to the group, at age 1 yearFootnote * (Numbers and percentages; mean values and standard deviations)

* n Indicates the number of responses recorded for each characteristic.
† Student’s t test or χ 2 was used (depending on categorical or continuous variables).
There were no differences between intervention and control groups with respect to glucose and insulin concentrations and HOMA-IR indices at 8 years of age, for both sexs (Table 2). In addition, there were no significant differences between intervention and control groups comparing BMI z-score changes from birth to 1 year, from age 1 to 4 years and from age 4 to 8 years, in girls and boys.
Table 2 Intervention effect on glucose, insulin and homoeostasis model assessment index of insulin resistance (HOMA-IR)Footnote * at 8 years of age and on BMI z-score variation from birth to 8 years of age, according to sex (Mean values and standard deviations; differences and 95 % confidence intervals)

* HOMA-IR: (insulin (μU/ml)×glucose (mmol/l)/22·5).
† Student’s t test was used.
‡ Non-normally distributed variables were log transformed.
The linear regression analyses evaluating the associations between anthropometric covariates and glucose, insulin and HOMA-IR values at 8 years of age are shown in Table 3. In multivariate analysis, birth weight and the increase in BMI z-scores between 1 and 4 years and between 4 and 8 years all contributed significantly to glucose and insulin concentrations at age 8 years and to rises in HOMA-IR. The increase in BMI during the 1st year of life was positively associated with rises in insulin and HOMA-IR. These findings persisted after adjustment for child sex and skin colour, total family income, maternal schooling, mode of delivery and exclusive or partial breast-feeding. Additional analyses were performed after further adjustment for total sleep and screen-time duration and total HEI score at 8 years of age and all results persisted.
Table 3 Linear regressions analysis of glucose, insulin and homoeostatic model assessment of insulin resistance (HOMA-IR)Footnote * at 8 years of age and independent variables (n 305) (β-Coefficients and 95 % confidence intervals)

* HOMA-IR: (insulin (μU/ml)×glucose (mmol/l)/22·5).
† Univariable model: β-coefficients for a separate model for each listed variable.
‡ Multivariate model adjusted for all variables together.
Discussion
To the best of our knowledge, this is the first randomised trial to examine the potential effects on children of maternal dietary counselling during the 1st year of life on insulin resistance at age 8. Contrary to our expectation, we did not see any changes in insulin resistance at this age.
We considered two possibilities that may explain the lack of effectiveness of the trial to affect metabolic outcomes in childhood. First, the change in dietary practices observed in this population( Reference Vitolo, Bortolini and Feldens 18 , Reference Vitolo, Bortolini and Campagnolo 21 , Reference Vitolo, Rauber and Campagnolo 22 ) might not be large enough to have any long-term impact on glucose metabolism at age 8 years. This supports the need to continue the dietary counselling even after the infancy period, to achieve adequate public policies. Second, any early changes in dietary practices may have been overridden by dietary and weight gain changes between 1 and 8 years of age, after the study intervention had ceased at age 1 year. The focus of the ‘Ten steps for the healthy feeding for Brazilian children from birth to two years of age’ intervention plan is on dietary counselling, and monitoring excessive weight gain is not emphasised by the plan. As insulin resistance is closely related to body weight( Reference Henderson, Benedetti and Barnet 3 , Reference Santiago-Torres, Cui and Adams 4 , Reference Nakasone, Miyakoshi and Sato 33 ), the lack of effectiveness of the trial to affect metabolic outcomes in older children could be related to weight gains between 1 and 8 years of age in all study participants, irrespective of dietary counselling in the 1st year of life. Although the effectiveness of breast-feeding and complementary feeding interventions to improve child nutrition, growth and development is well documented in many settings( Reference Dewey and Adu-Afarwuah 34 – Reference Lassi, Das and Zahid 37 ), less attention has been paid in clinical trials to the potential impact of weight gain in infancy and childhood as a contributor to the development of adverse metabolic outcomes, including insulin resistance in children.
In the absence of an intervention effect, we therefore also analysed the impact of selected anthropometric variables on the glucose profile and insulin resistance at ages 1, 4 and 8 years. Our findings show that increased birth weight and higher weight gain velocities from birth to 8 years of age all had a negative impact on the glucose profile and insulin resistance among school-aged children, irrespective of the dietary counselling in the 1st year of life. These findings support other observations that higher weight gain velocities at either 3–5 years( Reference Botton, Heude and Maccario 38 ) or 4–9 years of age( Reference Wells, Hallal and Wright 39 ) can lead to relatively larger increases in fat mass and insulin resistance, either through overproduction of NEFA or through increased synthesis and release of pro-inflammatory cytokines( Reference Koyama, Ichikawa and Kojima 40 ). Recently, a positive relationship was observed between BMI z-score increases and adverse insulin and HOMA-IR outcomes in Brazilian children followed-up from 4 to 10 years, although no impact was found on glucose concentrations( Reference Lourenço, Gimeno and Cardoso 41 ). Weight gain from birth to 2 years of age is already related to insulin resistance among young adults in several cohort studies conducted in developing countries( Reference Norris, Osmond and Gigante 42 ). There is also evidence that increases in fasting insulin between 3 and 6 years of age can be associated with a greater risk of type 2 diabetes, irrespective of adult BMI and parental history( Reference Sabin, Magnussen and Juonala 43 ). Although major metabolic diseases related to early insulin resistance may not become symptomatic until adulthood, our findings suggest that the detection and prevention of risk factors for insulin resistance should begin in childhood, when changes in lifestyle, including those related to weight gain, can still reduce the risk and severity of metabolic disease later in life.
This study has some limitations. Although we experienced losses to follow-up, we found no differences between the baseline characteristics of children who remained in the study and those who were lost to follow-up. Second, the mothers may have been aware of the intervention group to which they were assigned. This may have affected their responses to the study because of social desirability bias, as it is not possible to blind patients in studies that evaluate dietary advices. To minimise such bias, fieldworkers not involved in the intervention carried out the assessments. A limitation with respect to generalisability of the study findings is the choice of the study population, including only children between birth and 8 years of age from a low-income population. In response, we note that this target population is of specific interest for the introduction and qevaluation of large-scale dietary interventions to improve population health in regional or national health programmes. There could be self-report bias of pre-pregnancy weight; however, studies conducted in high-( Reference Han, Abrams and Sridhar 44 ) and low-income countries( Reference Natamba, Sanchez and Gelaye 45 ), including a study in Brazil( Reference Oliveira, Gadelha and Leal 46 ), have demonstrated that the BMI obtained using self-reported pre-pregnancy weight strongly correlated with those obtained using anthropometrics measured. Considering the outcome of this study (insulin resistance), another limitation is the fact that gestational diabetes data were not obtained and children born from those mothers could present metabolic alterations. However, this trial was conducted with healthy newborn babies, and the analyses were adjusted for pre-pregnancy weight, gestational weight gain and birth weight – variables highly related to gestational diabetes mellitus. As a further limitation, we were not able to measure insulin resistance before age 8 years, and other studies will be needed to fill this gap. This study clearly shows, however, that understanding the metabolic impact of dietary interventions and growth trends in infancy and childhood is extremely important to formulate public health strategies aimed at preventing chronic diseases and that the long-term follow-up even of study populations recruited at birth can be extremely important.
In summary, our study shows no impact of dietary counselling in the 1st year of life on metabolic profiles and insulin resistance at the age of 8 years. Our results do point, however, to the crucial relationship between infant and childhood weight gain and insulin resistance at age 8 years in school children from a low-income community in Brazil. We found that weight gains between birth and ages 1, 1–4 and 4–8 years all contributed significantly to adverse changes in insulin resistance at the age of 8 years. Our findings suggest that preventing excessive weight gain since early life is a relevant key to prevent insulin resistance in childhood.
Acknowledgements
The authors thank the families who participated in the study.
The present study was supported by the Brazil CNPq (National Council for Scientific and Technological Development).
C. S. C.: formulated the research question, analysed and interpreted the data, performed statistical analysis and wrote the manuscript; P. D. B. C.: conducted the study and critically reviewed the manuscript; L. H. L.: interpreted the data and statistical analysis and critically reviewed the manuscript; M. R. V.: designed and conducted the study, formulated the research question, interpreted the data and the statistical analysis, and critically reviewed the article. All authors: read and approved the final manuscript.
The authors declare that there are no conflicts of interest.







