Fe and Ca are two essential mineral nutrients of particular concern in women's health. Losses of Fe in menstruation and the added needs during pregnancy mean that the RDA for Fe in adult women are higher than the corresponding value for men(1). Meanwhile, adolescence and early adulthood mark the attainment of peak bone mass, with long-term consequences of increased risk of osteoporosis if Ca intakes are less than adequate(2). There is evidence that many adolescents and young adult women in North America and elsewhere may have inadequate intakes of Fe and/or Ca(1–Reference Theobald6). Adding to the complexity of managing optimal mineral nutrition are the known interactions whereby Ca can inhibit the absorption of dietary Fe(Reference Hallberg, Brune and Erlandsson7–Reference Hallberg9).
Body Fe status can be assessed by a number of biochemical indices including blood Hb concentration, serum ferritin (SF) concentration, transferrin saturation, serum transferrin receptor concentration and total Fe-binding capacity of serum. It is preferable to use a combination of these indices to improve specificity(Reference Looker, Gunter and Johnson10). A recent review of population intervention studies has indicated that SF and Hb are the most efficient combination of indicators for monitoring population change in Fe status(Reference Mei, Cogswell and Parvanta11). The level of body Fe stores is reflected by SF, with a typical ratio of 1 μg/l SF representing approximately 8–10 mg stored Fe(Reference Cook12). However, SF is also an acute-phase protein that increases in inflammation and infection; therefore, its association with Fe deficiency needs to be evaluated in combination with another indicator of acute-phase response, such as C-reactive protein (CRP)(Reference Cook, Flowers and Skikne13, Reference Gibson14).
As human subjects have no physiologically regulated mechanism for Fe excretion, the content of Fe in the body is controlled mainly by absorption(Reference McCance and Widdowson15). Dietary Fe absorption is strongly influenced by physiological factors including body Fe status, and by dietary factors (which can be categorised as enhancers or inhibitors) including inhibition by Ca(1). Inadequate Fe absorption, if uncorrected, may lead to declining body Fe stores and ultimately to functional consequences including altered erythropoiesis and Fe deficiency anaemia(Reference Zimmermann and Hurrell16).
Common methods for clinical investigation of Fe absorption may involve single- or multiple-day controlled trials. The relevance of the so-called ‘single-meal’ studies (which frequently compare absorption from two meals given on consecutive days) has been questioned because of evidence of decreased effect of enhancers or inhibitors on Fe absorption in the context of a complete diet with greater food variety than in the simple meals commonly used in the single-meal trials(Reference Cook and Reddy17). Nevertheless, single-meal studies remain a useful tool to identify the potential inhibitors or enhancers that affect Fe bioavailability in human subjects(Reference Reddy, Hurrell and Cook18). Such studies have permitted the identification of numerous determinants of Fe bioavailability including different chemical forms of Fe salts, aspects of meal composition (i.e. other components interacting with Fe as inhibitors or enhancers of absorption) and host-related factors such as body Fe status(Reference Singh, Sanderson and Hurrell19).
One interaction that has been subjected to much study is that between Ca and Fe(Reference Hallberg, Brune and Erlandsson7, Reference Gleerup, Rossander-Hulthen and Gramatkovski8, Reference Deehr, Dallal and Smith20–Reference Minihane and Fairweather-Tait22). Public education to increase dietary Ca intakes, Ca supplementation and food fortification with Ca are strategies to decrease the prevalence of Ca deficiency in young women and their risk of developing osteoporosis later in life. However, there is evidence that absorption of dietary or supplemental non-haem Fe can be compromised by the consumption of Ca at the same time(Reference Cook, Dassenko and Whittaker21, Reference Minihane and Fairweather-Tait22). Ca is also the only reported inhibitor for haem Fe absorption(Reference Hallberg, Brune and Erlandsson7). A variable depressing effect (from 0 to 80 %) of Ca on Fe absorption in a dose-related manner has been reported in most single-meal studies and short-term diet interventions in human subjects(Reference Bendich23). No inhibition was seen for an amount of Ca of < 50 mg in a meal (10 mg native and 40 mg added Ca). The inhibition was, however, maximal (80 %) at a content of 300 mg Ca per meal. No significant further inhibition was observed when increasing the Ca content from 300 to 600 mg(Reference Hallberg, Brune and Erlandsson7, Reference Hallberg, Rossander-Hultén and Brune24). The inhibitory effect of Ca on absorption of Fe has been shown to be modified by body Fe status; in one report, 600 mg Ca taken with 18 mg Fe as FeSO4 had no effect in a group of individuals with low Fe stores, whereas it caused a decrease of 9 % in Fe absorption in volunteers with normal Fe stores(Reference Cook, Dassenko and Whittaker21).
Most of the short- and long-term studies on the effect of Ca on Fe absorption conducted to date have been performed in groups of individuals with a wide range of Fe status (mostly with adequate Fe stores), which resulted in high inter-subject variability in Fe absorption. This variability has been attributed in some cases to dietary factors but mainly to the influence of Fe status. Correcting for such a difference in Fe stores to be able to compare dietary absorption in different studies requires adjusting individual absorption values to a common point. This can be done either by using a correction factor relative to an absorption of 40 % for a reference dose, using an average absorption value of 8 % for a standard meal or correcting absorptions using SF values and their known inverse relationship to Fe absorption(Reference Cook, Dassenko and Lynch25).
Few data, however, are available for such studies in pre-menopausal women with pre-existing low Fe stores. It seems important to investigate more in depth the effect of Ca supplementation in this population subgroup, which is at risk for developing Fe deficiency and Fe deficiency anaemia as result of inadequate Fe intake or bioavailability. Therefore, in the present study, our objective was to evaluate the effect of Ca on Fe absorption using singe-meal methodology in a group of woman with marginal Fe status, which represents about 15 % of Canadian women of childbearing age(Reference Cooper, Cockell and L'Abbé3). The null hypothesis was that pre-menopausal women with low Fe stores would show significant inhibition of non-haem Fe absorption with co-consumption of a typical Ca supplement tablet. The study also allowed us to evaluate whether pre-selecting a study population within a narrow range of SF would be useful to reduce variability in Fe absorption measurements in bioavailability studies. This secondary objective was addressed by comparison of results of the present study with observations published in the literature.
Subjects and methods
Subjects
Fe absorption with and without added Ca was measured in thirteen pre-menopausal women with pre-existing marginal Fe status defined as Hb between 120 and 160 g/l, SF between 12 and 24 μg/l and CRP < 7 mg/l; their mean age was 31·5 years (range 25–38). Potential participants were recruited through public advertising. Persons who responded to the advertisement were given an explanation of the study and were scheduled for screening. Before screening, written informed consent was obtained from each subject. The screening consisted of a brief health questionnaire to detect a history of haematological or gastrointestinal disorders and an assessment of Fe status. Subjects were excluded from the study if they had a history of haematological or gastrointestinal disorders, if their haematological indices fell outside the required range, if they were taking medications that could interfere with haematopoiesis or Fe absorption, if they were taking other supplements and refused to discontinue their use for the duration of the study, if they were pregnant, if they had reached menopause or if they had donated blood during the previous 6 months. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Human Research Ethics Board at Health Canada.
Study design
Each subject received stable iron isotope-labelled meals on two consecutive mornings. One meal was labelled with 5 mg added Fe as 57Fe, consumed without added Ca, while the other meal was labelled with 5 mg 58Fe, consumed with 500 mg added Ca in the form of a CaCO3 tablet. Subjects were randomly assigned to begin the study with either the 57Fe-labelled breakfast or 58Fe-labelled breakfast with the 500 mg Ca dose. Fourteen days after the second meal, a blood sample was taken from the subjects to measure the Fe isotopic enrichments for the two labels in comparison with a blood sample taken before the first labelled meal. Fe absorption from the two test meals, with or without Ca, was compared for each subject. The percentage absorption was calculated based on the total blood volume (BV) estimated from the height and weight(Reference Brown, Hopper and Hodges26). The red blood cell incorporation of absorbed stable isotopes was assumed to be 80 % in all subjects(Reference Kastenmayer, Davidsson and Gallan27).
Test meals
Each meal consisted of 2 × 50 g bread rolls and 250 g of deionised water as a beverage. The breakfast was extrinsically labelled with 5 mg 57Fe or 58Fe added as FeSO4 solution by accurate pipetting onto the first roll consumed. The rolls were made with white wheat flour, water, salt, sugar and yeast, and the dough was allowed to rise for 1 h after mixing the ingredients and again for 30 min after forming the rolls before baking. The two rolls consumed as a breakfast provided (as analysed by flame atomic absorption spectrophotometry) 3·8 mg Fe and 19·2 mg Ca, to which 5 mg Fe stable isotope was added for a total Fe intake of approximately 8·8 mg per meal.
On day 0 of the study, the subjects arrived at the study centre between 08.00 and 08.30 hours after an overnight fast. The subjects were administered a standard commercial pregnancy test to confirm non-pregnant status, and a blood sample was taken for the measurements of Hb, SF, CRP and basal Fe isotopic ratios. On day 1, the subjects were given the first labelled meal (randomised for order of 57Fe or 58Fe with Ca) at breakfast and were asked to refrain from eating or drinking any fluid for the next 3 h. On day 2, subjects were given the second labelled breakfast and were asked to refrain from eating or drinking any fluid for the next 3 h.
Stable isotope labels
Isotopic labels used in the present study were prepared from Fe metal isotopically enriched in 57Fe (95·38 % 57Fe) and 58Fe (92·8 % 58Fe; ISOFLEX USA, San Francisco, CA, USA) by dissolution in 3 m H2SO4 and dilution to appropriate concentration with ultrapure water (Millipore, Bedford, MA, USA). The exact isotopic composition of 57Fe and 58Fe solutions was measured by multicollector inductively coupled plasma MS. Fe concentrations of the solutions were determined by isotope dilution MS against a commercially available isotopic Fe standard material solution (IRMM-014b, EU Institute of Reference Materials, Geel, Belgium). The administered dose was determined by accurate weighing of the first wheat roll of each test meal before and after addition of the stable isotope solution by pipette.
Measurements of iron status
A total of 15 ml venous blood samples were drawn into EDTA-treated and serum separator tubes at day 0 and 14 d following the second labelled breakfast. Hb, SF and CRP were measured by a commercial medical laboratory service (LifeLabs, Inc., Toronto, ON, Canada) for the assessment of Fe status. Aliquots of EDTA-treated whole blood from day 0 and day 14 after the second breakfast were stored at − 80°C until analysis for Fe isotopic composition before and after stable isotope incorporation.
Blood sample preparation and isotopic analysis
To minimise risks of sample contamination during digestion and analysis, only high purity acids and ultrapure water were used throughout the present work. Baseline and stable isotope-enriched blood samples were prepared for isotopic analysis as previously reported(Reference Benkhedda, Chen and Dabeka28). Aliquots of 0·5 g thawed and homogenised venous whole blood were digested in a microwave system using a mixture of HNO3 and H2O2, and Fe was separated from the matrix using anion exchange resin AG 1-X8. Total Fe in the samples was determined by flame atomic absorption spectrometry.
The amount of incorporated labels in enriched blood samples was determined from the measurements of 57Fe/56Fe and 58Fe/56Fe isotopic ratios before and after administration of the enriched stable isotope labels. All isotopic analysis was carried out with a VG Axiom multicollector inductively coupled plasma MS (VG Elemental, Winsford, Cheshire, UK). An Aridus desolvating sample introduction system (CETAC Technologies, Omaha, NE, USA) with a microconcentric nebulizer T1H was used to reduce the interference of oxides, hydroxides and molecular ions on Fe isotopes determination. Instrumental mass bias was corrected by bracketing the samples with a standard reference material IRMM-014b.
Calculation of iron absorption
The amount of 57Fe and 58Fe isotopic labels present in blood of each subject was determined based on the shift of the isotopic ratios in the blood after red cell incorporation of the absorbed labels. The calculations were based on the principle of isotope dilution and considering the non-monoisotopic character of two isotopic labels and crossover contributions(Reference Walczyk, Davidsson and Zavaleta29). The fractional Fe absorption was thus determined from the circulating amount of isotopic label assuming 80 % incorporation of the absorbed iron into erythrocytes. The amount of circulating Fe was calculated based on BV and Hb(Reference Kastenmayer, Davidsson and Gallan27):
BV were calculated using an empirical formula based on height and weight according to Brown et al. (Reference Brown, Hopper and Hodges26):

Fe absorption values were expressed as unadjusted data and also as data adjusted to the group geometric mean SF and to a hypothetical SF of 40 μg/l. The adjusted data were calculated as follows:
where Aadj is the adjusted absorption; Aobs is the observed absorption; SFs is the subject's SF; and SFt is the target group geometric mean SF or hypothetical SF value of 40 μg/l.
Statistical analysis
Because of the skewed distribution of fractional Fe absorption data, statistical analysis was performed on log-transformed data and the results were reconverted by antilogarithm to recover the original units. Paired t test was used to compare Fe absorption in the presence or absence of Ca. The relationship between Fe stores (as SF) and Fe absorption was examined using a linear regression model. A significance level of P < 0·05 was used for all statistical tests.
Results
Subject characteristics and iron status
Serum CRP concentrations were < 7 mg/l for all subjects (data not shown), within the normal reference range used by the commercial medical laboratory doing the clinical blood analyses for the present study. When screened for inclusion in the study, the subjects had mean Hb of 132·8 (sd 6·4) g/l (range 120–143) and geometric mean SF of 17·8 μg/l (range 13–24; Table 1). Because of scheduling logistics with the study participants, there was an average lag time of 8 weeks (range 1–19 weeks) between screening and initiation of the study. On day 0, some of the participants showed a higher SF compared to their values from the screening test, with CRP values still < 7 mg/l. The mean Hb for the group was increased slightly to 134·8 (sd 9·8) g/l, and their geometric mean SF increased to 18·4 μg/l.
Table 1 Characteristics of young adult women participating in the present study at time of screening and at day 0 of the study
(Mean values and standard deviations with their ranges)

* Geometric mean, n 13.
Effect of calcium on iron absorption
Fractional Fe absorption was calculated as the ratio of the amount of 57Fe and 58Fe incorporated into red blood cells 14 d after the second test meal to the ingested amount of either stable isotope label, and is presented as geometric mean and as the ratio of absorption measured with v. without added Ca (Table 2). Fractional Fe absorption ranged from 2·2 to 40·6 % and from 0·7 to 18·9 % in the absence and presence of 500 mg Ca as the carbonate, respectively. The ratio of Fe absorption from the test meal containing Ca to that without (i.e. +Ca/ − Ca) was calculated as an expression of the effect of Ca on Fe absorption. The ratio varied from 0·16 to 1·08. The addition of CaCO3 supplement to the breakfast meal reduced group geometric mean absorption of the co-consumed Fe from 10·2 to 4·8 %, a significant (P = 0·0009) 53 % decrease (ranging from 0 to 84 %).
Table 2 Fractional iron absorption values for thirteen pre-menopausal women with pre-existing marginal iron status, measured in the presence and absence of 500 mg calcium (as CaCO3)

SF, serum ferritin.
* Geometric mean, n 13.
In an attempt to reduce inter-individual variations in Fe absorption, adjusted individual iron absorption was calculated by applying a correction equation using the group mean SF (18·4 μg/l) or using a hypothetical SF value of 40 μg/l (Table 3). There was no significant difference (P = 0·999) between the uncorrected mean values for 57Fe absorption (without added Ca) and those corrected for the group mean value of SF. The CV, calculated as percentage relative standard deviation using the log-transformed data, was reduced from 39 to 29 % and from 74 to 63 % in the absence and presence of Ca, respectively. It has been common practice in the studies on Fe absorption to correct for inter-individual variation using a hypothetical SF of 40 μg/l. In the present work, such correction, for 57Fe absorption, resulted in significant reduction (P = 0·00 013) of the mean fractional absorption value from 10·2 to 4·7 % with no improvement in the CV (44·6 % v. 39 % for non-corrected values).
Table 3 Iron absorption values for thirteen pre-menopausal women with pre-existing marginal iron status, arithmetically corrected to group geometric mean serum ferritin (SF) and to a hypothetical SF of 40 μg/l

* Geometric mean, n 13.
Correlation of iron status with iron absorption
Within the relatively narrow range of SF investigated in the present study (9–35 μg/l), there was a trend of increased Fe absorption for lower concentrations of SF in either the presence or absence of added Ca (Fig. 1). Significant inverse correlation was found in the absence (r − 0·67, P = 0·01) and presence (r − 0·58, P = 0·04) of Ca, respectively, between SF (Fe stores) and Fe absorbed.
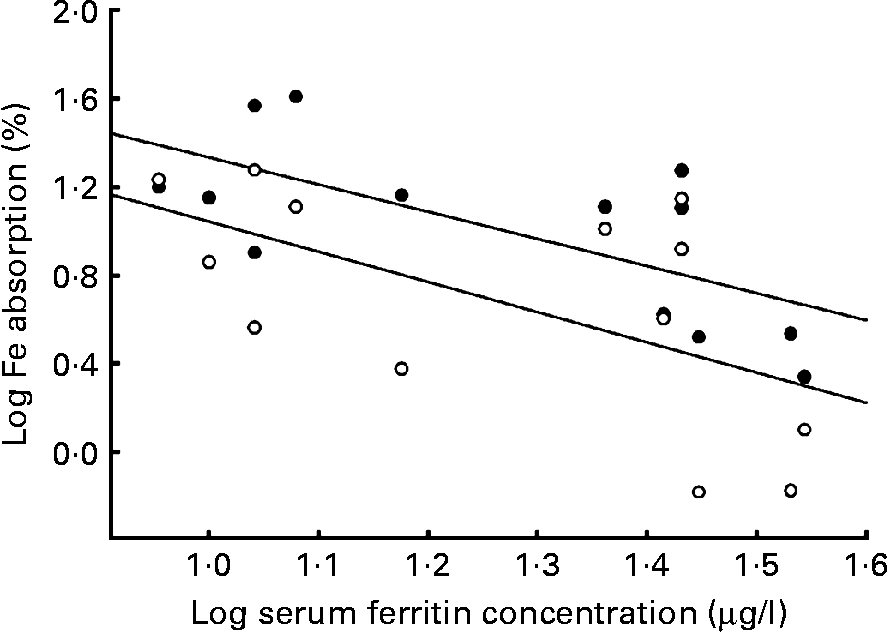
Fig. 1 Relationship between log serum ferritin and log absorption of iron in a single-meal study. The regression line for iron absorption without calcium (•) was y = − 1·2317x+2·5661 (r 2 0·4597) and for iron absorption with calcium (○) was y = − 1·3617x+2·4035 (r 2 0·3382).
Discussion
The present study, conducted in a group of pre-menopausal adult women with marginal body Fe stores, defined as SF (at screening) in the range 12–24 μg/l with normal Hb levels, showed a mean fractional non-haem Fe absorption from a simple bread meal to be 10·2 %. The results of the present study confirm the inhibitory effect of 500 mg Ca on non-haem Fe absorption in single-meal studies, with a magnitude of inhibition in the present study of 53 % (range 0–84 %). The range of inhibition found in the present study is comparable to the reductions of 0–80 % in Fe absorption reported in other studies in human subjects at doses between 165 and 300 mg Ca(Reference Hallberg, Brune and Erlandsson7, Reference Deehr, Dallal and Smith20, Reference Cook, Dassenko and Whittaker21, Reference Sokoll and Dawson-Hughes30). It is interesting to note that in the present study, two subjects with SF values of 9 and 26 μg/l showed no effect of the added Ca with absorption ratios of 0·96 and 1·08, respectively. Similar lack of inhibitory effect of Ca on non-haem Fe absorption in some individuals in a short-term test has been noted previously(Reference Cook, Dassenko and Whittaker21).
For some participants, a shift in SF values to higher values on day 0 of the study was observed compared to the time of initial screening. Up to several weeks had elapsed between screening and the start of the study (day 0). An attempt was made to control for the contribution of possible inflammation on the elevation of SF values by the concurrent measurement of CRP, for which the values were reported to be normal (ranging from < 3 to up to 7 mg/l) for the participants included in the study. The variation in Fe status between screening day and study start day may have been attributable to biological intra-individual variability, which has been estimated to be about 20 % in females(Reference Cooper and Zlotkin31). Moreover, it has been reported that even a very mild infection might influence the level of SF(Reference Hulthén, Lindstedt and Lundberg32), which might also have contributed to the shift of SF concentrations to slightly higher values. Despite the observed changes in SF between the time of screening and the start of the study, the participating women had low iron stores (SF range 9–35 μg/l) at day 0.
In comparison to radioisotope tracer studies, the use of stable isotopes often necessitates the administration of relatively large amounts of isotope to yield a measurable change in isotope ratios. Human studies of Fe absorption commonly use several milligrams of iron stable isotope in a dose(Reference Kastenmayer, Davidsson and Gallan27, Reference Walczyk, Davidsson and Zavaleta29, Reference Roe, Collings and Dainty33, Reference Young, Glahn and Ariza-Nieto34). However, stable isotopes do not involve the same ethical constraints as radioisotopes for use in human subjects(Reference Fairweather-Tait, Fox, Harvey, Lowe and Jackson35). The 5 mg stable isotope doses used in the present study had been shown in a pilot study in our laboratory to be amply adequate for quantification of enrichment following absorption from the simple wheat roll meal matrix(Reference Benkhedda, Chen and Dabeka28).
In the present study, we have focused on pre-menopausal women with low Fe stores for investigation of the effect of Ca on Fe absorption, with one objective being to minimise inter-individual variations in Fe absorption caused by variability in body Fe stores. The results obtained in the present study show large inter-individual variations in Fe absorption, with (0·7–18·9 %) and without added Ca (2·2–40·6 % Fe absorption), despite the selection of participants within a relatively narrow range of initial SF values. In a study by Cook et al. (Reference Cook, Dassenko and Whittaker21), ten young men with SF 19–171 μg/l had Fe absorption measurements ranging from 1·6–34·0 % from a standard hamburger meal. In an earlier study(Reference Lynch, Skikne and Cook36), twenty-two men and women, heterozygous relatives of haemochromatosis patients with SF ranging from 7–221 μg/l, had non-haem Fe absorption measurements ranging from 1·4–23·3 % from a standard hamburger meal.
Investigation of individuals within a wide range of body Fe stores, as found in other studies, has generally employed correction of Fe absorption using a reference dose, or correction equations involving SF, to be able to compare absorption values obtained in different studies under different experimental conditions. We found, in the present study on pre-menopausal women with low Fe stores, that such calculations did not improve the interpretation of results. A similar conclusion was reached by Davidsson et al. (Reference Davidsson, Ziegler and Kastenmayer37) in earlier studies on erythrocyte incorporation of Fe by infants.
There is a little information in the literature on the inhibitory effect of Ca specifically in pre-menopausal women with low Fe stores. Cook et al. (Reference Cook, Dassenko and Whittaker21) reported on the effect of different Ca salts on Fe absorption, taken with or without food, in volunteers with low or normal Fe stores. In a group of eight women and one man with low Fe stores (mean SF 24 μg/l), they reported a slight increase in Fe absorption from 18 to 21·5 % of a dose of 18 mg Fe as FeSO4 in the absence or presence of 600 mg Ca as CaCO3 without food (given with water only). The amount of Fe given was much larger than the amount of Fe in our test breakfast, and two of their subjects had SF values of 73 and 75 μg/l, so the results may not be directly comparable to the present work. A similar study in the same report(Reference Cook, Dassenko and Whittaker21), where the effect of 600 mg Ca (as carbonate) was tested in the context of a hamburger meal, revealed an average 44 % inhibition (range 10–65 %).
There are relatively few reports relating body Fe stores to non-haem Fe absorption in single-meal studies. A highly significant inverse correlation between SF and non-haem Fe absorption was reported for forty-seven normal control subjects (with SF values ranging from < 10 to >200 μg/l) in a study of the effects of haemochromatosis(Reference Lynch, Skikne and Cook36). Roughead et al. (Reference Roughead, Zito and Hunt38) have found that non-haem Fe absorption was inversely correlated with SF in either the presence or absence of added Ca, in either low- or high-Fe bioavailability diets. SF values for male and female participants in that study ranged widely (4–219 μg/l), in contrast to the relatively narrow range of SF in the present work. In a study on Fe absorption from soyabean in women with low body Fe stores(Reference Murray-Kolb, Welch and Theil39), there was a significant inverse correlation between SF and absorption of Fe from plant (soyabean) ferritin. Over half of the women in that study were Fe deficient, having SF < 10 μg/l, which contrasts to the marginal, but not frankly deficient, iron stores of women in the present work.
In a study focusing on women with a smaller range of SF (4–73 μg/l) and incorporating a radioactive Fe tracer into all meals over a 2-d period, inverse correlation of Fe absorption with SF was found only in the context of a high-bioavailability diet, but not a low-bioavailability diet(Reference Hunt40). Similar correlation had been found in an earlier study in men, where the range in SF concentrations had been 22–336 μg/l, although the pattern reversed (i.e. became significant with the low- bioavailability diet, but not the high-bioavailability diet) after 10 weeks(Reference Hunt and Roughead41). Subjects in the present study were consuming their habitual diets, apart from the simple test breakfasts used in the Fe absorption test (which did not contain important amounts of enhancers or inhibitors apart from Ca).
It has been demonstrated in several studies on dietary Fe bioavailability, within a wide range of body Fe status (for example SF in the range 8–242 μg/l)(Reference Cook, Dassenko and Lynch25), that Fe status rather than dietary bioavailability is the major factor determining the extent of Fe absorption from the diet. However, the regulation of body Fe stores is complex and still not completely understood(Reference Beard, Dawson and Piñero42). Recent work has shown a strong correlation between concentrations of SF and plasma hepcidin, a protein involved in the regulation of Fe absorption(Reference Roe, Collings and Dainty33). Circulating hepcidin has been shown to be inversely correlated with iron absorption in men(Reference Roe, Collings and Dainty33) and women(Reference Young, Glahn and Ariza-Nieto34).
The size of body Fe stores is one of the main factors believed to modulate the rate of intestinal Fe absorption, such that Fe absorption increases several fold in states of Fe deficiency and decreases in Fe overload(Reference Testa43). However, in the present study, there was still a significant variation in Fe absorption despite the relatively narrow range of body Fe stores of the subjects. The present findings on the relationship between Fe stores and Fe absorption seem to suggest that Fe stores may not be the main physiological factor determining Fe (or more particularly non-haem Fe) absorption in subjects with low body Fe stores. Interestingly, our observations are supported by the report of Reddy et al. (Reference Reddy, Hurrell and Cook18), who have analysed by multiple regression the results from twenty-five radioisotopically labelled meals in human subjects. They concluded that dietary factors such as inhibitors and enhancers accounted for 16 % of variability in Fe absorption, and body Fe stores (as indicated by SF) accounted for 32 % of variability, while about half of the variation was due to unexplained factors. The large variations observed in menstrual Fe losses in women of reproductive age are known to affect body Fe stores(Reference Harvey, Armah and Dainty44). It would be interesting to investigate whether there might be some more direct impact of this variable on Fe absorption. Further studies including hepcidin and other proteins involved in Fe metabolism may also prove fruitful.
In conclusion, we have confirmed the null hypothesis that pre-menopausal women with low Fe stores show significant inhibition of non-haem Fe absorption with co-consumption of a typical Ca supplement tablet. Pre-selection of a study group with a narrow initial range of SF values did not appear to reduce inter-individual variability in measured Fe absorption. The results of the present study and reported observations from previous studies suggest that additional physiologic or genetic factors, besides the levels of body Fe stores and type of diet consumed, have strong influences on Fe absorption in individuals with similar body Fe stores.
Acknowledgements
Financial support for the present work was obtained through internal funding of Health Canada. The authors declare no conflict of interest regarding the present work, financial or otherwise. The study described in the present paper was planned by K. A. C. and M. R. L. K. B. was responsible for day-to-day conduct of the study. K. B. and K. A. C. wrote the manuscript, with editorial input by M. R. L.






