Routine Fe supplementation in pregnancy is a common practice to prevent Fe deficiency and Fe-deficiency anaemia, although expert opinion on this practice is divided. While pregnant women in the USA(1) are routinely advised to take Fe supplements of 30–60 mg/d, the policy in Australia(2) and the UK(3) is to screen pregnant women for anaemia and only treat those with Fe-deficiency anaemia. A reason for this is that although Fe supplementation in pregnancy improves maternal Fe status, there is a paucity of evidence of a clear clinical benefit for mothers(4, Reference Mahomed5). The long-term effects of routine Fe supplementation on child development is also unclear(4, Reference Mahomed5). We recently published a 4-year follow-up of the Adelaide Mothers' and Babies' Iron Trial (AMBIT), the first human intervention trial designed specifically to assess the effect of routine low-dose Fe supplementation (20 mg/d) on childhood intelligence quotient (IQ) and behaviour(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6). Our findings indicated that routine Fe supplementation in pregnancy had no effect on IQ at 4 years. There was, however, a higher incidence of abnormal behaviour reported by parents of children in the Fe group than in the placebo group(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6). Although this may have been a chance finding, the possibility that it reflects a true adverse effect of routine Fe supplementation in pregnancy in well-nourished populations cannot be overlooked. Animal studies have highlighted that both inadequate Fe nutrition in pregnancy and prenatal Fe overload are associated with persistent behavioural changes in offspring(Reference Kwik-Uribe, Golub and Keen7Reference deUngria, Rao, Wobken, Luciana, Nelson and Georgieff8Reference Fredriksson, Schroder, Eriksson, Izquierdo and Archer9 regardless of later Fe status or later Fe supplementationReference Kwik-Uribe, Golub and Keen(7). The aim of the present study was to assess whether routine Fe supplementation in pregnancy in a well-nourished population affects child behaviour at early school age through further follow-up of the children born in the AMBIT.
Subjects and methods
Participants
Participants were children born in the AMBIT, a double-blind randomised controlled trial of Fe supplementation in pregnancy(Reference Makrides, Crowther, Gibson, Gibson and Skeaff10). The original trial was conducted between 1997 and 1999. Four hundred and thirty non-anaemic pregnant women receiving antenatal care at the Children, Youth and Women's Health Services in Adelaide, Australia were recruited to participate. Women were randomly allocated to receive either low-dose Fe (20 mg/d) or placebo from 20 weeks gestation until delivery. The low-dose supplement was designed to increase Fe intake to the recommended dietary intake for pregnant women in Australia at that time, 22–36 mg/d(11). The compliance rate was 86 % for both groups and the dietary intake of Fe during pregnancy also did not differ between groups (13·0 (sd 5·4) mg/d in the Fe group and 13·6 (sd 5·4) mg/d in the control group; P = 0·299)(Reference Makrides, Crowther, Gibson, Gibson and Skeaff10). The incidence of Fe-deficiency anaemia and Fe deficiency in women at delivery was significantly lower in the Fe group than in the placebo group (Table 1). The present follow-up was conducted between April and November 2006 when the children born in the AMBIT were between 6 and 8 years old. Participation in the 4-year follow-up(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6) was not a prerequisite for participation in this school-age follow-up and an attempt was made to contact all mothers and children enrolled in the original study. In the present paper children are referred to as belonging to the Fe or the placebo group according to their mother's group allocation in the AMBIT. The present study was approved by the Children, Youth and Women's Health Services Research Ethics Committee. Informed consent was obtained from the families of all participants.
Table 1 The demographic characteristics of participating families

AMBIT, Adelaide Mothers' and Babies' Iron Trial; ID, Fe deficiency (defined as ferritin < 12 μg/l); IDA, Fe-deficiency anaemia (defined as Hb < 110 g/l and ferritin < 12 μg/l).
* Ranked according to the scale by Daniel(Reference Daniel25). The higher the score, the lower the skilled occupation.
Assessments
The primary outcome was child behaviour assessed using parent-rated and teacher-rated versions of the Strengths and Difficulties Questionnaire (SDQ)(Reference Goodman12) for 4–10-year-olds. The SDQ is an internationally validated brief screening measure used to assess behavioural problems(Reference Goodman13). It was chosen as the primary outcome because it was used to assess behaviour in the 4-year AMBIT follow-up in which a higher incidence of total difficult behaviours was reported by parents in the Fe group compared with control(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6). The SDQ has five subscales (conduct problems, peer problems, hyperactivity, emotional symptoms and prosocial behaviour) in addition to a combined total difficulties scale. Total difficulties scores and subscale scores are defined as ‘normal’ and ‘abnormal’ according to SDQ cut-offs(14). The questionnaire has moderate-to-strong internal reliability, test–retest reliability and external validity(Reference Goodman13, Reference Hawes and Dadds15). In addition to the SDQ, parents were also asked whether they had ever consulted a healthcare professional about difficulties with their child's behaviour and, if they had, to provide details of the consultation.
The secondary outcome was child temperament assessed using the parent-rated Short Temperament Scale for Children (STSC)(Reference Prior, Sanson, Smart and Oberklaid16). This was included to support the SDQ because temperament reflects behavioural style. Children with a history of behavioural problems have more consistently difficult temperaments than their counterparts without behavioural problems(Reference Prior, Sanson, Smart and Oberklaid16). The STSC has four subscales (approach, persistence, rhythmicity and inflexibility) in addition to a total easy–difficult temperament scale. The STSC has strong internal consistency and was developed after factor analysis of the longer Childhood Temperament Questionnaire(Reference Sanson, Smart, Prior, Oberklaid and Pedlow17). A total easy–difficult temperament score of > 1 sd above the population mean is indicative of a difficult temperament(Reference Prior, Sanson, Smart and Oberklaid16).
To assess the effect of potential covariates, parents were also asked to complete two brief measures of risk factors for behavioural problems in children. A Recent Life Events questionnaire(18) was used to assess the number and impact of life events in the family, while the General Functioning Subscale of the McMaster Family Assessment Device(Reference Epstein, Baldwin and Bishop19) was used to assess the healthiness of the family unit. Information regarding sex, gestational age at birth, birth order, duration of breast-feeding, maternal smoking in pregnancy, parental education and occupation were extracted from the original AMBIT dataset(Reference Makrides, Crowther, Gibson, Gibson and Skeaff10).
Research staff involved in data collection were blinded to the group assignment until all primary analyses were completed. Mothers had the opportunity to learn their group allocation in the AMBIT at the conclusion of the 4-year follow-up(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6) but were not reinformed during the present follow-up.
Statistical analysis
The data were analysed using SPSS software (version 10.0.1; SPSS Inc., Chicago, IL, USA). The primary analyses were based on intention to treat. Mean scores for parent-rated child behaviour, child temperament and teacher-rated child behaviour were compared between the Fe and placebo groups using independent samples t tests. Differences between categorical variables such as SDQ scores dichotomised to ‘normal’ and ‘abnormal’ were compared using χ2 tests. Statistical significance was set at a P value < 0·05 for all statistical tests. Regression analyses were conducted to examine the influence of potential covariates on group comparisons. Selection of potential covariates was based on independent predictors cited in the literature as influencing child behaviour and those maternal and child characteristics where the P value for comparison between groups was 0·2 or less.
Results
Characteristics of the participants
A total of 61 % (264 out of 433 subjects) of the children born in the AMBIT were assessed at follow-up (Fig. 1). A total of 89 % (236 out of 264 subjects) of the children who participated in this follow-up also participated in the 4-year follow-up. All children had at least 1 year of schooling at the time of assessment because children start school at age 5 years in Adelaide where the study was conducted. The social and demographic characteristics of participating families did not differ between the Fe and placebo groups (Table 1). The difference in maternal Fe status at the conclusion of the original trial (at birth) between the Fe and placebo groups remained significant among follow-up participants. There were significant differences between the socio-demographic characteristics of participants and non-participants. Mothers of children who did not participate were generally younger than participating mothers (P = 0·016). They were also more likely to have a lower skilled occupation and to have smoked during pregnancy, and less likely to have breastfed their child, than the mothers of participating children (P < 0·05 for all).

Fig. 1 Flow diagram of the original Fe trial and the early school-age follow-up.
Child behaviour
The mean scores for parent-rated and teacher-rated SDQ scores did not differ between the Fe and placebo groups (Table 2). There was no difference in the proportion of children with abnormal parent-rated behaviour scores in the two groups (Table 3). There was a higher incidence of abnormal teacher-rated peer problems scores in the Fe group than the placebo group (Table 3). The relative risk was 3·70 (Table 3). Adjustment for potential covariates including family functioning and sex of the child did not affect the statistical significance of any analyses for the behaviour outcome. There were strong positive correlations between parent-rated and teacher-rated total difficulties scores (r 0·587; P < 0·001) as well as parent-rated total difficulties scores between the 4-year follow-up(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6) and the present follow-up (r 0·593; P < 0·001). Among the thirty-six children who had an abnormal parent-rated total difficulties score at the 4-year follow-up(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6), twenty-five of them participated in the present study and ten of the twenty-five remained abnormal. There was no significant association between a behavioural consultation and group allocation (twenty-seven of 132 (20 %) children in the Fe group compared with twenty-one of 132 (16 %) children in the placebo group; P = 0·353). There were significant differences between the behaviour and temperament scores of those children who had a behavioural consultation and those who had not. Children who had had a consultation for their behaviour had higher parent-rated total difficulties scores than those who had not (16·0 (sd 5·9) compared with 7·3 (sd 4·9) for the parent-rated SDQ; mean difference 8·74 (95 % CI 7, 11); P < 0·001); they also had higher teacher-rated total difficulties scores than those who had not had a consultation (13·0 (sd 7·9) compared with 5·3 (sd 7·4) for the teacher-rated SDQ; mean difference 10·25 (95 % CI 5, 10); P < 0·001).
Table 2 Parent-rated and teacher-rated behaviour scores (Mean values and standard deviations)

AMBIT, Adelaide Mothers' and Babies' Iron Trial; MD, mean difference; SDQ, Strengths and Difficulties Questionnaire.
Table 3 Proportion of children with abnormal behaviour ratings on the Strengths and Difficulties Questionnaire (SDQ)

AMBIT, Adelaide Mothers' and Babies' Iron Trial; RR, relative risk.
Child temperament
There was no difference between either mean temperament scores or the proportion of children with difficult temperament scores in the Fe and placebo groups (Tables 4 and 5). There was a strong correlation between parent-rated behaviour total difficulties scores and total temperament scores (r 0·637; P < 0·001). Children who had consulted a health professional for behavioural problems had more difficult temperament ratings than those who had not (3·5 (sd 0·6) compared with 2·8 (sd 0·5); mean difference 0·74 (95 % CI 0·6, 0·9); P < 0·001).
Table 4 Child temperament: parent-rated temperament score (Mean values and standard deviations)
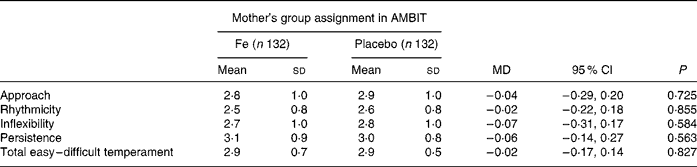
AMBIT, Adelaide Mothers' and Babies' Iron Trial; MD, mean difference.
Table 5 Child temperament: difficult temperament*
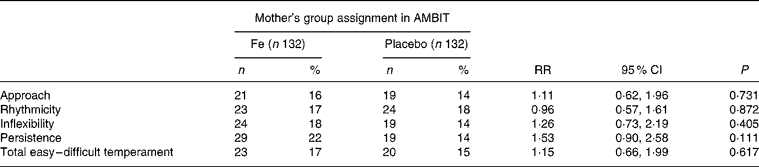
AMBIT, Adelaide Mothers' and Babies' Iron Trial; RR, relative risk.
* Defined as a score 1 sd above the sample mean(Reference Prior, Sanson, Smart and Oberklaid16).
Discussion
This is the first human intervention trial to assess the long-term effects of routine Fe supplementation in pregnancy on child behaviour and development. The present results demonstrate that Fe supplementation in pregnancy had no clear beneficial or adverse effect on parental reports of child behaviour and temperament when the children were 6–8 years of age. There was also no difference in the incidence of teacher-rated total abnormal behaviour scores in the two groups. However, significantly more children in the Fe group had an abnormal teacher-rated peer problems score than in the placebo group.
Although the majority of data collected in the present study from multiple assessments indicate no effect of Fe supplementation in pregnancy of childhood behaviour at early school age, our single report of a higher incidence of abnormal peer problems rated by teachers in the Fe group deserves some attention because the effect is consistent with our earlier observations when the children were 4 years of age(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6). The only other human study to suggest that a high Fe supply to the fetus may adversely affect early childhood development was a cohort study involving largely small-for-gestational-age infants(Reference Tamura, Goldenberg, Hou, Johnston, Cliver, Ramey and Nelson20). They reported that children with cord serum ferritin levels in the highest quartile scored lower on developmental tests including Full Scale IQ than children with cord serum ferritin levels in the two middle quartiles. Children with cord ferritin in the lowest quartile also scored lower than the median quartiles on some measures. While the investigators suggested that ferritin levels in the highest quartile may have been falsely elevated due to maternal infection, it is plausible that the high cord ferritin levels (high Fe status) adversely affected child development. These data are supported by an animal study that showed excessive Fe exposure during the neonatal period altered long-term neurobehavioural outcome, including motor behaviour and radial arm maze learning(Reference Fredriksson, Schroder, Eriksson, Izquierdo and Archer9).
On the other hand, the incidence of abnormal teacher-rated peer problems was relatively small and it is not possible to exclude that this is a chance effect because of limited sample size. The difference in teacher-rated abnormal peer problems scores was not reflected in the corresponding peer problems subscale in the parent-rated SDQ. The number of teacher questionnaires returned was less than the number of parent questionnaires and it is possible that an increased teacher response rate might have reduced the variance in scores to the level of the parent-rated scores. Conversely, it is likely that teachers witness a range of peer interactions that parents are not privy to, and this may be why the ratings vary between the two versions of the SDQ. When multiple informants are used, clinician and researcher assessments can differ depending on the information sources used in assessment and the weight given to information from different sources where disagreement exists(Reference Sawyer, Baghurst and Clark21). However, Goodman(Reference Goodman13) reported better inter-rater correlations between the parent and teacher versions of the SDQ than for other behavioural measures such as the Child Behaviour Checklist.
The parent-rated SDQ and STSC scores of the children reflect normative data for the Australian population(Reference Hawes and Dadds15, Reference Sanson, Smart, Prior, Oberklaid and Pedlow17). The parent-rated SDQ total difficulties score and the STSC easy–difficult temperament score correlated well, reflecting consistency in parent answers and increasing confidence in the ability of these measures to identify common themes in both behaviour and temperament. Adjustment for the difference in potential confounding factors of behaviour between groups including family functioning did not alter the study findings.
A potential limitation of the present study was a relatively high attrition rate although it is better or comparable with that of other long-term follow-up studies of perinatal nutrition interventions(Reference Helland, Smith, Saarem, Saugstad and Drevon22, Reference Tamura, Goldenberg, Ramey, Nelson and Chapman23). There were also significant differences between the socio-demographic characteristics of participants and non-participants. Similar differences have also been documented in other long-term nutritional studies(Reference Schoetzau, Gehring, Franke, Grubl, Koletzko, von Berg, Berdel, Reinhardt, Bauer and Wichmann24). Women with a young maternal age, low education level, who smoke during pregnancy or do not breastfeed their child are less likely to comply with the study treatment and more likely to withdraw or be lost to follow-up(Reference Schoetzau, Gehring, Franke, Grubl, Koletzko, von Berg, Berdel, Reinhardt, Bauer and Wichmann24). Despite these factors, the present study had high internal validity. Participation rates were equal across the two groups, there were no differences in the baseline characteristics of the groups and the effect of the intervention (the incidence of Fe deficiency and Fe-deficiency anaemia at delivery) remained significant and equivalent in size to that seen in the original report(Reference Makrides, Crowther, Gibson, Gibson and Skeaff10). These points highlight the overall strength of the present report as a long-term follow-up of a randomised controlled trial designed to investigate causal relationships.
In summary, our data suggest that routine Fe supplementation in pregnancy in an otherwise well-nourished population has no effect on behaviour and may even have a negative influence. However, our findings may not generalise to other populations where Fe deficiency in pregnancy is more severe. Our earlier follow-up of the same children showed no effect on IQ(Reference Zhou, Gibson, Crowther, Baghurst and Makrides6), and other randomised controlled trials of prophylactic Fe supplementation in pregnancy suggest that Fe does not improve pregnancy outcomes(4, Reference Mahomed5). Taken together, these data indicate that routine Fe supplementation of well-nourished women in pregnancy has no detectable benefit in terms of clinical measures beyond improved maternal Fe status and raise the possibility of an adverse outcome on child behaviour. It is therefore prudent that this widespread practice be re-evaluated in industrialised countries.
Acknowledgements
We thank the families who participated in the study and the Child Health Research Institute for supporting the study. We thank Heather Garreffa, Vanessa Derecki and Jenni Scambiatterra for their administrative support.
All authors contributed to the study design. Under the supervision of M. M., N. J. S. and S. J. Z. A., G. P. collected and analysed the data. A. G. P. wrote the manuscript with contributions from all co-authors. M. M. occasionally provides advice to manufacturers of prenatal nutrition supplements. Other authors had no known conflict of interest.
M. M. was supported by a National Health & Medical Research Council Senior Research Fellowship (ID 298902). The Fe and placebo tablets used in the trial were manufactured and donated by Soul Pattinson Manufacturing (Kingsgrove, NSW, Australia). The funding organizations and Soul Pattison Manufacturing had no role in the design and conduct of the study, the analysis and interpretation of the data, or the preparation, review and approval of the manuscript.








