In 2015, the older adult population (over 65 years of age) reached 617 million people worldwide( Reference He, Goodkind and Kowal 1 ), with those aged greater than 80 years being the fastest growing segment of the global population( Reference Wasay, Grisold and Carroll 2 ). The trend towards an increase in lifespan has led to a rapidly growing interest in the treatment and prevention of diseases associated with ageing, especially neuropsychological conditions, including mild cognitive impairment (MCI) and dementia( Reference Ngandu, Lehtisalo and Solomon 3 ). Pharmacological interventions for these conditions currently provide symptomatic relief, leaving large gaps in the ability to slow dementia disease progression( Reference Rountree, Chan and Pavlik 4 ) and improve the quality of life (QoL)( Reference Meguro, Kasai and Akanuma 5 ). The worldwide economic burden of dementia is high and increasing, particularly in developing countries, presenting challenges for the public health and aged care systems( Reference Wasay, Grisold and Carroll 2 , Reference Takizawa, Thompson and van Walsem 6 ). The situation has led to a demand for alternative treatments by individuals, their families and carers attempting to improve the QoL in affected individuals by alleviating the burden of disease( Reference Siddiqui, Min and Verma 7 ).
The theory underpinning the pathophysiology of MCI and dementia include severe neuronal apoptosis, excessive deposition of amyloid plaque, presence of neurofibrillary tangles, atrophy of the hippocampus and impaired brain glucose metabolism( Reference Harrington 8 – Reference Willette, Bendlin and Starks 10 ). The consequence of these result in symptoms including decreased cognitive function, memory loss and impaired executive function( Reference Naeini, Elmadfa and Djazayery 11 ). Increasingly with age, but particularly in those with dementia and MCI, social isolation and poor QoL ensues, with a gradual decrease in the ability to carry out daily functions independently( Reference Opara 12 ). Dementia and MCI are also associated with depressive symptoms and depressive disorders which contribute to a decline in QoL( Reference Djernes 13 ). In addition, the risk of developing these pathologies is increased in carriers of the apoE e4 (APOE4) allele, an established genetic risk factor for the most common form of dementia, Alzheimer’s disease (AD)( Reference Sando, Melquist and Cannon 14 ).
Recently, it has become more evident that MCI and dementia are predominantly associated with many different factors including the influence of non-modifiable (age, sex and genetics), and modifiable risk (education, physical activity and dietary intake) factors( Reference Xu, Tan and Wang 15 ). Dietary patterns and intake are of particular interest in the light of promising evidence about the usefulness of the Mediterranean diet in mitigating cognitive decline and dementia( Reference van de Rest, Berendsen and Haveman-Nies 16 , Reference Canevelli, Lucchini and Quarata 17 ). This evidence has led to increasing scientific interest in evaluating the role of diet and nutrition and starting to view food as a source of consumer-driven management approach of disease( Reference Mogendi, De Steur and Gellynck 18 ). Therefore, to boost nutrient intake and increase diet quality, elderly commonly use nutraceuticals and dietary supplements, as a strategy to promote well-being and prevent disease. A recent study( Reference Qato, Wilder and Schumm 19 ), identified a sharp rise in the use of nutraceuticals and dietary supplements in the elderly between 2005 and 2011, especially among females. In addition, females are more commonly turning to nutraceutical use, especially those aged over 60 years of age( Reference Gahche, Bailey and Burt 20 ). However, questions remain regarding their safety( Reference Petroczi, Taylor and Naughton 21 ), quality( Reference Gabriels and Lambert 22 ), potential interactions with conventional medication( Reference Heuberger 23 ) and their efficacy, that is, the ability to meet certain health claims made by manufacturers( Reference Gabriels and Lambert 22 ). An overall lack of regulation of the dietary supplements industry in most jurisdictions poses numerous challenges associated with safety monitoring, particularly due to transactions occurring through the internet( Reference Petroczi, Taylor and Naughton 21 , Reference Gabriels and Lambert 22 ). In addition, certain over the counter supplements may be contaminated by prohibited substances( Reference Gabriels and Lambert 22 ), including oestrogenic endocrine disruptors and melamine( Reference Deldicque and Francaux 24 ). In the USA, use of dietary supplements is associated with an estimate of more than 23 000 emergency room visits per year( Reference Geller, Shehab and Weidle 25 ). Numerous nutraceuticals, including multivitamins and iron, are associated with higher risk of total mortality in elderly females( Reference Mursu, Robien and Harnack 26 ). Despite this, the dietary supplements industry sales continue to grow with a thriving niche market for products that claim to improve cognitive function and promote brain health.
Typically nutraceuticals and dietary supplements are concentrated bioactive substances derived from foods or herbs and are usually, but not exclusively, consumed in a pill or powder form( Reference Das, Bhaumik and Raychaudhuri 27 ). The term ‘nutraceuticals’ commonly includes drugs, food ingredients and dietary supplements generally without patent protection( Reference Nasri, Baradaran and Shirzad 28 ). For this review, ‘nutraceuticals’ are defined as non-patented products and substances that do not contribute to overall energy intake but provide a potential physiological benefit or protection against chronic disease( Reference Das, Bhaumik and Raychaudhuri 27 , Reference Nasri, Baradaran and Shirzad 28 ). Although often used interchangeably with nutraceuticals, not all dietary supplements are sold with claims of protection against chronic disease. Therefore, this review included only dietary supplements with nutraceutical properties. The term nutraceutical and functional foods have also been used interchangeably( Reference Kalra 29 ), and it should be noted that functional foods may also include fortified food products; such as margarine with added n-3 fatty acids( Reference Geleijnse, Giltay and Kromhout 30 ). The main categories of nutraceuticals and dietary supplements are vitamins, minerals, herbal medicines, probiotics, n-3 PUFA, antioxidants and polyphenol supplements. Some of the claims include memory enhancement( Reference McDougall, Austin-Wells and Zimmerman 31 ), maintenance of memory function( Reference McDougall, Austin-Wells and Zimmerman 31 ), and protection from cell degeneration( 32 ), whereas others suggest that nutraceuticals exhibit beneficial outcomes in certain conditions such as AD( Reference Zandi, Anthony and Khachaturian 33 ). Nevertheless, the perception of efficacy in relation to the scientific literature available requires a systematic analysis and assessment.
This systematic review aims to analyse the recent evidence concerning the use of nutraceuticals and dietary supplements over the last 10 years and their associated effects on cognition in elderly individuals in recent, large randomised, double-blinded, placebo-controlled, human trials of at least 1-year duration and with large participant cohorts.
Methods
A systematic review of published literature was performed to identify the evidence for the long-term use of common nutraceuticals and dietary supplements to improve cognition in elderly participants (aged≥65 years).
Search strategy
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guidelines( Reference Moher, Liberati and Tetzlaff 34 ), literature searches were conducted across four electronic databases (PubMed, Scopus, CINAHL and Web of Science), to identify the most recent studies published in the last 10 years. The study was established on the 15 June 2016, therefore only articles published from 15 June 2006 to 14 June 2016 were eligible for inclusion. The search strategy was re-run on the 2 September 2017, and one further article from within our date range was located for inclusion into the systematic review. The popularity of dietary supplements has increased since 2005 and 2006( Reference Qato, Wilder and Schumm 19 ), whereas evidence surrounding the long-term use of nutraceuticals and dietary supplements has strengthened since 2006, in particular in relation to the evidence surrounding B-vitamins and cognitive function( Reference Clarke, Bennett and Parish 35 , Reference Oulhaj, Jernerén and Refsum 36 ). Observational studies are limited by heterogeneity, a reliance on self-reported nutritional data and confounding due to socioeconomic factors( Reference Canevelli, Lucchini and Quarata 17 ). Therefore, to attempt to avoid such limitations and to best examine the potential presence of causality, only randomised, double-blind, placebo-controlled human trials examining the effects of nutraceuticals and dietary supplements on cognition in older adults were identified. The database search used keywords relating to the aim of the paper; that is ‘Alzheimer*’, ‘dementia’ or ‘cognitive impairment’; ‘supplement*’ or ‘nutraceutical*’, and ‘trial*’; and ‘control*’ or ‘random*’. Where applicable, the search option ‘clinical trial’ or ‘controlled trial’ was selected to narrow the search results before the screening. During the search, the reference lists of other review articles were also manually searched( Reference Clarke, Bennett and Parish 35 , Reference Ford, Flicker and Alfonso 37 ).
Eligibility criteria
To best determine the efficacy of nutraceuticals and dietary supplements, only human, double-blinded, randomised, placebo-controlled studies >1 year in duration, with participant cohorts consisting of 100 or more elderly human subjects aged over 65 years, were eligible for inclusion. The duration of 1 year was considered essential as a minimal time period for nutraceutical interventions to potentially influence cognitive function( Reference Rainey-Smith, Brown and Sohrabi 38 ). Studies published in peer-reviewed journals only in English language were selected for the title and abstract screening. Studies were included if the primary or secondary outcomes pertained to cognitive function, cognitive performance, cognitive impairment, QoL, brain atrophy and the prevention of cognitive decline. A range of outcomes were included such as: Screening measures for cognitive impairment (i.e. Mini-Mental State Examination (MMSE)); cognitive batteries (i.e. cognitive subscale of Alzheimer’s Disease Assessment Scale (ADAS-cog)); and tests of cognitive function (i.e. Rey Auditory Verbal Learning Test (RAVLT) and California Verbal Learning Test (CVLT)). If the eligibility criteria could be met or not be determined based on the information provided in the title and abstract, then the article was retrieved and screened for inclusion.
Selection criteria and quality assessment
The reasons for exclusion of full-text articles included not sustaining a placebo arm for the full duration of the study, a mean age of <65 years and no measurement of cognitive outcomes. Articles testing the effects of medications in combination with a nutraceutical were excluded due to the inability to distinguish the beneficial properties of the nutraceutical per se. The included studies contained measures of cognitive function or screening measures as either a primary or secondary outcome. Although some studies included additional measures for depression, mood and behavioural difficulties, these results were not of interest to this review. The flow chart describing the process of the study selection is represented in Fig. 1. All full-text articles were reviewed and independently assessed for risk of bias by two reviewers (N. M. D. C. and E. N. G.) (Table 5) using the criteria suggested in the Cochrane guidelines( Reference Higgins and Green 39 ).
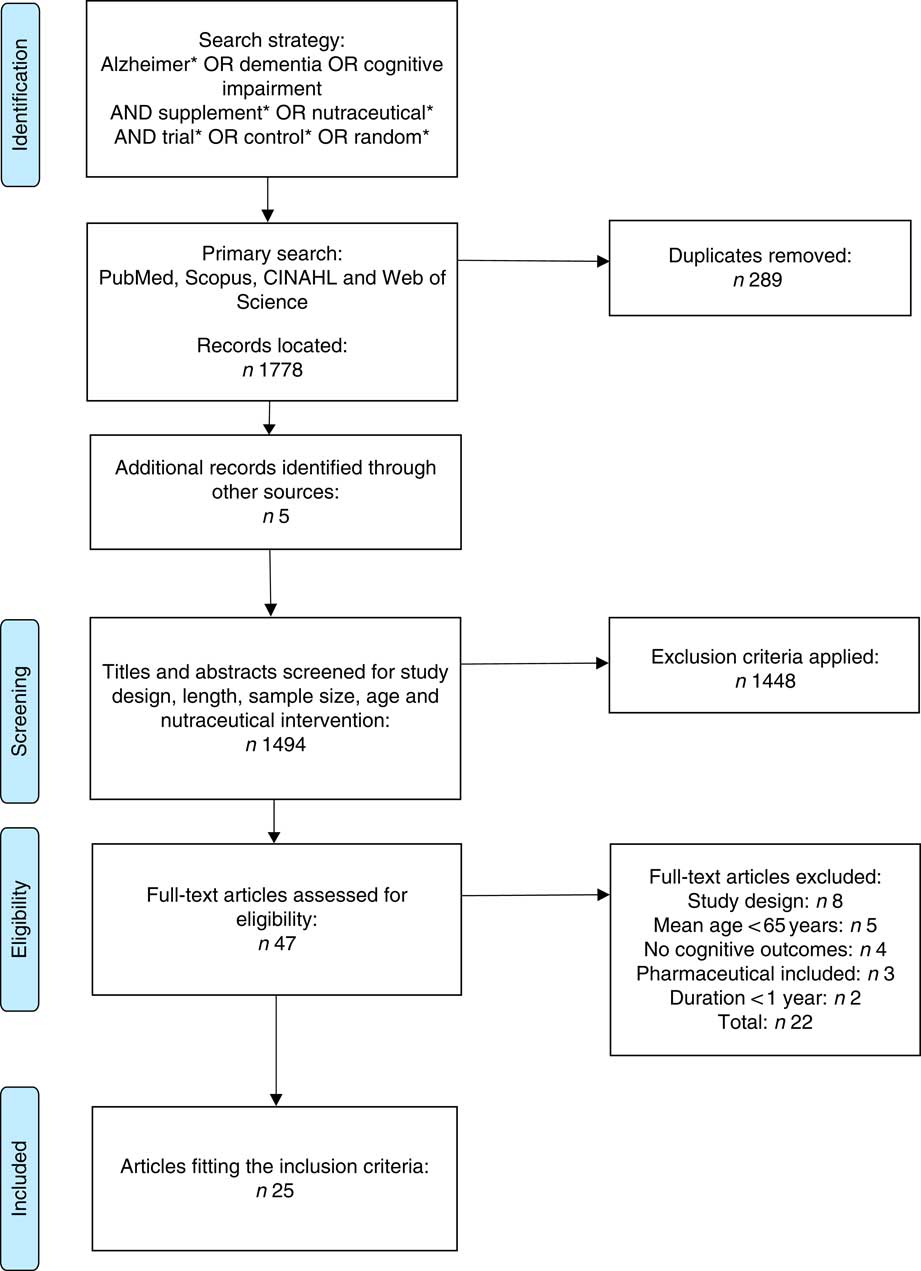
Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart summary of systematic review search process.
Results
Description of studies
A total of 1489 records were identified from initial electronic database searches, and five were identified through hand searching. In all, twenty-five studies were included following both title and abstract screening and subsequent assessment of the full-text. For this review, articles were categorised into four categories:
-
(1) products containing more than one type of nutraceutical or dietary supplements (Table 1);
-
(2) B-vitamins (products containing B-vitamins only or individual B-vitamins) (Table 2);
-
(3) n-3 fatty acids (or a combination of) (Table 3); and
-
(4) nutraceuticals or dietary supplements that did not fall within the first three categories (Table 4).
Table 1 The effect of combinations of nutraceuticals and dietary supplements on cognition in elderly participants (Mean values and standard deviations; medians and interquartile ranges (IQR))
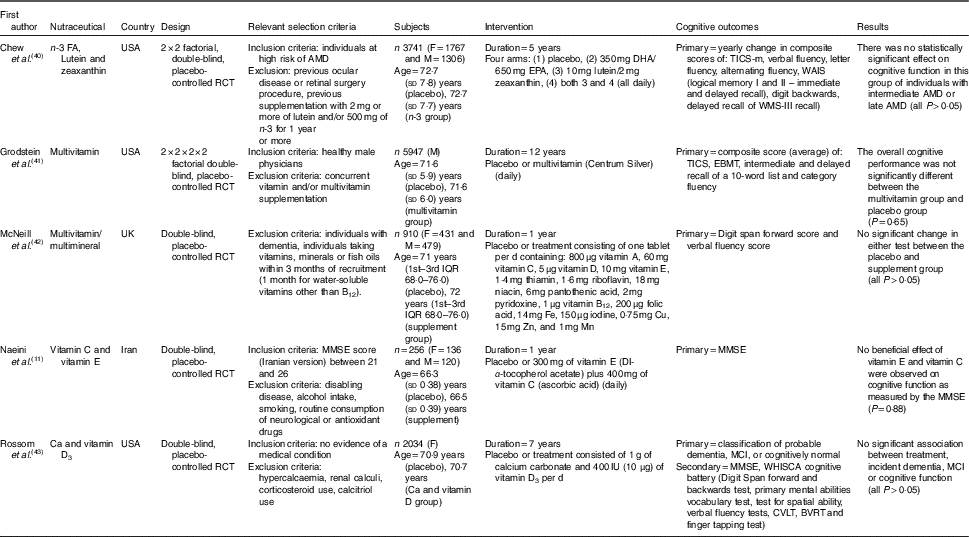
FA, fatty acids; F, female; M, male; RCT, randomised controlled trial; AMD, age-related macular degeneration; TICS, Telephone Interview of Cognitive Status; WAIS, Wechsler Adult Intelligence Scale; EBMT, East Boston Memory Test; MMSE, Mini-Mental State Examination; MCI, mild cognitive impairment; WHISCA, Women’s Health Initiative Study of Cognitive Aging; CVLT, California Verbal Learning Test; BVRT, Benton Visual Retention Test; WMS, Wechsler Memory Scale.
Table 2 The effect of B-vitamins on cognition in elderly participants (Mean values and standard deviations)

F, female; M, male; RCT, randomised controlled trial; MMSE, Mini-Mental State Examination; ADAS-cog, Alzheimer’s Disease Assessment Scale-Cognitive; ADCS-ADL, Alzheimer’s Disease Cooperative Study-activities of daily living; CDR, Clinical Dementia Rating; QOL-AD, Quality of life-Alzheimer’s Disease; Hcy, homocysteine; CVLT, California Verbal Learning Test; MCI, mild cognitive impairment; TICS, Telephone Interview of Cognitive Status; HVLT-R, Hopkins Verbal Learning Test-recall; BDI, Beck Depression Inventory; BTACT, Brief Test of Adult Cognition by Telephone; CAMDEX, Cambridge Mental Disorders of the Elderly Examination; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery; CLOX, Executive Clock Drawing Test; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; IPAQ, International Physical Activity Questionnaire; K10, Anxiety and Depression Checklist; RAVLT, Rey Auditory Learning Test; tHcy, total homocysteine; TSH, thyroid-stimulating hormone; WAIS, Wechsler Adult Intelligence Scale.
Table 3 The effect of n-3 PUFA supplementation on cognition in elderly participants (Mean values and standard deviations)
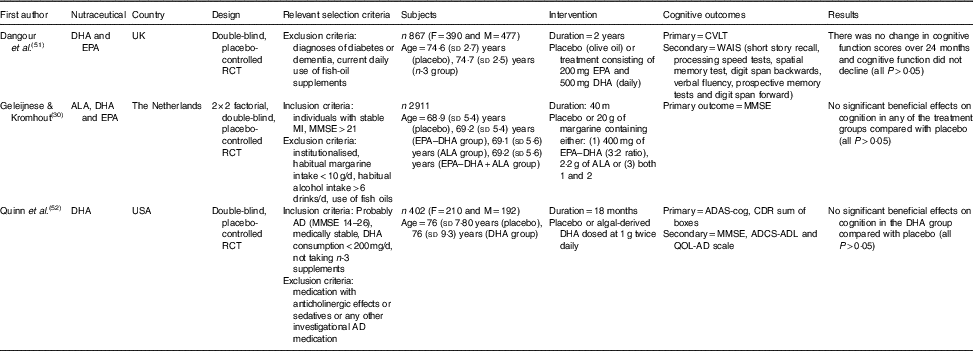
RCT, randomised controlled trial; F, female; M, male; CVLT, California Verbal Learning Test; ALA, α-lipoic acid; MI, myocardial infarction; MMSE, Mini-Mental State Examination; AD, Alzheimer’s disease; ADAS-cog, Alzheimer’s Disease Assessment Scale-Cognitive; ADCS-ADL, Alzheimer’s Disease Cooperative Study-activities of daily living; CDR, Clinical Dementia Rating; QOL-AD, quality of life-Alzheimer’s disease; WAIS, Wechsler Adult Intelligence Scale.
Table 4 The effect of ‘Other’ nutraceuticals and dietary supplements on cognition in elderly participants (Mean values and standard deviations)
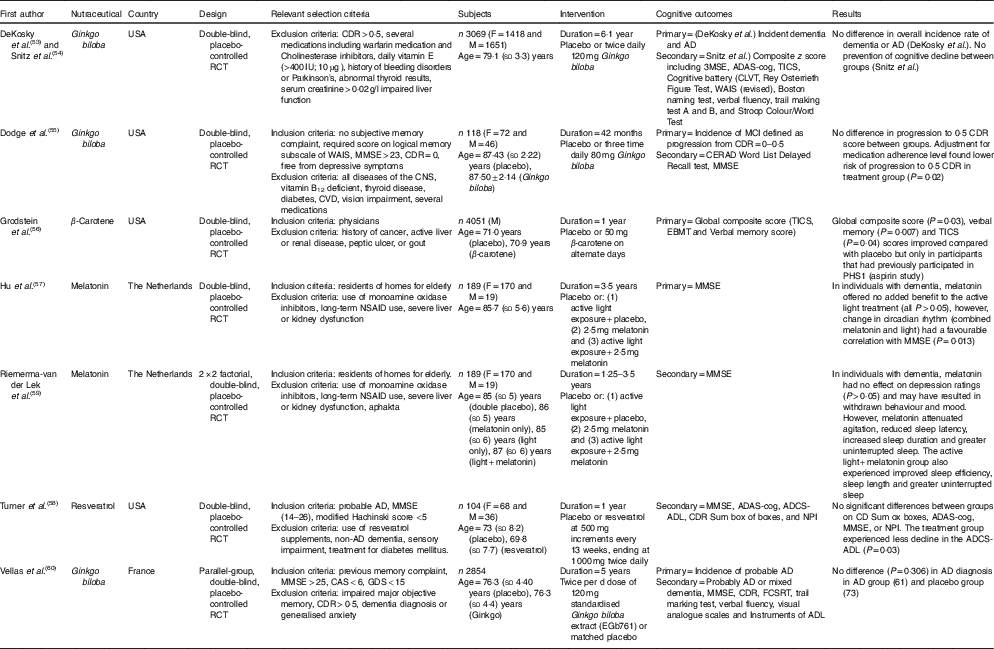
RCT, randomised controlled trial; M, male; F, female; CVLT, California Verbal Learning Test; CDR, Clinical Dementia Rating; AD, Alzheimer’s disease; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive; ADCS-MCI ADL, Alzheimer’s Disease Cooperative Study-mild cognitive impairment activities of daily living; MMSE, Mini-Mental State Examination; TICS, Telephone Interview of Cognitive Status; WAIS, Wechsler Adult Intelligence Scale; MCI, mild cognitive impairment; CAS, Cognitive Assessment System; CERAD, Consortium to Establish a Registery for Alzheimer’s disease; CLOX, Executive Clock Drawing Test; EBMT, East Boston Memory Test; PHS I, Physicians’ Health Study I; FCSRT, Free and Cued Selective Reminding Test; GDS, Geriatric Depression Scale; NPI, Neuropsychiatric Inventory; NSAID, nonsteroidal anti-inflammatory drug; NYU, New York University; RAVLT, Rey Auditory Verbal Learning Test.
Outcome measures
These main outcome measures included but were not limited to:
-
(1) MMSE score: 30-point questionnaire commonly used to measure cognitive impairment;
-
(2) Telephone Interview of Cognitive Status (TICS): global mental status test administered over the telephone;
-
(3) ADAS-cog: most frequently used test to measure cognition in clinical trials, consisting of eleven memory, language, and attention tasks;
-
(4) RAVLT: common test for episodic declarative memory;
-
(5) The Clinical Dementia Rating Scale (CDR): used to quantify the severity of symptoms of dementia (i.e. its ‘stage’);
-
(6) CVLT: commonly used to measure episodic verbal memory;
-
(7) Alzheimer’s Disease Cooperative Study-activities of daily living inventory: used for the assessment of severe cases of AD; and
-
(8) East Boston Memory Test (EBMT): short story recollection task.
The MMSE (or equivalent variant) was the most commonly used, which was applied in eighteen studies( Reference Naeini, Elmadfa and Djazayery 11 , Reference Geleijnse, Giltay and Kromhout 30 , Reference Oulhaj, Jernerén and Refsum 36 , Reference Ford, Flicker and Alfonso 37 , Reference Rossom, Espeland and Manson 43 , Reference Aisen, Schneider and Sano 44 , Reference de Jager, Oulhaj and Jacoby 46 – Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 , Reference Quinn, Raman and Thomas 52 – Reference Dodge, Zitzelberger and Oken 55 , Reference Hu, Riemersma-van der Lek and Patxot 57 – Reference Vellas, Coley and Ousset 60 ); whereas the TICS or TICS-modified (TICS-m) was used in nine studies( Reference Oulhaj, Jernerén and Refsum 36 , Reference Ford, Flicker and Alfonso 37 , Reference Chew, Clemons and Agron 40 , Reference Grodstein, O’Brien and Kang 41 , Reference de Jager, Oulhaj and Jacoby 46 , Reference Walker, Batterham and Mackinnon 50 , Reference DeKosky, Williamson and Fitzpatrick 53 , Reference Snitz, O’Meara and Carlson 54 , Reference Grodstein, Kang and Glynn 56 ) and the ADAS-cog was used in six studies( Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 , Reference Quinn, Raman and Thomas 52 – Reference Snitz, O’Meara and Carlson 54 , Reference Turner, Thomas and Craft 58 ), respectively.
Effect of nutraceuticals and dietary supplements on cognitive function in elderly individuals
Combinations of different nutraceuticals
Two included studies( Reference Grodstein, O’Brien and Kang 41 , Reference McNeill, Avenell and Campbell 42 ) tested the implementation of a broad-spectrum, once daily, multivitamin supplement on cognitive function in elderly individuals. The 12-year long study by Grodstein et al.( Reference Grodstein, O’Brien and Kang 41 ) was a subgroup of the Physicians’ Health Study II, which enrolled 5947 male physicians (age: 71·6 years and supplied them with Centrum® Silver, a commercially available multivitamin (composition in Table 1). This highly educated, health-conscious cohort reported no benefits in overall cognitive performance over three follow-up visits (mean duration between assessments: 2 years between 1st and 2nd, 4 years between 2nd and 3rd, 4 years between 3rd and 4th) based on a composite score including TICS and EBMT (both, P>0·05). In the global composite score, the difference in cognitive change between placebo and multivitamin groups over the follow-up period was −0·01 statistical unit (SU). To our knowledge, to date, this is the longest study investigating the effect of a nutraceutical on cognition. However, there are numerous limitations found with this study including selection bias. Male physicians by their vocation are required to have excellent cognition and may already have been consuming a healthy diet and thus derived little to no benefit from additional vitamins and minerals. They are also wealthier and rarely smoke, drink or use recreational drugs. A large proportion of the participants may have also benefited from previous successful long-term treatment with aspirin in the Physicians’ Health Study I( Reference Veronese, Stubbs and Maggi 61 ). It is also possible that higher doses of certain vitamins with may be necessary to elicit the desired effect, whereas multivitamin absorption and bioavailability has been questioned( Reference Yetley 62 ). It was also reported that the participants had 83·5 and 84·2 % compliance at least two-thirds of the time, which were likely to influence the results. However, a trial published in 2007 using a multivitamin or placebo, given to 910 participants including 431 females, also reported no benefits on cognitive function in older participants( Reference McNeill, Avenell and Campbell 42 ). In addition, no benefit for digit span forward and verbal fluency scores was found over 1 year. Further analysis suggested a weak benefit in verbal fluency scores for participants aged over 75 years (mean difference of 2·8 SU, p for the difference between groups >0·05) who were also at risk of micronutrient deficiency based upon a simple 17 item questionnaire at baseline. Compared with the 12-year study in physicians( Reference Grodstein, O’Brien and Kang 41 ), this study( Reference McNeill, Avenell and Campbell 42 ) had lower compliance (78 %). However, it is likely that participants experiencing a decline in cognition may have been lost to follow-up. In addition, only short-term memory and executive function were assessed. Therefore it is difficult to draw broad conclusions from this study. The initial cognitive testing was conducted face to face; however, the end point tests were performed over the phone, potentially limiting the utility of these findings.
In 2015, a study by Chew et al.( Reference Chew, Clemons and Agron 40 ) assessed cognition in participants with both intermediate and late age-related macular degeneration, consuming either placebo or supplement containing n-3 fatty acids DHA and EPA, along with antioxidants lutein and zeaxanthin. Over 5 years (median), this supplementation demonstrated no beneficial cognitive effects as measured by yearly visits and 30-min telephone interview cognitive testing sessions (P=0·63) at 6 months between visits. A strength of this study was the administration of the hearing inventory conducted at the commencement of each bi-annual phone call and the consistent progression of the cognitive tests. Participants were well-nourished and highly educated, but adherence was 75 % during the 5-year follow-up. A major limitation of this study was that all participants had a neurodegenerative condition, therefore, no significant cognitive improvements was a rather predictive outcome.
A 1-year, 2014 Iranian randomised controlled trial (RCT), supplemented vitamin C and E or placebo in elderly individuals with MCI to investigate the effect on cognition as measured by the MMSE( Reference Naeini, Elmadfa and Djazayery 11 ). Despite improved biomarkers of oxidative stress and improved performance in the MMSE, no cognitive enhancement was observed in the vitamin group compared with the placebo group (P=0·88). The MMSE was administered at three time points during the study, and strength was the homogeneity of the sample to attempt to control for confounding variables. However, despite being conducted in person, the MMSE as the sole cognitive measure is a weakness of this study and perhaps using additional cognitive tests would have uncovered specific cognitive benefits to parallel the improvements in biomarkers. The 1-year length of the study was also a limitation, especially evident by the improvements in MMSE performance by both groups.
Post hocanalysis of 2034 females in the Women’s Health Initiative 7-year Ca and vitamin D trial in the USA, revealed no associated between supplementation of calcium carbonate and vitamin D3 with incident dementia or MCI( Reference Rossom, Espeland and Manson 43 ). The primary outcome was the incidence of dementia or MCI, whereas secondary outcomes were measured by MMSE and a cognitive battery (Table 1). No significant differences were observed between groups for dementia risk (P=0·64) or MCI (P=0·72). A strength of the study was the annual assessment of domain-specific cognitive functions undertaken by a subset of 1420 participants. However, only thirty-nine in the treatment group and thirty-seven in the placebo group developed dementia during the study which limited the statistical power of the analysis and the 10 µg/d dose of vitamin D may not have been adequate to raise serum levels significantly( Reference Scragg, Stewart and Waayer 63 ). In addition, participants in the placebo group were free to supplement with Ca and vitamin D on their terms, and the treatment group consisted of significantly more current or former smokers (P=0·007). These results highlight the relatively unsuccessful outcomes of combined long-term vitamin and antioxidant supplementation to improve cognition. Although the reviewed studies admirably included trials of 5, 7 and 12 years in length, the results do not support combinations of nutraceuticals at the doses investigated.
B-vitamins
The hypothesis of an increased homocysteine (Hcy) and B-vitamin status being a risk factor for dementia and AD has generated nine studies that fell within the inclusion criteria( Reference Oulhaj, Jernerén and Refsum 36 , Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 – Reference Walker, Batterham and Mackinnon 50 ), which have been included in this review. A variety of doses and different forms of B-vitamins were used in these studies, and are described in Table 2. All of these studies included vitamin B12 as part of the intervention, with the addition of vitamin B9 (folic acid) included in eight studies( Reference Oulhaj, Jernerén and Refsum 36 , Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 , Reference de Jager, Oulhaj and Jacoby 46 – Reference Walker, Batterham and Mackinnon 50 ) and vitamin B6 in five studies ( Reference Oulhaj, Jernerén and Refsum 36 , Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 , Reference de Jager, Oulhaj and Jacoby 46 , Reference McMahon, Green and Skeaff 48 ). Three included studies( Reference Kwok, Lee and Law 47 , Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 , Reference Walker, Batterham and Mackinnon 50 ) tested the effect of folic acid and vitamin B12, whereas one study investigated the effect of vitamin B12 alone( Reference Dangour, Allen and Clarke 45 ). Between 2006 and 2012, four included studies were published with folic acid, vitamin B6 and vitamin B12 interventions( Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 , Reference de Jager, Oulhaj and Jacoby 46 , Reference McMahon, Green and Skeaff 48 ). Three of the studies reported increased plasma folate( Reference Aisen, Schneider and Sano 44 , Reference de Jager, Oulhaj and Jacoby 46 , Reference McMahon, Green and Skeaff 48 ) , two serum folate( Reference Oulhaj, Jernerén and Refsum 36 , Reference Kwok, Lee and Law 47 ) and two erythrocyte folate( Reference Ford, Flicker and Alfonso 37 , Reference Walker, Batterham and Mackinnon 50 ). Increased vitamin B12 was reported in eight of the included studies( Reference Oulhaj, Jernerén and Refsum 36 , Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 – Reference McMahon, Green and Skeaff 48 , Reference Walker, Batterham and Mackinnon 50 ). Despite reductions in plasma Hcy in eight of the B-vitamin groups in eight of the studies( Reference Oulhaj, Jernerén and Refsum 36 , Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 – Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 ), there were limited reports of cognitive improvements in the treatment groups.
The Hcy and B-vitamins in cognitive impairment (VITACOG) study has revealed numerous findings within two studies( Reference Oulhaj, Jernerén and Refsum 36 , Reference de Jager, Oulhaj and Jacoby 46 ) included in this systematic review. The VITACOG trial included relatively high doses of folic acid (0·8 mg), vitamin B6 (2 mg) and vitamin B12 (0·5 mg). Hcy was decreased in the treatment group (from 11·3 to 8·7 µmol/l), and levels of folate, vitamin B12 and holotranscobalamin were all increased. The study by de Jager et al.( Reference de Jager, Oulhaj and Jacoby 46 ) included individuals with MCI, and reported an improvement in cognition as measured by the Executive Clock Drawing Test compared with placebo (P=0·015). This study( Reference de Jager, Oulhaj and Jacoby 46 ), also found better performance in the MMSE (P<0·001), Hopkins Verbal Learning Test-delayed recall (P=0·001) and semantic memory (category fluency) (P=0·037) tests in those with higher Hcy levels (>11·3 µmol/l). In the same study, an increase of Hcy levels was reported in the placebo group (before: 11·6 µmol/l; after: 12·4 µmol/l), which made the results more reliable and robust. Further analysis from the VITACOG study( Reference Oulhaj, Jernerén and Refsum 36 ) was published by Oulhaj et al.( Reference Oulhaj, Jernerén and Refsum 36 ) to analyse plasma concentrations of n-3 fatty acids. The groups were categorised into three tertiles, revealing higher performance in the third tertile of the B-vitamin group compared with placebo for episodic memory score (P=0·047), better outcome in the CDR score (P=0·043) and CDR sum-of-boxes score (P=0·04). In the B-vitamin group, the highest tertile of baseline n-3 fatty acids reported improved performance compared with the lowest tertile in episodic memory score (P=0·01) and TICS-m (P=0·035). The authors also propose more significant effects with higher baseline concentrations of DHA compared with EPA. However, the authors report this data with the suggestion to use these results for hypothesis generation of future trials due to co-variation between DHA and EPA. Importantly, this study provides data recognising a lack of effect of B-vitamins relative to low baseline plasma n-3 fatty acids. Both included VITACOG studies( Reference Oulhaj, Jernerén and Refsum 36 , Reference de Jager, Oulhaj and Jacoby 46 ) were part of a secondary analysis of cognitive function conducted as a follow-up to previous findings of a reduced rate of brain atrophy (0·76 %/year) in the B-vitamin group compared with placebo (1·08 %/year)( Reference Smith, Smith and de Jager 64 ). Despite these significant findings, these are still only preliminary results, and larger studies are required.
Out of all eight included studies, the two largest studies by van der Zwaluw et al.( Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 ) (n 2919; cognitive subsample: n 856) and Walker et al.( Reference Walker, Batterham and Mackinnon 50 ) (n 900) both reported small improvements in cognition. The study by van der Zwaluw et al.( Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 ) reported a significant improvement in the MMSE (P=0·05) by the treatment group compared with the placebo group (MMSE decline of only 0·1 in the treatment group and 0·3 in the placebo group). Walker et al.( Reference Walker, Batterham and Mackinnon 50 ) detected an improvement in overall cognitive function score in a general population sample with elevated psychological distress. Participants were assessed by the TICS-m (P=0·032), with the treatment group reporting improvements in immediate (P=0·046) and delayed recall (P=0·013) when compared with the placebo group at 24 months( Reference Walker, Batterham and Mackinnon 50 ). It should be noted that TICS is not as sensitive as an in-person interview. The study by Walker et al.( Reference Walker, Batterham and Mackinnon 50 ) reported increased Hcy over 24 months, although this increase was lower (P<0·001) in the treatment group (from 9·6 to 10·4 µmol/l), compared with the placebo group (from 9·8 to 12·0 µmol/l). Both interventions consisted of 400 µg folic acid. However, van der Zwaluw et al.( Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 ), used 500 µg of vitamin B12 compared with Walker et al.( Reference Walker, Batterham and Mackinnon 50 ) who used 100 µg. These two studies incorporated the lowest dose of folic acid (400 µg) used in the eight included RCT, suggesting that smaller doses potentially provide benefit to the elderly. The overall conclusions in the additional five studies did not report cognitive improvements with B-vitamin treatment( Reference Ford, Flicker and Alfonso 37 , Reference Aisen, Schneider and Sano 44 , Reference Dangour, Allen and Clarke 45 , Reference Kwok, Lee and Law 47 , Reference McMahon, Green and Skeaff 48 ). However, Kwok et al.( Reference Kwok, Lee and Law 47 ), reported reduced rate of cognitive decline in the Mattis dementia rating scale with B-vitamins in individuals with plasma Hcy>13 µmol/l (P=0·003).
There were several limitations apparent in these studies. Four included studies included participants with mean Hcy levels >13 µmol/l at baseline( Reference Ford, Flicker and Alfonso 37 , Reference Dangour, Allen and Clarke 45 , Reference McMahon, Green and Skeaff 48 , Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 ). Only two of these( Reference Dangour, Allen and Clarke 45 , Reference McMahon, Green and Skeaff 48 ) including individuals with mean levels considered to be hyperhomocysteinaemia at baseline (>15 µmol/l). Five of the included studies did not include participants that had experienced a cognitive decline( Reference Ford, Flicker and Alfonso 37 , Reference Dangour, Allen and Clarke 45 , Reference McMahon, Green and Skeaff 48 – Reference Walker, Batterham and Mackinnon 50 ). The two VITACOG studies included participants with MCI( Reference Oulhaj, Jernerén and Refsum 36 , Reference de Jager, Oulhaj and Jacoby 46 ), and only two studies included participants with dementia( Reference Aisen, Schneider and Sano 44 , Reference Kwok, Lee and Law 47 ). The placebo groups in the studies did not experience cognitive decline, limiting the ability for meaningful statistical comparisons to be made. Furthermore, the use of the MMSE by six of the studies( Reference Ford, Flicker and Alfonso 37 , Reference Dangour, Allen and Clarke 45 , Reference de Jager, Oulhaj and Jacoby 46 , Reference McMahon, Green and Skeaff 48 – Reference Walker, Batterham and Mackinnon 50 ), is not suitable to detect subtle changes in cognition. Four of the studies excluded individuals based upon low blood baseline B-vitamin levels( Reference Aisen, Schneider and Sano 44 , Reference Dangour, Allen and Clarke 45 , Reference Kwok, Lee and Law 47 , Reference Walker, Batterham and Mackinnon 50 ). Several studies referred individuals with low blood B-vitamin levels for medical treatment. It is possible that supplementation with B-vitamins only benefits those with low B-vitamin status. However, only the study( Reference Dangour, Allen and Clarke 45 ) of vitamin B12 by Dangour et al.( Reference Dangour, Allen and Clarke 45 ) used moderately deficient participants. Some heterogeneity of blood B-vitamin levels may have occurred in the studies not excluding participants due to baseline B-vitamin levels( Reference Ford, Flicker and Alfonso 37 , Reference de Jager, Oulhaj and Jacoby 46 , Reference McMahon, Green and Skeaff 48 , Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 ).
It is also noteworthy that mandatory folic acid fortification has been enacted in the USA since 1998. The inclusion of synthetic folic acid in foods may have contributed to the overall intake of participants in the study by Aisen et al.( Reference Aisen, Schneider and Sano 44 ), thus lessening any potential effect on cognition( Reference Aisen, Schneider and Sano 44 ). Aisen et al.( Reference Aisen, Schneider and Sano 44 ) also included individuals consuming B-vitamins (folic acid in multivitamin <400 µg/d), as did the VITACOG studies( Reference Oulhaj, Jernerén and Refsum 36 , Reference de Jager, Oulhaj and Jacoby 46 ) (folic acid <300 µg/d, pyridoxine <3 mg/d and vitamin B12 <3 mg/d) and Van Der Zwaluw et al.( Reference Van Der Zwaluw, Dhonukshe-Rutten and Van Wijngaarden 49 ) (folic acid <300 µmol/l). In one study( Reference Kwok, Lee and Law 47 ), it was unclear whether individuals consuming B-vitamins were excluded. Larger sample sizes may be required to assess the potential of B-vitamin formulations to improve cognition in the elderly. It has also been suggested that focus on only vitamins B6, B9 and B12 has ignored the impact of the lesser known B-vitamins involved in Hcy metabolism that may preserve brain health( Reference Kennedy 65 ). In summary, while the link between B-vitamin intake and blood levels of Hcy and folate with dementia is well recognised, the benefits due to B-vitamin supplementation remain unclear. However, research involving the interactions between the B-vitamins and biomarkers, relative to other nutrients, nutraceuticals such as the n-3 fatty acids( Reference Oulhaj, Jernerén and Refsum 36 ), and genetic polymorphisms( Reference Yassine, Rawat and Mack 66 ) remain an important area of focus.
n-3 PUFA
A study by Dangour et al.( Reference Dangour, Allen and Elbourne 51 ) conducted a 2-year trial of daily 200 mg EPA and 500 mg DHA supplementation in comparison to an olive oil placebo in cognitively healthy adults. Participant cognition did not decline during this time (Table 3), and no benefit was seen in the treatment group (P>0·05). The strengths of this study( Reference Dangour, Allen and Elbourne 51 ) included measurement of significantly higher serum EPA (treatment: 49·9 (sd 2·7) mg/l; placebo: 39·1 (sd 3·1) mg/l; P=0·009) and DHA (treatment: 95·6·9 (sd 3·1) mg/l; placebo: 70·7 (sd 2·9) mg/l; P<0·001) concentrations in the treatment group, but this suggests that higher serum levels are not associated with improved cognition. The choice of olive oil as a placebo is questionable, as olive oil itself is known to contain bioactive compounds and have beneficial health properties( Reference Panagiotakos 67 ) and as such has been associated with improved cognition( Reference Martinez-Lapiscina, Clavero and Toledo 68 ). As participants were cognitively healthy and there was a lack of decline in the control arm, it is likely that 2 years was not sufficient for any cognitive benefits to be observable.
Likewise, Geleijnse et al.( Reference Geleijnse, Giltay and Kromhout 30 ) found no benefit of daily supplementation of 400 mg EPA and DHA (3:2 ratio) added to 20 g of margarine compared with placebo in stable individuals having previously suffered a myocardial infarction. In addition, 2 g of α-lipoic acid (ALA) did not result in cognitive benefits (P=0·44), nor did a combination of ALA and the n-3 fatty acids in the margarine (P=0·12). Certainly, strengths of this study were its large sample size (n 2911), a 40-month study duration and especially reporting that no patients were lost to follow-up. In addition, participants were instructed to not use the margarine in cooking or baking, limiting the likelihood that the margarine would be degraded under high heat conditions and then consumed. However, the main limitation of this study was the sole use of MMSE as the measure of cognitive outcome. The study also employed a moderate dose of EPA and DHA, although shorter-term studies with higher doses have yielded mixed results( Reference Freund-Levi, Eriksdotter-Jonhagen and Cederholm 69 , Reference Freund-Levi, Basun and Cederholm 70 ). Furthermore, the margarine also contained other fatty acids including SFA palmitic acid and stearic acid as well as trans-fatty acids, which could lessen any potential benefits. The margarine was consumed with 4·3 slices of bread per d, potentially confounding the results further, although the authors state that this level of bread consumption is common in the study recruitment area.
The final study, Quinn et al.( Reference Quinn, Raman and Thomas 52 ) supplemented with a higher dose than the previous two studies, consisting of 1 g of algal-derived DHA twice daily (2 g total) for 18 months. Again, no significant benefits were observed in the treatment group compared with placebo, with improvements seen in the ADAS-cog in both groups. As measured in the VITACOG study( Reference Smith, Smith and de Jager 64 ), the rate of brain atrophy was also measured by volumetric MRI in a subsample of 102 participants with no reduction in the rate of total volume brain decline being observed compared with placebo. A strength of this study( Reference Quinn, Raman and Thomas 52 ) was the high dose use of DHA, however, as a plant source of DHA, it may not be absorbed as well as fish sources of DHA( Reference Ketz, Rodavich and Barnes 71 ). Despite this, plasma DHA (and a subsample of cerebrospinal fluid (CSF)) was increased in the treatment group. Interestingly, when APOE4 carriers were excluded from the analysis (n 232), APOE4 negative participants (n 170) had a slower rate of decline in the ADAS-cog and MMSE compared with the placebo group (both, P=0·03) but was not evident in other measures. As this study consisted of a high percentage of APOE4 carriers (57·7 %), this analysis sets the stage for future trials to conduct such subgroup analysis. A limitation of this study was the high attrition rate of 28 % in the DHA group and 24 % of the placebo group not completing the study with the main reason reported being due to adverse effects. The included studies have found only minor benefits to n-3 fatty acid supplementation yet future long-term trials with higher doses, and consideration of the APOE4 genotype may promote cognitive benefits.
Other nutraceuticals (β-carotene, melatonin, resveratrol and Ginkgo biloba)
As part of the Physicians’ Health Study II, 4051 physicians consumed placebo or 50 mg of β-carotene on alternate days and undertook cognitive testing by telephone( Reference Grodstein, Kang and Glynn 56 ). Analysis following 1 year of supplementation found improved performance in global and verbal memory but only in participants who also took part in the Physicians’ Healthy Study I (all P’s <0·05) which found positive effects from aspirin use( Reference Veronese, Stubbs and Maggi 61 ). Strengths of this study( Reference Grodstein, Kang and Glynn 56 ) included a dose of β-carotene being equivalent to that found in over ten medium sized carrots, and high participation rates (over 92 % in both groups). The relatively short 1-year follow-up was a limitation of this study as cognitive testing only commenced shortly before the β-carotene arm was terminated and not all baseline cognitive data were available. In addition, various confounders apply to the use of physicians as participants in cognitive studies as previously discussed.
Two included studies investigated the long-term use of melatonin and active light therapy in the same, mostly female, Netherlands nursing home dementia participants( Reference Hu, Riemersma-van der Lek and Patxot 57 , Reference Riemersma-van der Lek, Swaab and Twisk 59 ). Although melatonin is considered to be a pharmacologic agent in many jurisdictions, it is also commonly sold as an over the counter supplement to assist with sleep quality, and in Canada it is regulated as a natural health product( 72 ). One study( Reference Riemersma-van der Lek, Swaab and Twisk 59 ) reported that bedtime melatonin only treatment had no effect on cognition as measured by the MMSE (P>0·05). However, some benefits were observed regarding sleep duration and fewer sleep disruptions when combined with light therapy. Another study( Reference Hu, Riemersma-van der Lek and Patxot 57 ) reported no benefit of bedtime melatonin supplementation, only chronic effects of treatment as it applied to changes of temporal correlations with physical activity. The lack of cognitive improvements observed in these studies may be due to the sole use of the MMSE as a cognitive measure but is likely attributable to most participants being in the late stages of dementia (age: 85·7 (sd 5·6) years). This trial was a novel long-term investigation of active light therapy on the circadian rhythm, sleep and activity in dementia patients. The authors noted that the dose of melatonin (2·5 g) may be too high and thereby impacted on mood behaviour.
The study by Turner et al.( Reference Turner, Thomas and Craft 58 ) investigated whether an escalating dose of resveratrol in individuals with AD affected levels of Aβ40 and brain volume. Participants in the treatment group commenced the trial with a 500 mg dose. The dose was increased every 13 weeks resulting in a final dose of 1000 mg twice daily during weeks 39 and 52. Resveratrol may cause gastrointestinal stress at higher doses, but in this study it was well tolerated by participants with no significant differences between groups for adverse events aside from weight loss (P=0·038) in the treatment group (−0·92 (sd 4·9) kg) compared with the placebo group (+0·54 (sd 3·2) kg). The study was underpowered to detect differences in the secondary cognitive outcomes that were measured which included MMSE and the ADAS-cog. However, no benefits were identified in the treatment group except less decline in the activities of daily living score (P=0·03). Greater reductions in levels of CSF Aβ40 and plasma Aβ40 were identified in the placebo group v. the treatment group (P=0·002 and P=0·024, respectively). The level of Aβ40 has been shown to decrease as dementia advances; hence there may be a role for resveratrol supplementation to ameliorate this decline. The results also provide evidence that resveratrol crosses the blood–brain barrier as resveratrol metabolites were measurable in the CSF. However, neuroimaging found that the treatment group had greater brain volume loss (P=0·025), and higher ventricular volume compared with the placebo group (P=0·05). Ventricular enlargement has been proposed to be an important short-term marker of AD severity, including risk of progression from MCI to AD( Reference Nestor, Rupsingh and Borrie 73 ). A key strength of this study was the inclusion of analysis by APOE4genotype which revealed an effect of resveratrol on plasma Aβ40 in the APOE4carriers (P=0·04), and a greater decline in brain volume (P=0·02). More research is required to understand the significance of the findings, including if the loss in brain volume is related to the potential weight loss properties of resveratrol. A larger, longer study, powered to assess cognitive outcomes will better examine if the loss in brain volume is related to a reduction in inflammation and brain swelling.
Ginkgo biloba has been studied extensively for its potential health benefits and four publications reporting on three trials fulfilled our inclusion criteria. Two publications by DeKosky et al.( Reference DeKosky, Williamson and Fitzpatrick 53 ) and Snitz et al.( Reference Snitz, O’Meara and Carlson 54 ) investigated 3069 individuals using a twice daily dose of 120 mg Ginkgo biloba extract for a medium follow-up of 6·1 years. At enrolment, thorough exclusion criteria were applied. The majority of participants (n 2587) were determined to have normal cognition and 482 were classified as having MCI. Dekosky et al. reported no difference in incidence of dementia between the placebo group (n 246) and the treatment group (n 277). Cognitive improvements were measured by composite z score as published by Snitz et al.( Reference Snitz, O’Meara and Carlson 54 ), and also found no benefit to annual rates of cognitive decline. The rates of dropout for the study were 6·3 %, which may be considered strength of the study, however, adherence to both assignments dropped to 60·3 % of participants by the end of the study. Compliance was similar between groups but this may be a strong source of bias confounding the final results.
The 2012 study by Vellas et al.( Reference Vellas, Coley and Ousset 60 ) found no reduction in the risk of progression to AD over 5 years in participants that reported memory complaints to their physician. The study was a large and age-matched with the treatment group also receiving 120 mg of standardised Ginkgo biloba extract for consumption twice per d. The strength of this study includes the involvement of physicians in identifying participants that may be at risk of developing AD. However, progression to AD was only observed in sixty-one participants in the treatment group, and seventy-three in the placebo group, out of 2854 individuals with an average age of 76·3 years (both groups, sd ±4·4). Another strength is the exploratory subgroup analysis that tested for relationships between progression of AD and some factors including APOE4 positive status and hypertension at baseline. The analysis found fewer males progressed to AD with treatment (treatment=14, placebo=32, P=0·011) The authors suggested the overall absence of protective effect in this study may have been due to the higher level of education in the sample compared with the general population and exclusion of individuals receiving an MMSE score <25. In the smallest included Ginkgo biloba study (n 118), Dodge et al.( Reference Dodge, Zitzelberger and Oken 55 ) applied a dose of 80 mg three times daily, and found no difference between groups in progression from a CDR score of 0 to 0·5 over 42 months. Secondary analysis, found benefit when controlling for baseline medication adherence during the first 6 months of the study (hazard ratio=0·33; 95 % CI 0·12, 0·89) for progression to 0·5 CDR. This analysis demonstrated there may be benefit to older individual (mean age >87 years in both groups), however, concern was expressed for seven cases of stroke/transient ischaemic attack in the treatment group compared with none in the placebo group. All included Ginkgo biloba studies included individuals in late life, therefore, it is plausible that benefits may exist when supplementing earlier in the life. In addition, the trials also did not include individuals with dementia. A 1997 study found modest improvements with 120 mg/d Ginkgo extract in the ADAS-cog (P=0·04) in individuals with mild to severe AD( Reference Weinmann, Roll and Schwarzbach 74 ), therefore it is premature to rule out Ginkgo biloba as an effective treatment to improve cognition.
The remaining articles in this review not categorised in the first three sections found limited overall evidence of efficacy in long-term trials. Despite this, further research is warranted to investigate melatonin in younger at-risk individuals, whereas questions remain regarding the usefulness of Ginkgo biloba and resveratrol to treat or prevent impaired cognition.
At this point, it should be mentioned that no attempt was made to meta-analyse the data, due to the heterogeneity of the included studies and cognitive assessment tools used, as well as the number of different nutraceuticals listed in the systematic review.
Discussion
The use of nutraceuticals and dietary supplements with a long-term view to improving cognition in elderly individuals aged over 65 years is not supported by the currently published data. This review included randomised, double-blinded, placebo-controlled clinical trials. Only trials consisting of over 100 participants were included in an attempt to make inferences about data from studies that had more statistical power. In addition, the duration of all included studies was between 1 and 12 years, again strengthening the overall findings of this review through the exclusion of short-term studies. Long-term nutritional trials are particularly difficult to conduct due to the associated costs, yet they represent an important aspect of nutrition research due to the time it can take for positive or adverse effects to become symptomatic. There was an overall low risk of bias for the majority of studies included (Table 5). However, the longer-term focus of included studies in this review still failed to find compelling evidence that currently warrants the use of dietary supplements and nutraceuticals in the elderly. Of the included literature, none reported notable memory enhancement in the participants, and evidence of maintenance of memory function was scant at best. In addition, no studies reported a reduction in the progression towards MCI or dementia.
Table 5 Risk of bias summary for studies included in this systematic review (Mean values and standard deviations)
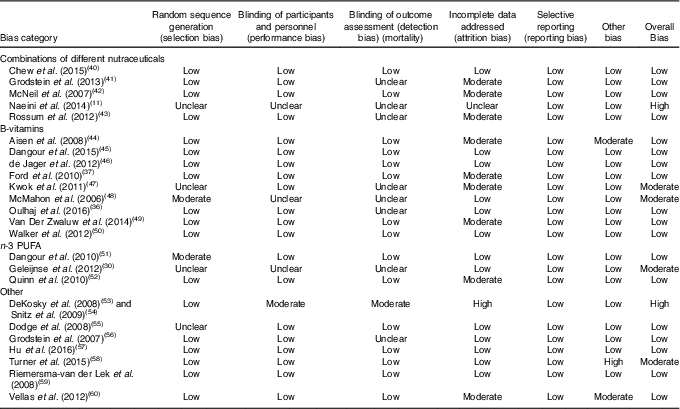
The RCT is generally regarded as the highest quality clinical trial study design in nutrition( Reference Byers 75 ), however, there are several general limitations to be considered when evaluating the ability of nutraceuticals and dietary supplements to improve cognition in the included studies (Table 5). Many included studies investigated cognitively healthy people who may not experience cognitive decline in life but may instead suffer mortality from another cause( Reference Wang, Naghavi and Allen 76 ). The included studies examined individuals aged over 65 years during a stage of their life where the health quality may have already started to diminish. Importantly, nutraceutical interventions may be more effective if treatment begins earlier in life. In the case of B-vitamin supplementation, benefits resulting from 3 years of treatment have been observed in older individuals under 65 years that were recruited based on elevated Hcy levels (>13 µmol/l)( Reference Durga, van Boxtel and Schouten 77 ). RCT investigating nutraceutical intake in individuals already diagnosed with dementia and AD may be undertaken too late to be able to measure noticeable changes in neuropsychological or pathological features. Moving forward, the focus of long-term dietary supplements and nutraceutical use should focus longitudinally on individuals at increased risk of developing MCI or AD at an earlier life stage. This review has suggested mixed results with benefits to cognition associated with B-vitamin use especially in studies with larger cohorts and benefits related to n-3 fatty acids. The VITACOG study has revealed reduced brain atrophy with B-vitamin use( Reference Smith, Smith and de Jager 64 ), and an important recent finding suggesting that efficacy of B-vitamins to improve cognition may be dependent on plasma n-3 fatty acid status( Reference Oulhaj, Jernerén and Refsum 36 ). DHA was also found to have a greater effect on cognition in individuals without a copy of the APOE4 gene( Reference Quinn, Raman and Thomas 52 ). In addition, the quality of supplemental fish oil is a determinant of their health effects( Reference Rundblad, Holven and Ottestad 78 ). Taken together, these findings highlight the complicated nature of elucidating the potential benefits of individual nutrients and categories of nutrients, relative to blood biomarkers and genetic risk factors. This systematic review has uncovered only limited evidence supporting the use of nutraceuticals and dietary supplements to improve cognition in the elderly. However, future trials sufficiently powered to investigate specific cognitive risk factors are likely to find more benefits in particular groups including the elderly.
When assessing the viability of nutraceutical and dietary supplement use in the elderly, a number of considerations must be taken into account. Firstly, there exists a poorly defined area between the use of specific compounds from food that potentially assert health benefits, and those that are considered formally as pharmaceutical agents. Synthetic versions of nutraceuticals may hold different physiological properties than naturally occurring forms found in food( Reference Burton, Traber and Acuff 79 ). Second, all trials included in this review used sample populations at varying life stages and degrees of cognitive health, as indicated by the heterogeneity between selection criteria. For example, MMSE cut-offs varied throughout studies with >23 being considered to exclude significant cognitive impairment in one study( Reference Dodge, Zitzelberger and Oken 55 ), and 24 in another( Reference Dangour, Allen and Clarke 45 ). In one study( Reference Naeini, Elmadfa and Djazayery 11 ), participants were classified to have MCI with an MMSE score between 21 and 26, whereas another classified probable AD as being between 14 and 26( Reference Quinn, Raman and Thomas 52 ). Variation between different stages of cognitive impairment across the included trials makes it difficult to make conclusions regarding the overall usefulness of the nutraceuticals. Only seven studies in this review included individuals with dementia( Reference Aisen, Schneider and Sano 44 , Reference Kwok, Lee and Law 47 , Reference Quinn, Raman and Thomas 52 , Reference Hu, Riemersma-van der Lek and Patxot 57 – Reference Riemersma-van der Lek, Swaab and Twisk 59 ), and three included studies( Reference Naeini, Elmadfa and Djazayery 11 , Reference Oulhaj, Jernerén and Refsum 36 , Reference de Jager, Oulhaj and Jacoby 46 ) used individuals with MCI. Certainly, there is difficulty in conducting trials in individuals with cognitive impairment and several studies in this review required caregiver assistance or approval to participate from a proxy. It is unrealistic to expect trials conducted worldwide to utilise the same selection criteria, however, standardised criteria would be highly advantageous moving forward to assist in determining the cognitive outcomes in nutritional intervention studies. This may include standardised goal criteria for the baseline cognitive status, a hierarchy of suitable cognitive outcome measures, and also guidelines to inclusion criteria based on important biomarkers. Further, there exists difficulty in assessing the optimal doses for treatment with nutraceuticals and supplements. For example, folate toxicity may be breached through supplementation, a relatively high folate containing diet, and also the addition of folic acid through fortified foods in many countries. Although folic acid fortification has been determined important to prevent neural tube defects, risks of excessive B-vitamin intake exist with risk of chromosomal and nerve damage , particularly with certain gene variants( Reference Lucock and Yates 80 ). Moreover, individuals with normal serum vitamin B12 and high folate levels have been demonstrated to be more likely to have impaired cognition( Reference Moore, Ames and Mander 81 ).
Genetics play a significant role in cognitive performance in the elderly. The APOE4gene is not only a major risk factor for AD but even in non-demented individuals, it predicts a higher rate of decline in verbal memory and abstract reasoning( Reference Schiepers, Harris and Gow 82 ). The studies in this review mostly overlooked the effect of the APOE4 genotype on cognition in their analysis. Quinn et al.( Reference Quinn, Raman and Thomas 52 ) found that when APOE4 genotype was excluded, a slower rate of cognitive decline was observed in participants that did not carry the APOE4 gene( Reference Quinn, Raman and Thomas 52 ). As APOE4 is recognised as the most powerful genetic predictor of AD( Reference Liu, Kanekiyo and Xu 83 ), it is possible that APOE genotype is confounding the results in some studies and that greater benefits may have been observed in the treatment groups with APOE4 carriers excluded. Nutrient status in the brains of APOE4 carriers may differ, as evident by differences in Se concentrations in AD brains of APOE4 carriers( Reference B, Hare and Lind 84 ). In addition to APOE genotype, several different polymorphisms of the fatty acid desaturase (FADS) gene cluster compromise n-3 status by slowing the conversion of alpha-linolenic acid to EPA and DHA( Reference Minihane 85 ). Other FADS variants also increase the number of long-chain PUFA that may be synthesised from plant-based foods( Reference Lucock, Martin and Yates 86 ). Although expression of FADS may be moderated hormonally( Reference Meyer, Onyiaodike and Brown 87 ), further research is required to determine if there is a causal relationship between FADS and n-3 fatty acid metabolism. Higher intakes of n-3 fatty acids may be required to elicit benefits in individuals with specific FADS polymorphisms. Future studies on the effects of nutraceuticals on cognition in specific genetic subgroups are warranted, particularly in APOE4 carriers, due to the relatively high number of individuals diagnosed with AD( Reference Farrer, Cupples and Haines 88 ).
Presently, older people and their carers must apply caution when considering the use of nutraceuticals and dietary supplements. The majority of nutraceuticals consumed at the recommended doses are generally regarded as safe in most cases( Reference Hathcock 89 ). However, toxic side effects are still common, and caution is advised( Reference Nasri, Baradaran and Shirzad 28 , Reference Guallar, Stranges and Mulrow 90 ), including their potential to interact with prescribed drugs( Reference Heuberger 23 ). Relative to cognition, only a limited number of nutraceuticals have been systematically studied long-term for safety. Numerous studies have found no benefit to mortality from dietary supplements and nutraceutical use. In fact, a 2013 review of seventy-eight RCT( Reference Bjelakovic, Nikolova and Gluud 91 ) suggested that β-carotene, vitamin E and high dose vitamin A may be associated with increased all-cause mortality in participants with an average age of 63 years (range: 18–103). Meta-analyses of multivitamin-mineral supplementation and vitamin E usage (up to 5500 IU/d) both reported no effect on all-cause mortality( Reference Abner, Schmitt and Mendiondo 92 , Reference Macpherson, Pipingas and Pase 93 ). Another study( Reference Mursu, Robien and Harnack 26 ) found that greater nutraceutical and dietary supplements usage may be associated with an increased total mortality risk, particularly in association with iron supplementation. However, a small cohort study suggested that antioxidant vitamins may reduce cancer and all-cause mortality( Reference Li, Kaaks and Linseisen 94 ), with a 2013 systematic review( Reference Fortmann, Burda and Senger 95 ) also reporting on two trials with a small significant benefit from multivitamins on cancer in men. Overall, there is a lack of high-quality evidence to support the long-term use of nutraceuticals and dietary supplements, especially in those over 65 years of age, and epidemiological evidence suggests that their use may even be detrimental to health( Reference Guallar, Stranges and Mulrow 90 ).
It is also noteworthy that a number of important published trials did not fall under our inclusion criteria. The nutraceutical formulation Souvenaid® (Nutricia N.V.) includes ingredients hypothesised to support synaptogenesis such as n-3 fatty acids, Cl and uridine monophosphate, and has been shown to promote improvements in verbal recall in individuals with early AD( Reference Onakpoya and Heneghan 96 ). A larger and longer trial of Souvenaid® (Nutricia N.V.) is ongoing, and preliminary results have shown promise with reduced brain atrophy reported( Reference Soininen, Visser and Kivipelto 97 ). In addition, the recent 3-year Multidomain Alzheimer Preventative Trial( Reference Andrieu, Guyonnet and Coley 98 ) was published outside of our included search dates. Despite being one of the most comprehensive lifestyle and cognition studies conducted to date, only very minimal benefits were found comparing a multidomain intervention (cognitive training, nutritional counselling and physical activity) and n-3 fatty acids (225 mg EPA and 800 mg DHA), n-3 fatty acids alone, multidomain intervention with placebo, to placebo alone. In response to this study, it has been suggested that higher dose DHA of 2 g for longer than 3 years is necessary to promote an increase in CSF levels of DHA to promote dementia prevention( Reference Yassine and Schneider 99 ). Finally, a study investigating curcumin including ninety-six participants found mild benefits in the Montreal Cognitive Assessment in a 12-month RCT( Reference Rainey-Smith, Brown and Sohrabi 38 ). This finding is expected to compliment upcoming trials exploring combined effects of curcumin with yoga, and curcumin with fish oil.
Limitations of cognitive tests administered
The study of cognition and cognitive decline is fraught with challenges. Although several measures of cognitive function are available, these tests rely on significant time spent on administration, which may not be feasible for use in research settings. In such contexts, brief screening measures are favoured. Screening measures allow for the detection of cognitive disorders without the need for significant time spent completing actions and is the preferred method of detecting cognitive decline and dementia within large-scale studies. However, these screening measures do not allow for the detection of subtle and domain-specific cognitive changes. Although some studies made efforts to detect changes in specific cognitive domains through the use of domain-specific measures such as those that included Wechsler Adult Intelligence Scale-IV subscales, many studies relied upon broad screening measures (such as the MMSE) which lack sensitivity in the detection of domain-specific change and cognitive impairment. In the research setting, it is cost and time-prohibitive to conduct a thorough battery of cognitive tests to determine the cognitive state of participants fully. As evident in this review, the most common of these is the MMSE, followed by the TICS and ADAS-Cog. Most of the included studies conducted the tests over the telephone, which is not as effective as face-to-face assessment. Even considering these obvious limitations, brief instruments such as these are recognised to detect dementia( Reference Yassine, Rawat and Mack 66 ) adequately, but despite considerable effort in some studies, the majority included in this review may not have been suitably designed to detect subtle, but significant, changes in cognitive performance. It is also possible that detection of slowed rates of overall decline, as well as buffering of decline from nutraceutical use, was not measurable in the majority of the studies. Lastly, the dropout rates varied across the included studies and a small number considered the cause of death (by dementia) in the analysis. However, overall, it can be speculated that some participants did not complete the study due to cognitive decline, therefore, slightly skewing results towards participants capable of adhering to the protocols and follow-up.
Future directions
Future large RCT aiming to determine the effectiveness of dietary supplements and nutraceuticals to improve cognition in the elderly should include comprehensive neuropsychological testing. Although general screening measures may be optimal for physicians, to detect subtle changes across cognitive domains requires sensitive testing that may take from 2 h to 2 d to administer. This degree of assessment provides challenges, as more than just screening tests for dementia are needed to measure cognitive outcomes. A focus on the overall dietary pattern has been proposed as an intervention strategy to prevent cognitive decline, yet the current evidence from RCT is relatively weak overall( Reference Canevelli, Lucchini and Quarata 17 ). However, dietary supplements use is associated with greater compliance to healthy dietary and lifestyle habits( Reference Pouchieu, Andreeva and Peneau 100 ). Therefore, the combination of dietary and nutraceutical interventions may be a promising strategy to improve cognitive function. Advances in molecular nutrition, nutrigenetics and nutrigenomics must be considered in designing future studies investigating dietary supplements and nutraceuticals( Reference Schwartz 101 ), particularly regarding APOE4 genotyping of participants in studies of cognitive function. The consideration of several blood-based biomarkers, such as n-3 fatty acid status( Reference Oulhaj, Jernerén and Refsum 36 ), will be important in future trials. It also remains to be seen whether mandatory fortification measures, such as folic acid, will result in long-term cognitive benefits( Reference Beckett, Martin and Boyd 102 ). Curcumin is a botanical of current interest, proposed to improve cognition, but to date, mixed results have been reported( Reference Rainey-Smith, Brown and Sohrabi 38 , Reference Baum, Lam and Cheung 103 ). We suggest consideration of less-known botanicals, such as Yamabushitake( Reference Mori, Inatomi and Ouchi 104 ), which has shown benefit in a short-term trial. The absorption and bioavailability of such botanicals both in the gastrointestinal tract and across the blood–brain barrier must also be considered before long-term trials commence. Lastly, interest in nootropic supplements is also increasing, with short-term benefits in young healthy individuals( Reference Solomon, Leech and deBros 105 ) but the potential of these supplements to improve cognition in the elderly is worthy of future research.
Conclusion
The popularity of dietary supplements and nutraceuticals continues to increase; however, this systematic review has failed to locate sufficient evidence to justify long-term use to improve cognitive function in elderly individuals aged over 65 years. However, there is limited evidence regarding the interaction of blood biomarkers such as n-3 fatty acid status and genetic risk factors including APOE4, which suggests the potential benefits of nutraceuticals may be dependent on multiple factors. Until rigorous long-term clinical trials are conducted, claims made by manufacturers and under qualified health advisors, must be interpreted with extreme caution as to avoid potential side effects and future health complications. Advances in molecular nutrition and nutrigenomics will continue to uncover novel compounds, and may allow for re-investigation of well-known nutraceuticals. These findings may result in benefit to the elderly, especially those suffering from impaired cognition. This review suggests that future long-term clinical trials utilise sensitive neuropsychological testing of participants when consuming nutraceuticals, and the recruitment of individuals at high risk of developing MCI or dementia, to determine if benefits exist to improving cognitive function or delaying cognitive decline.
Acknowledgements
This study was supported by the University of Canberra Health Research Institute – Research and Support Development Program.
J. K. and N. N. designed the study and devised the research questions, N. N., N. M. D. C, E. N. G. and L. D. carried out the literature review and data analysis. N. M. D. C. wrote the manuscript with contributions from D. B. P., J. T., A. J. M. K., D. D. M. and N. N. All authors read and contributed to the finalisation of the manuscript.
The authors declare that there are no conflicts of interest.









