Obesity is a serious health problem in industrialized countries and it has become prevalent in industrialized countries as a result of changes in lifestyle, especially in eating habits. Obesity is implicated in various diseases, including type II diabetes, hypertension, cancer and CHDReference Kopelman1. Obesity is characterized at the cell biological level by an increase in the number and size of adipocytes differentiated from fibroblastic pre-adipocytes in adipose tissueReference Furuyashiki, Nagayasu, Aoki, Bessho, Hashimoto, Kanazawa and Ashida2. Adipose tissue is vital for maintaining whole body energy homeostasis and consists of adipocytes, which store TAG as a fuel for the body. Excessive adipose tissue deposition is attributed to an imbalance between energy intake and energy expenditureReference Prins and O'Rahilly3. The major health consequences of obesity are predictable given an understanding of the pathophysiology of increasing body fat. Therefore, prevention and treatment of obesity are relevant to health promotion.
Phenolic acids such as hydroxybenzoic acids and hydroxycinnamic acid are antioxidant compounds in fruits and vegetablesReference Bagchi, Hassoum, Bagchi and Stohs4. Gallic acid (3,4,5-trihydroxybenzoic acid; GA) is a naturally abundant phenolic compound in vegetables, such as asparagus, broccoli and aubergineReference Yeh and Yen5. It is reported to have antioxidant, antimutagenic and anticarcinogenic activity and is expected to reduce the risk of disease and brings health benefits through daily intakeReference Galati and O'Brien6. Epidemiological evidence has shown that dietary antioxidants play a role in the prevention of several chronic diseases such as cancer, CVD and diabetesReference Cadenas and Packer7, Reference Willcox, Ash and Catignani8. A number of studies have demonstrated that antioxidants may act as a regulator of obesity in mice or rats with high fat-diets (HFD)Reference Han, Sumiyoshi, Zhang, Liu, Zhang, Zheng, Okuda and Kimura9, Reference Kuda, Iwai and Yano10. Research on phenolic acids is of current interest due to their important biological and pharmacological propertiesReference Tanaka, Kojima, Kawamori, Yoshimi and Mori11. Our previous in vitro data indicated that GA has the highest inhibition of 3T3-L1 pre-adipocyte population growth (63·9 %) among the fifteen phenolic acids testedReference Hsu, Huang and Yen12. Nevertheless, the literature data regarding the in vivo effects of GA on anti-obesity effects are limited.
Obesity has been shown to be one of the conditions that decrease antioxidant capacityReference Asayama, Nakane, Dobashi, Kodera, Hayashibe, Uchida and Nakazawa13, Reference Carmiel-Haggai, Cederbaum and Nieto14. Obesity seems to decrease antioxidant defence by lowering the levels of antioxidant enzymes (catalase, glutathione peroxidase (GPx) and glutathione reductase (GRd))Reference Carmiel-Haggai, Cederbaum and Nieto14. Cellular antioxidant defences against such oxidative stress involve antioxidant enzymes such as GPx, catalase and two forms of superoxide dismutase: a cytosolic Cu/Zn-dependent superoxide dismutase and a mitochondrial Mn-dependent superoxide dismutaseReference Tribble, Aw and Jones15–Reference Sies17. Antioxidants have been reported to play an important role in enzymatic and non-enzymatic protection against oxidative stress-induced toxicityReference Anjaneyulu, Tirkey and Chopra18, Reference Singh, Chander and Chopra19. However, the effect of GA on HFD-induced oxidative stress in obese rats has not yet been investigated. We focused on the GA-enhanced antioxidant defence levels in the hepatic tissue of HFD-induced obese rats.
In the present paper we investigated the effect of GA on HFD-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Thus, in the present study, growth parameters, weights of organ and adipose tissues, serum biochemical parameters, histology and the antioxidant defence system were measured in rats fed a normal diet (ND) and a HFD with or without GA.
Methods
Animals, diets and experimental design
Male Wistar rats (4 weeks old) were purchased from the National Science Council Animal Center, Taipei, Taiwan. Animals were housed individually in stainless steel cages in an air-conditioned room at 23 ± 2°C, 55–60 % relative humidity and a 12 h light–dark cycle and they were given a laboratory rodent chow diet for 1 week. The rats were divided into normal and obese groups (six per group) and then fed with ND and HFD, respectively. The HFD group was divided into three groups according to whether they received supplemental GA for 10 weeks: the HFD group were fed HFD only; the HFD+GA (LD) group were fed HFD with a low dose (LD) of GA (50 mg/kg rat); the HFD+GA (HD) group were fed HFD with a high dose (HD) of GA (100 mg/kg rat). These rats were provided with semi-synthetic diets (Table 1) and water ad libitum throughout the experimental period. These animals should normally be able to consume 5 % of their body weight in daily diets. The diets were stored in a 4°C cold chamber. Body weights, food intakes and food efficiency ratios were measured every day for 10 weeks. The food efficiency ratio, which is the ratio of body weight gain (g/d) to food intake (g/d), assesses utilization of food consumed. After overnight fasting, blood was withdrawn from the abdominal aorta under diethyl ether anaesthesia and serum was collected. The visceral tissues were excised immediately, rinsed, weighted and frozen in liquid N. All experimental procedures involving animals were conduced in accordance with the guidelines of the National Institutes of Health. The present experiment was approved by the Institutional Animal Care and Use Committee of National Chung Hsing University, Taichung, Taiwan.
Table 1 Composition of experimental high fat-diets (HFD)*
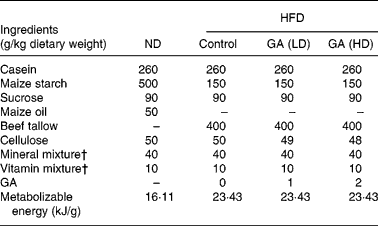
* For details of diets and procedures, see Methods.
† Mineral and vitamin mixtures (AIN-76) were purchased from Oriental Yeast (Tokyo, Japan).
ND, normal diet; GA, gallic acid; LD, low dose; HD, high dose.
Measurement of serum parameters
Blood was placed into a sterile Vacutainer plastic tube (BD Vacutainer, Plymouth, UK). Serum was separated by centrifugation (4000 rpm, 10 min) and transferred to Eppendorf tubes. All serum samples were stored at − 80°C until analysis. The concentrations of TAG, glucose, phospholipid, total cholesterol, LDL-cholesterol, HDL-cholesterol, aspartate aminotransferase, alanine aminotransferase, uric acid, creatinine, Na+, K+ and Cl− in serum were measured with commercial kits (Bayer Corporation, Tarrytown, NY, USA). The concentrations of insulin and leptin in the serum were measured with rat insulin ELISA kits (Mercodia AB, Uppsala, Sweden) and leptin ELISA kits (BioVendor, Brno, Czech Republic).
Haematoxylin/eosin and oil-red O staining
The studied animals were killed and liver samples were collected, fixed in 10 % formalin buffered solution, cut into 5-μm sections and stained with haematoxylin/eosin. Moreover, livers from the animals were frozen in liquid N, embedded in an optimal temperature-cutting compound and cut into 5 μm sections and stained with oil-red O. Haematoxylin/eosin and oil-red O staining was performed using standard techniques.
Hepatic lipid analysis
Liver lipids were extracted according to the methods of Folch et al. Reference Folch, Lees and Sloane-Stanley20 and the concentrations of TAG and cholesterol were measured by the methods of FletcherReference Fletcher21 and Sperry & WebbReference Sperry and Webb22.
Determination of GSH and GSSG in the liver
GSH and GSSG were determined after hepatic tissue was homogenized with PBS at a pH of 7·4. GSH and GSSG were determined using a glutathione assay kit (Cayman Chemical Company, Ann Arbor, MI, USA). The amounts of total GSH and GSSG were photometrically determined using a microplate reader (Awareness Technology, Palm City, FL, USA) at 405 nm and the amounts of GSH and GSSG were calculated.
Assay of antioxidant enzymes in liver
All antioxidant enzyme activities were determined after hepatic tissue was homogenized with PBS at a pH of 7·0. GPx activity was determined according to the method of Lawrence & BurkReference Lawrence and Burk23. Liver homogenate solution (100 μl) was mixed with 800 μl 100 mmol/l potassium phosphate buffer (pH 7·4), containing 1 mmol/l EDTA, 1 mmol/l NaN3, 0·2 mmol/l NADPH, 1 U/ml GRd and 1 mmol/l GSH. After 5 min, 2·5 mmol/l H2O2 (100 μl) was added to start the reaction. The absorbance change at 340 nm in 3 min was recorded. The enzyme activity was calculated using the value of E 340 = 6220/m per cm and the result was expressed in units of nmol NADPH/min per mg protein.
GRd activity was determined according to the method of Bellomo et al. Reference Bellomo, Mirabelli, Dimonte, Richelmi, Thor, Orrenius and Orrenius24. Liver homogenate solution (100 μl) was mixed with 900 μl 100 mmol/l potassium phosphate buffer (pH 7·0), containing 1 mmol/l MgCl2.6H2O, 50 mmol/l GSSG and 0·1 mmol/l NADPH, and was incubated at room temperature for 3 min. The absorbance change at 340 nm in 3 min was recorded. The enzyme activity was calculated using the value of E 340 = 6220/m per cm and the result was expressed in units of nmol NADPH/min per mg protein.
GSH S-transferase (GST) activity was determined according to the method of Habig et al. Reference Habig, Pabst and Jakoby25. Liver homogenate solution (100 μl) was well mixed with 880 μl 100 mmol/l potassium phosphate buffer (pH 6·5), containing 100 mmol/l GSH and 50 mmol/l 1-chloro-2,4-dinitrobenzene. The absorbance change at 340 nm in 3 min was recorded. The enzyme activity was calculated using the value of E 340 = 9·6/mm per cm and the result was expressed in units of nmol 1-chloro-2,4-dinitrobenzene-GSH conjugated formed/min per mg protein.
Statistical analysis
All data are presented as means and standard deviations. ANOVA were performed using ANOVA procedures. Differences were considered to be significant at 95 % CI.
Results
Effect of gallic acid on growth parameters
The composition of the diets is shown in Table 1. GA was added as 0·1 % (50 mg/kg rat) and 0·2 % (100 mg/kg rat) of the HFD. All rats were allowed free access to the test diets and water throughout the test period. The effect of GA on body weights, food intake and food efficiency in rats fed with a ND or HFD is shown in Fig. 1. After 10 weeks of feeding, body weights in the HFD group were heavier than those of the ND group. Moreover, the body weights of the HFD+GA groups were significantly decreased as compared with the HFD group (P < 0·05). Food intake in the HFD group was as high as that in the ND or HFD+GA groups, but there were no significant differences among the four groups. The food efficiency in the HFD group was significantly higher than that in both the ND and HFD+GA groups (P < 0·05).

Fig. 1 Effect of gallic acid (GA) on (A) body weights (–●–, normal diet (ND); –○–, high-fat diet (HFD); –▾–, HFD+GA (low dose; LD); –Δ–, HFD+GA (high dose; HD); (B) food intake, (C) food efficiency ratio of rats with obesity induced by a HFD. Food efficiency ratio = body weight gain (g/d)/food intake (g/d). Values are means and standard deviations for six rats. a,b Mean values with unlike letters were statistically significantly different (P < 0·05). For details of diets and procedures, see Methods.
Effect of gallic acid on organ and adipose tissue weights
The organ and adipose tissue weights of the four groups are depicted in Table 2. There were no significant differences in the organ weights of the heart, spleen, lung and kidney among the four groups. However, the weight of the liver in the HFD group was significantly increased as compared with the ND and HFD+GA groups (P < 0·05). The adipose tissue weights of peritoneal fat and epididymal fat in the HFD+GA groups were significantly decreased as compared with those of the HFD group (P < 0·05).
Table 2 Effect of gallic acid (GA) on the weights of organ and adipose tissue of rats with obesity induced by high fat-diet (HFD)* (Mean values and standard deviations for six rats per group)
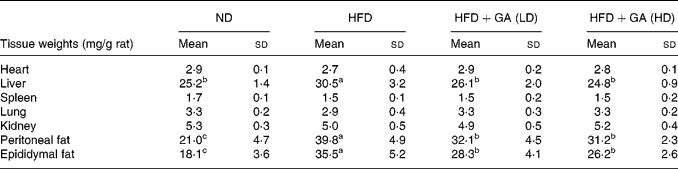
* For details of diets and procedures, see Methods.
a,b,c Mean values within a row with unlike superscript letters were significantly different by ANOVA (P < 0·05).
ND, normal diet; LD, low dose; HD, high dose.
Effect of gallic acid on serum biochemical parameters
The serum biochemical parameters of the four groups are depicted in Table 3. Serum TAG levels in the HFD+GA groups were significantly decreased as compared with those in the HFD group (P < 0·05). Serum glucose levels were not significantly different among the four groups. Serum phospholipid levels in the HFD group were significantly increased as compared with those in the other groups (P < 0·05). Total cholesterol levels in the HFD group were higher than those in the other groups (P < 0·05), but there were no significant differences between the ND and HFD+GA groups. LDL-cholesterol levels in the HFD+GA groups were significantly decreased as compared with those in the HFD group (P < 0·05), but there were no significant differences between the ND and HFD+GA (HD) groups. HDL-cholesterol levels were not significantly different among the four groups. There were no significant differences in serum aspartate aminotransferase, alanine aminotransferase, uric acid, creatinine, Na+, K+ and Cl− levels among the four groups.
Table 3 Effect of gallic acid (GA) on the serum biochemical parameters of rats with obesity induced by high fat-diet (HFD)* (Mean values and standard deviations for six rats per group)

* For details of diets and procedures, see Methods.
a,b,c Mean values within a row with unlike superscript letters were significantly different by ANOVA (P < 0·05).
ND, normal diet; LD, low dose; HD, high dose; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Effect of gallic acid on serum leptin and insulin levels
The effect of GA on serum insulin and leptin levels of obese rats induced by a HFD is shown in Fig. 2. Serum insulin levels in the HFD+GA groups were significantly decreased as compared with those in the HFD group (P < 0·05) (Fig. 2(A)). These levels in the HFD+GA (HD) group were lower than those in the HFD+GA (LD) group. Serum leptin levels in the HFD+GA groups were significantly decreased as compared with those in the HFD group (P < 0·05) (Fig. 2(B)). The serum leptin levels were not significantly different between the ND and HFD+GA (HD) groups.

Fig. 2 Effect of gallic acid (GA) on (A) levels of serum insulin and (B) leptin of rats with obesity induced by a high-fat diet (HFD). ND, normal diet; LD, low dose; HD, high dose. Values are means and standard deviations for six rats. a,b,c,d Mean values with unlike letters were statistically significantly different (P < 0·05). For details of diets and procedures, see Methods.
Effect of gallic acid on hepatosteatosis
As demonstrated in Fig. 3(A), haematoxylin and eosin staining showed normal liver architecture in the ND group. The HFD group showed more profound steatosis with macrovesicular fat accumulation. The HFD+GA groups showed microvesicular fat accumulation. However, this phenomenon in the HFD+GA (HD) group was less significant than in the HFD+GA (LD) group. These changes were confirmed with oil-red O staining. Staining with oil-red O confirmed the presence of lipid droplets within hepatocytes of the rats fed HFD (Fig. 3(B)). The number of lipid droplets of the HFD+GA groups was smaller than that of the HFD group.

Fig. 3 Effect of gallic acid on hepatosteatosis of rats with obesity induced by a high fat-diet. Livers were stained with (A) haematoxylin and eosin or (B) oil-red O. Original magnification × 200. For details of diets and procedures, see Methods.
Hepatic TAG levels in the HFD+GA groups were significantly decreased as compared with the HFD group (P < 0·05) (Fig. 4(A)). Hepatic TAG levels in the HFD+GA (HD) group were lower than those in the HFD+GA (LD) group (P < 0·05). Liver cholesterol levels were higher in the HFD group (P < 0·05), but there was no significant difference between the ND and HFD+GA groups (Fig. 4(B)).

Fig. 4 Effect of gallic acid on (A) hepatic TAG and (B) cholesterol of rats with obesity induced by a high fat-diet. Values are means with their standard deviations for six rats. a,b,c,d Mean values with unlike letters were statistically significantly different (P < 0·05). For details of diets and procedures, see Methods.
Effect of gallic acid on GSH and GSSG contents
GSH constitutes the first line of defence against free radicals. As shown in Table 4, the HFD group showed a significant reduction in GSH content (P < 0·05) in the livers of rats with obesity induced by a HFD. The GSH content in the HFD+GA groups was significantly increased as compared with that of the HFD group (P < 0·05). The GSSG content in the HFD+GA groups was significantly decreased as compared with that of the HFD group (P < 0·05).
Table 4 Effect of gallic acid (GA) on the contents of GSH and GSSG in liver of rats with obesity induced by high fat-diet (HFD)* (Mean values and standard deviations for six rats per group)
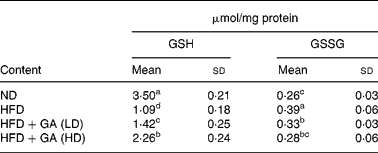
* For details of diets and procedures, see Methods.
a,b,c,d Mean values within a column with unlike superscript letters were significantly different by ANOVA (P < 0·05).
ND, normal diet; LD, low dose; HD, high dose.
Effect of gallic acid on antioxidant enzymes
As shown in Table 5, the HFD group showed a significant reduction in the activities of antioxidant enzymes (P < 0·05) in the livers of rats with obesity induced by a HFD. The GSH-dependent antioxidant enzymes activities GPx, GRd and GST showed 20, 27 and 44 % reductions, respectively, in the HFD group when compared with the ND group. Antioxidant enzymes (GPx, GRd and GST) in the HFD+GA groups were significantly increased as compared with those in the HFD group (P < 0·05).
Table 5 Effect of gallic acid (GA) on antioxidant enzymes in liver of rats with obesity induced by high fat-diet (HFD)* (Mean values and standard deviations for six rats per group)
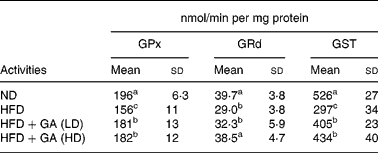
* For details of diets and procedures, see Methods.
a,b,c Mean values within a column with unlike superscript letters were significantly different by ANOVA (P < 0·05).
GPx, glutathione peroxidase; GRd, glutathione reductase; GST, glutathione S-transferase; LD, low dose; HD, high dose.
Discussion
Dietary fat is one of the most important environmental factors associated with the incidence of CVD; high cholesterol and saturated fat diets have been shown to promote atherosclerosisReference McNamara26. Han et al. Reference Han, Takaku, Li, Kimura and Okuda27 indicated that increases in body weight, fat storage, hepatic TAG content and the frequency of fatty liver are all noted. The present study was designed to establish if short periods of alternations between the various diets (ND, HFD and HFD+GA) in rats would result in different levels of dyslipidaemia, hepatosteatosis and oxidative stress. The present in vitro data indicated that the inhibition of phenolic acids on the growth of 3T3-L1 pre-adipocytes is well correlated to the antioxidant activity of the phenolic acidsReference Hsu, Huang and Yen12. In the present study, we focused on the effect of GA on rats with obesity induced by a high-energy diet. Many studies indicated that obesity is induced in mice and rats by feeding a high-energy diet containing 40 % beef tallowReference Han, Sumiyoshi, Zhang, Liu, Zhang, Zheng, Okuda and Kimura9, Reference Han, Xu, Kimura, Zheng and Okuda28, Reference Han, Kimura, Kawashima, Takaku, Taniyama, Hayashi, Zheng and Okuda29. In the present study, GA was given as a supplement at the levels of 50 and 100 mg/kg rat for a period of 10 weeks. The range of doses used in the present study was consistent with those in other studies on the effect of GA on ratsReference Niho, Shibutani, Tamura, Toyoda, Uneyama, Takahashi and Hirose30, Reference Yeh and Yen31. We found that feeding GA for 10 weeks suppressed the increases in body weight, organ weight of the liver and adipose tissue weights of peritoneal fat and epididymal fat induced by a HFD (Fig. 1 and Table 2). However, the plant-derived phenolic compound genistein is known to exhibit several biological properties. The reports indicated that genistein can reduce fat deposition in the adipose tissue of miceReference Naaz, Yellayi, Zakroczymski, Bunick, Doerge, Lubahn, Helferich and Cooke32, Reference Kim, Sohn, Lee and Lee33.
Lavie & MilaniReference Lavie and Milani34 indicated that obesity adversely affects plasma lipids, especially by increasing TAG and decreasing the level of HDL-cholesterol. We also found that the HFD+GA groups had significantly decreased levels of TAG, phospholipids, total cholesterol and LDL-cholesterol (Table 3). The HFD might lead to an increase in the synthesis of phospholipids and cholesterol esters in ratsReference Jayakumar, Nalini and Venugopal35. Hyson et al. Reference Hyson, Schneeman and Davis36 indicated that the blood level of LDL-cholesterol and its oxidation are related to cardiovascular risk and the LDL-cholesterol level of blood is an index of health. Serum insulin and leptin levels in the HFD+GA groups were significantly decreased as compared with those in the HFD group (Fig. 2). Fried et al. Reference Fried, Ricci, Russell and Laferrere37 indicated that basal levels of leptin are known to be strongly positively correlated with body fat on a HFD. The report indicated that leptin might contribute to hepatic steatosis by promoting insulin resistance and also by altering insulin signalling in hepatocytes, so as to promote increased intracellular fatty acidsReference Uygun, Kadayifci and Yesilova38. Therefore, GA prevents the increase of these levels due to its decrease of the body fat content of rats fed with HFD. However, serum glucose levels were not significantly different among the four groups. The present data indicated that intake of GA (50 and 100 mg/kg rat) for 10 weeks in Wistar rats did not affect the serum biochemical data (aspartate aminotransferase, alanine aminotransferase, uric acid, creatinine, Na+, K+ and Cl− ). Niho et al. Reference Niho, Shibutani, Tamura, Toyoda, Uneyama, Takahashi and Hirose30 reported that intake of GA (119 mg/kg per d) for 13 weeks is determined to be a no-observed-adverse-effect level in male rats. The dosage used in the present study was consistent with those in many other studies on the inhibitory effect of intake of phenolic acids in ratsReference Choi, Park, Choi and Lee39, Reference Jadon, Bhadauria and Shukla40. The data of the serum biochemical parameters (aspartate aminotransferase, alanine aminotransferase, uric acid, creatinine, Na+, K+ and Cl− ) might also support the dosage used to be a no-observed-adverse-effect level in male rats. Thus, we assume that GA has an anti-obesity effect via suppression of dyslipidaemia, hepatosteatosis and oxidative stress in obese rats.
The liver is the central organ for cholesterol, phospholipid, TAG and lipoprotein metabolism. In the histology study (haematoxylin–eosin staining and oil-red O staining), the number of lipid droplets of the HFD+GA groups was significantly reduced as compared with that of the HFD group (Fig. 3). In obesity, the liver is the receiver of large amounts of fatty acids, which cause its steatosisReference Festi, Colecchia, Sacco, Bondi, Roda and Marchesini41. The present data indicated that intake of GA for 10 weeks suppressed the increases in the levels of hepatic TAG and cholesterol induced by a HFD (Fig. 4). Moreover, reactive oxygen species have been found to be one of the main factors causing liver steatosisReference Laurent, Nicco and Tran Van Nhieu42, Reference Pessayre, Fromenty and Mansouri43. Reports have indicated that adiponectin reduces atherosclerosis by activating the insulin effectReference Pajvani and Scherer44, Reference Tschritter, Fritsche, Thamer, Haap, Shirkavand, Rahe, Staiger, Maerker, Haring and Stumvoll45 and adiponectin increases fatty acid β-oxidation and energy expenditure through an improvement in insulin resistanceReference Nagasawa, Fukui, Funahashi, Maeda, Shimomura, Kihara, Waki, Takamatsu and Matsuzawa46.
In animal and human studies, obesity is associated with a decrease in tissue or plasma antioxidant capacityReference Olusi47, Reference Ozata, Mergen, Oktenli, Aydin, Sanisoglu, Bolu, Yilmaz, Sayal, Isimer and Ozdemir48. Phenolic compounds have been considered to play an important antioxidant role as dietary antioxidants for the prevention of oxidative damage in living systemsReference Hertog, Feskens, Hollman, Katan and Kromhout49. GSH constitutes the first line of defence against free radicals in the liver and is also responsible for the maintenance of protein thiols and acts as a substrate for GPx and GSTReference Prakash, Gupta, Kochupillai, Singh, Gupta and Joshi50. The present data indicate that GSH content was depleted in the rats with obesity induced by a HFD and were restored after the treatment of GA (Table 4). Enzymatic antioxidants, such as superoxide dismutase, catalase or GPx, can scavenge reactive oxygen species and free radicals or prevent their formationReference Husain, Mejia, Lalla and Kazin51. The present results showed that antioxidant enzyme activities (GPx, GRd and GST) in the HFD group were significantly decreased, and the HFD+GA groups had significantly increased activities of antioxidant enzymes in the liver (Table 5). The reports indicate that antioxidants can modify cholesterol absorption and increase antioxidant statusReference Nicolle, Cardinault and Aprikian52, Reference Ko, Lee and Lim53. Several studies, such as that by Liou et al. Reference Liou, Chang, Geuze, Strous, Crapo and Slot54, have shown that hyperlipidaemia reduces the strength of the antioxidative defence system. Mehta et al. Reference Mehta, Van Thiel, Shah and Mobarhan55 indicated that HFD lead to liver injury and insulin resistance through oxidative stress. Thus, we hypothesize that the possible explanation for reducing obesity following the consumption of GA is a reduction in oxidative stress in rats fed with HFD.
In conclusion, the present results demonstrate for the first time that the addition of GA to the diet decreases body weight gain, the weights of liver and adipose tissue, serum parameters (TAG, phospholipid, total cholesterol, LDL-cholesterol, insulin and leptin) and hepatic steatosis. GA reduced oxidative stress (reduced GSSG and enhanced GSH, GPx, GRd and GST) in rats with obesity induced by a HFD. These results provide initial evidence that GA may be useful for the treatment of obesity and raise the possibility of a new application as a health supplement.
Acknowledgements
This research was partially supported by the Department of Health (DOH95-TD-F-113-002), Taiwan, Republic of China.











