One of the primary contributors to vascular dysfunction and pro-thrombotic progression in CVD is due to hyperactivation and hyper-aggregation of platelets( Reference Santhakumar, Kundur and Fanning 1 ). Lack of physical activity has been proposed as an independent cardiovascular risk factor( Reference Laufs, Wassmann and Czech 2 ). Sedentary lifestyle is defined as an individual who performs less than 3 h of aerobic exercise per week( Reference Pate, O’Neill and Lobelo 3 ). However, during irregular acute exercise or absence of physical activity, an increase in free radical mediated oxidative stress and pro-thrombotic progression has been observed( Reference Laufs, Wassmann and Czech 2 , Reference Chevion, Moran and Heled 4 ). During increased oxidative stress and endothelial vessel wall damage in such pro-thrombotic conditions, platelets adhere, activate and aggregate at the site of injury( Reference Harrison 5 ). Consequently, platelet activation targets activation-dependent surface receptors: P2Y1/P2Y12 (ADP receptor), GPVI/ α2 β 1 (collagen receptor); and thrombotic pathways: cyclo-oxygenase-1 (COX-1) arachidonic acid pathway( Reference Gachet 6 ). During endothelial vessel wall damage and platelet activation, leucocytes and primarily monocytes form aggregates with platelets at the site of injury( Reference Freedman 7 , Reference Michelson, Barnard and Krueger 8 ). This mechanism of leucocyte adhesion to activated platelets is an important contributor to atherosclerotic plaque development in a high oxidative stress environment( Reference Freedman 7 ) such as in sedentary individuals( Reference Ford and Caspersen 9 ). Current antiplatelet therapeutics are designed to blunt such specific thrombotic pathways thereby reducing platelet activation and the subsequent risk of CVD( Reference Gachet 6 , Reference Ferreiro and Angiolillo 10 ). Though current anti-platelet drugs demonstrate platelet activation and aggregation inhibiting properties, there have been reports on increased resistance and side effects in target populations( Reference Roth and Calverley 11 ). Research focusing on natural dietary antioxidants and polyphenols, with anti-platelet benefits has been increasingly investigated( Reference Santhakumar, Kundur and Fanning 1 , Reference Thompson, Pederick and Santhakumar 12 – Reference Benn, Kim and Park 15 ). Recent evidence suggests polyphenols and their subclasses are effective in reducing CVD risk factors by demonstrating anti-hypertensive effects, endothelial function improvement and blocking of specific platelet pathways, therefore inhibiting platelet activation and aggregation( Reference Chong, Macdonald and Lovegrove 16 ). Anthocyanins (ACN), a subclass of the polyphenol family, flavonoids, have demonstrated the ability to reduce the risk of thrombosis via its free radical scavenging and metal chelating properties( Reference Santhakumar, Bulmer and Singh 17 , Reference Dembinska-Kiec, Mykkanen and Kiec-Wilk 18 ). Recent in vitro and in vivo studies have shown ACN-rich foods to improve endothelial function( Reference Amiot, Riva and Vinet 19 ), decrease lipid peroxidation( Reference Basu, Du and Leyva 20 ), demonstrate potent antioxidant capabilities( Reference Bell and Gochenaur 21 ) and reduce inflammatory markers( Reference Edirisinghe, Banaszewski and Cappozzo 22 , Reference Farrell, Norris and Ryan 23 ). This study aims to investigate the potential of 4-week ACN capsule supplementation in inhibiting specific mechanistic pathways of thrombogenesis in sedentary pro-thrombotic individuals.
Methods
Study participants and experimental design
This study was performed in compliance with the guideline laid down in the Declaration of Helsinki and was approved by the Central Queensland University Human Research Ethics Committee, QLD, Australia (approval no: H15/07-154). All participants provided informed consent before the commencement of the study. This study was also registered with the Australian New Zealand Clinical Trials Registry (ACTRN12615000797572). In all, sixteen sedentary participants (three males and thirteen females) with a BMI of <25 kg/m2 were recruited from the local community. Screening via interviews and questionnaires was conducted to ensure participants were sedentary, healthy, non-smokers, were not taking any anti-inflammatory, antiplatelet medications or health supplements and had no history of metabolic or CVD. The definition of sedentary population has been established as participants who perform less than 3 h of aerobic exercise per week( Reference Pate, O’Neill and Lobelo 3 , Reference Ford and Caspersen 9 ). Standard dietary intake and antioxidant questionnaires were conducted and reviewed before testing to ensure participants involved in the study did not consume a diet high in antioxidants. A randomised, double-blind, placebo (PBO) controlled, cross-over design was applied. Baseline fasting blood samples were collected from all participants to evaluate any pre-existing abnormalities in full blood count parameters, biochemical analysis, platelet activity and aggregation, oxidative stress and inflammatory markers as well as overall haemostatic function. Blood pressure and anthropometric measurements were also recorded to determine participant’s BMI. Upon initial screening, participants were grouped into A and B and supplemented with ACN extract or PBO capsules accordingly. An external body that worked independently to the study investigators randomly assigned participants to each group. Supplementation began after baseline testing, for 28 d, where they consumed two capsules in the morning and two capsules in the afternoon per day (80 mg each) of either ACN or PBO. Capsule intake and compliance were monitored upon return of the capsule strips after each supplementation bout. After 28 d of supplementation (either ACN or PBO), blood pressure, body measurements and blood testing were repeated on day 29 followed by a 2-week wash-out period, then supplementation cross-over was performed and analysis repeated, that is on days 43 and 71. Due to the rapid absorption and elimination of most anthocyanins, the 2-week wash-out period was deemed sufficient to avoid carryover( Reference Manach, Williamson and Morand 24 ). This wash-out period was utilised by our group in a previous study evaluating the consumption of ACN-rich Queen Garnet plum juice on platelet activation-related thrombogenesis in healthy volunteers( Reference Santhakumar, Kundur and Fanning 25 ).
Blood sample collection
Blood was collected at each visit from the median cubital vein by a trained phlebotomist using a 21-gauge butterfly needle into, two tri-potassium EDTA (1·8 mg/ml) anticoagulant tube for full blood count analysis, four tri-sodium citrate (28·12 g/l concentration) tubes for platelet aggregation, surface marker expression and coagulation assays, and serum separation tube (SST) for biochemical analysis. Careful handling of citrate tubes was ensured to minimise agitation and to prevent any artifactual platelet activation. SST tubes were left standing upright for >30 min to clot then centrifuged at 3000 rpm for 10 min. Samples were visually inspected for clots, and none were obtained from traumatic blood collection.
Supplementation capsules
The ACN supplements were produced by Medapalett Pharmaceuticals, Biolink. The supplements consisted of a hemicellulose capsule containing purified ACN extract (80 mg) from wild Norwegian bilberries (Vaccinium myrtillus) and black currants (Ribes nigrum) – ACN capsules contained 33·0 % of 3-Ο-β-glucosides, 3-Ο-β-galactosides and 3-Ο-β-arabinosides of cyanidin; 58·0 % of 3-Ο-β-glucosides, 3-Ο-β-galactosides and 3-Ο-β-arabinosides of delphinidin; 2·5 % of 3-Ο-β-glucosides, 3-Ο-β-galactosides and 3-Ο-β-arabinosides of petunidin; 2·5 % of 3-Ο-β-glucosides, 3-Ο-β-galactosides and 3-Ο-β-arabinosides of peonidin; 3·0 % of 3-Ο-β-glucosides, 3-Ο-β-galactosides and 3-Ο-β-arabinosides of malvidin; and 1·0 % of 3-Ο-rutinoside of cyanidin and delphinidin. In addition, the ACN capsules also contained pullulan, maltodextrin, and citric acid (which took up 4 % per capsule for the stability of the ACN). The PBO capsules were composed of maltodextrin and a blue colour additive which contained no phenolic compounds and appeared identical to ACN capsules. ACN supplementation of 320 mg/d has been shown as an effective treatment amount in previous studies owing to its bioavailability.
Dietary intake and physical activity monitoring
Upon commencement of the study, participants were instructed not to make any adjustments to their usual diet. In order to monitor diet and physical activity during their supplementation periods, participants were required to record 24 h full food intake of: (1) type of food eaten, (2) preparation involved, (3) amount and (4) time, once a week throughout each period of their 4-week supplementation bout (8 weeks in total). Records of dietary intake (food diary) were monitored using FoodWorks® (Xyris Software Pty Ltd) based on the Australian Food Composition database. The monitoring process also consisted of a physical activity log, a record of which was required to ensure participants performed less than 3 h of exercise per week and maintained a sedentary lifestyle.
Biochemical profile
Biochemical analysis including electrolytes, inflammatory markers, liver/kidney function, oxidative stress markers and lipid profiles were performed on a Beckman Coulter AU680 spectrophotometry and potentiometry biochemistry analyser (Beckman Coulter Inc.) with colorimetry, turbidimetry, latex agglutination, homogenous enzyme immunoassay and indirect ion selective electrode analytical capability methods. Troubleshooting, reagent management and machine maintenance were performed before testing along with quality control to ensure accurate analyser capability.
Full blood count and coagulation profile
Full blood count analysis was performed using a Sysmex XT-1800i (Sysmex Canada, Inc.) haematology analyser to evaluate general haematological parameters such as; Hb, haematocrit, erythrocyte count, leucocyte count, platelet count, mean platelet volume and platelet distribution width. A Sysmex CA-600 (Siemens Healthineers) coagulation analyser was used for coagulation profile analysis consisting of prothrombin clotting time, activated partial thromboplastin time, fibrinogen concentration and fibrinogen degradation product (FDP)/innovance D-dimer via clot detection. Tri-sodium citrate tubes were centrifuged at 3000 rpm for 10 min to produce platelet poor plasma used for coagulation profile testing.
Biomarkers of thrombogenesis and platelet activation
The analysis was performed and interpreted using a BD FACVerse™ flow cytometer (BD Biosciences). Platelet activity and thrombogenic indicators were assessed via activation-dependent platelet monoclonal antibodies and their respective isotype controls. Procaspase activating compound-1 (PAC-1)-fluorescein isothiocyanate-fluorescein isothiocyanate was used to detect platelet activation related conformational change, P-selectin/CD62P-allophycocyanin-allophycocyanin highlighted activation dependent de-granulation, CD42b-phycoerythrin/CD14-phycoerythrin-cyanin 7 expression determined monocyte-platelet aggregate formation and platelet endothelial cell adhesion molecule-1 (PECAM-1)/CD31-allophycocyanin-cyanine 7 expression represented cellular adhesion. Monocyte-platelet aggregates were expressed as percentage parent (%), and activated platelets were expressed as mean fluorescence intensity. A decreased expression of mAb exhibits alleviation of thrombogenesis. Within 5 min of collection tri-sodium citrated whole blood was used for assay preparation to avoid artifactual activation of platelets. A mixture of all monoclonal antibodies (16 μl) was added to 50 μl of whole blood and incubated for 15 min at room temperature in the dark. To induce platelet activation, ADP (1·65 μl) was added, and samples were incubated for a further 15 min in the dark at room temperature, after which erythrocytes were lysed (532 μl of 10 % lysing solution). Samples were thoroughly vortexed to ensure homogeneity and incubated in the dark at room temperature for a further 10 min and then analysed. In all, 10 000 platelet events were acquired, gated on the basis of light scatter and CD42b mAb expression.
Platelet aggregation
Whole blood platelet aggregation studies were performed on a Chrono-log model 700 (DKSH Australia Pty. Ltd) aggregometer and analysed via electrical impedance (Ω) to evaluate platelet aggregation occurring in the sample over a 6-min period. Citrated whole blood was stimulated with ADP (10 μm–10 μl) 30 min after collection, collagen (1 μg/ml–1 μl) and arachidonic acid (0·5 mm–10 μl). A quantity of 500 μl of blood and 500 μl saline were incubated for 5 min, the sample cuvette was then analysed by inserting the probe, setting the baseline and once stability was maintained, the platelet agonist (ADP, collagen or arachidonic acid) was added. All samples were analysed in duplicates.
Statistical analysis
GraphPad Prism version 7.0 for Mac OS X (GraphPad Software) was used to analyse all data. A repeated-measures ANOVA followed by Newman–Keuls post hoc multiple comparisons was carried out. A minimum sample size of fourteen subjects in each group was required for 80 % power to detect a 5 % variation in the laboratory parameters (platelet aggregation) measured, where a 3–5 % sd exists in the population, assuming an alpha error of 0·05. All data has been expressed as a means and standard deviations. Differences between the groups were considered significant when the value for P<0·05. Any significant statistical interactions were included in the analysis where applicable.
Results
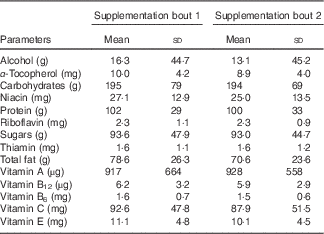
The baseline parameters of the participants in the study were all within normal reference ranges as established by the Royal College of Pathologists of Australasia( 26 ). There were no significant changes to the full blood counts; coagulation profile including plasma fibrinogen concentration and FDP (D-dimer) (Table 1); biochemical and inflammatory markers following ACN or PBO supplementation (online Supplementary Table S1). Assessment of physical activity records demonstrated that participants did not perform more than 3 h of exercise per week throughout the study (data not shown). Evaluation of the food intake did not demonstrate any variation in the micronutrients or macronutrient levels across the two supplementation bouts (Table 2). The results are presented in the form of homogenised data (males and females) and there were no significant differences between the two populations.
Table 1 Effect of anthocyanin (ACN) and placebo (PBO) supplementation on full blood count and coagulation profile (Mean values and standard deviations)

BP, blood pressure; PDW; platelet distribution width; MPV, mean platelet volume; PT, prothrombin time; APTT, activated partial thromboplastin time; TCT, thrombin clotting time.
Table 2 Distribution of volunteer micronutrient and macronutrient intake during supplementation bouts (Mean values and standard deviations)
Biomarkers of thrombogenesis and platelet activation
ACN supplementation alleviated thrombotic progression and endothelial dysfunction associated leucocyte migration/adhesion by demonstrating a reduction in ADP-induced monocyte-platelet aggregate formation (Fig. 1) and PECAM-1 expression (Fig. 2) by 39 and 14 %, respectively. Initial platelet activation associated conformational change represented by PAC-1 expression (Fig. 3) and activation-dependent platelet degranulation represented by P-selectin expression (Fig. 4) was also reduced by 10 and 14 %, respectively post-ACN supplementation. PBO supplementation did not affect thrombogenic biomarkers or platelet activation.

Fig. 1 Effect of anthocyanin (ACN) supplementation on monocyte-platelet aggregate formation using CD14/CD42b surface marker expression. ACN supplementation, 4 weeks, reduced monocyte-platelet aggregate formation by 39 % (↓0·28 (sd 0·19), P=0·0079). Data are represented as supplementation type v. percentage parent population. Values are means (n 16), and standard deviations represented by vertical bars. PBO, placebo. ** Statistical significance (P<0·01).

Fig. 2 Effect of anthocyanin (ACN) supplementation on CD31 (platelet endothelial cell adhesion molecule-1) surface marker expression. ACN supplementation, 4 weeks, reduced CD31 expression by 14 % (↓2041 (sd 832), P=0·0202). Data are represented as type of supplement v. mean fluorescence intensity (MFI). Values are means (n 16), and standard deviations represented by vertical bars. PBO, placebo. * Statistical significance (P<0·05).

Fig. 3 Effect of anthocyanin (ACN) supplementation on procaspase activating compound-1 (PAC-1) expression. PAC-1 showed a decreased expression post 4-week ACN supplementation by 10 % (↓626 (sd 188), P=0·0023). Data are represented as type of supplement v. mean fluorescence intensity (MFI). Values are means (n 16), and standard deviations represented by vertical bars. PBO, placebo. ** Statistical significance (P<0·01).

Fig. 4 Effect of anthocyanin (ACN) supplementation on CD62P (P-selectin). ACN supplementation, 4 weeks, reduced CD62P expression by 14 % (↓1673 (sd 723), P=0·0285). Data are represented as type of supplement v. mean fluorescence intensity (MFI). Values are means (n 16), and standard deviations represented by vertical bars. PBO, placebo. * Statistical significance (P<0·05).
Platelet aggregation
ADP-induced platelet aggregation was reduced by 29 % (Fig. 5) post 4-week ACN supplementation. Arachidonic acid and collagen-induced platelet aggregation showed no significant changes post-ACN supplementation (data not shown). PBO supplementation did not affect platelet aggregation induced by any of the platelet agonists.

Fig. 5 Effect of 4-week anthocyanin (ACN) supplementation on ADP-induced whole blood platelet aggregation. ACN supplementation reduced platelet aggregation by 29 % (↓3·8 (sd 1·2), P=0·0039). Data are represented as type of supplementation v. amplitude (Ω). Values are means (n 16), and standard deviations represented by vertical bars. PBO, placebo. ** Statistical significance (P<0·01).
Discussion
The objective of this dietary intervention trial was to evaluate the effect of ACN supplementation on mechanistic pathways of thrombogenesis in sedentary pro-thrombotic population. It was observed that 320 mg/d of ACN capsule for 4 weeks had the ability to alleviate both ADP-induced platelet activation and aggregation; biomarkers of thrombogenesis such as monocyte-platelet activation and PECAM-1 expression, demonstrating the potential to blunt a similar pathway targeted by current anti-platelet drugs such as clopidogrel.
Although there were no significant changes in full blood count, coagulation profile, biochemical profile or inflammatory markers post-ACN supplementation in this study, there have been other in vivo studies that have demonstrated diverse systemic effects. In a randomised, double-blind PBO-controlled dietary intervention trial of 200 ml/d Queen Garnet plum juice consumption for 28 d Santhakumar et al. demonstrated an increase in prothrombin time and a decrease in fibrinogen concentration( Reference Santhakumar, Kundur and Fanning 1 ). Another in vivo study evaluating the effect of blueberry supplementation on forty-eight obese men and women with metabolic syndrome demonstrated no significant change in glucose and lipid profiles( Reference Basu, Du and Leyva 20 ). However, an in vivo study observing the effects of freeze-dried strawberry powder on sixteen females who presented with features of metabolic syndrome demonstrated a decrease in total cholesterol and LDL-cholesterol, but no significant changes to the inflammatory marker (C-reactive protein) level post 4-week supplementation( Reference Basu, Wilkinson and Penugonda 27 ). Furthermore, a decrease in LDL-cholesterol and increase in HDL-cholesterol concentrations were observed post 320 mg/d of ACN supplementation for 12 weeks in 120 dyslipidaemic subjects( Reference Qin, Xia and Ma 28 ). It can be deduced from previous studies focusing on various ACN-rich supplementations that there have been divergent outcomes associated with haemostatic, biochemical and inflammatory markers( Reference Santhakumar, Kundur and Fanning 1 , Reference Farrell, Norris and Ryan 23 , Reference Basu, Wilkinson and Penugonda 27 ).
Biomarkers of thrombogenesis such as monocyte-platelet aggregate formation and PECAM-1 were inhibited post 4-week ACN supplementation. Monocyte-platelet aggregate formation has been established as a reliable marker of thrombus development( Reference Freedman 7 ). This study demonstrated a decrease in monocyte-platelet aggregate formation by 39 % (P<0·01) post 4-week ACN supplementation thereby exhibiting a potential role in reducing thrombus acceleration. Due to its ability to mediate leucocyte infiltration, PECAM-1 when highly expressed on leucocyte population has been linked with atherosclerotic involvement( Reference Veach, Hosking and Thompson 29 ). The decreased expression of PECAM-1 in this study by 14 % (P<0·05) demonstrates ACN ability to reduce leucocyte migration and aggregate formation in the damaged endothelium that would typically occur during atherogenesis( Reference Chong, Macdonald and Lovegrove 16 ). In an ex vivo study evaluating the anti-thrombogenic properties of a phenolic metabolite, shikimic acid (SA), a reduced expression of PECAM-1 was observed post-treatment with 1 mm of SA, also demonstrating the ability of polyphenols in minimising thrombus growth( Reference Veach, Hosking and Thompson 29 ).
Platelet activation related conformational changes occur via the GPIIb/IIIa complex which reveals a ligand binding site specific for fibrinogen, von Willebrand factor, fibronectin and vitronectin( Reference Calvete 30 ). This binding site is vital for promoting platelet aggregation (secondary haemostatic response), which is recognised by PAC-1 and is exclusive for its ability and specificity to bind to only activated platelets( Reference Calvete 30 ). In this current study, PAC-1 expression was inhibited by 10 % (P<0·01) and therefore demonstrates ACN supplementation capacity to exhibit antiplatelet activation potential induced by ADP platelet activation receptor. An in vivo study performed on thirteen participants who consumed ACN-rich QGPJ with and without exercise-induced oxidative stress for 28 d demonstrated no significant change in PAC-1 expression( Reference Santhakumar, Kundur and Sabapathy 31 ). In an ex vivo study focusing on hippuric acid and its ability to reduce activation related thrombogenesis also showed a reduction in PAC-1 expression( Reference Santhakumar, Stanley and Singh 13 ). During platelet activation by agonists such as ADP, collagen or arachidonic acid in vivo, platelets release α-granules. These secretory granules then translocate to the plasma membrane of endothelial cells, playing a crucial role in leucocyte recruitment to the site of injury( Reference Veach, Hosking and Thompson 29 ). In this study involving pro-thrombotic sedentary population, P-selectin expression was reduced by 14 % (P<0·05), hence alleviating platelet activation related degranulation and subsequently blunting platelet-fibrin/ platelet-platelet binding (aggregation). In an in vitro study involving delphinidin-3-glucoside on three healthy volunteers, P-selectin and PAC-1 expression were also reduced( Reference Yang, Shi and Reheman 32 ). Another in vitro study observing the effects of black soyabean extracts on healthy males also demonstrated a reduction in P-selectin expression( Reference Kim, Lim and Kim 33 ). Whole blood ADP-induced platelet aggregation was also notably reduced post-ACN supplementation in this study by 29 % (P<0·01). Current antiplatelet therapeutics such as clopidogrel, ticagrelor and prasugrel target the P2Y1/P2Y12 pathways of platelet activation and aggregation( Reference Thompson, Pederick and Santhakumar 12 , Reference Becker, Bassand and Budaj 34 ). But these antiplatelet therapeutics have been associated with increased bleeding risk and resistance in pro-thrombotic populations( Reference Thompson, Pederick and Santhakumar 12 , Reference Bhatt 35 ). The anti-radical and anti-thrombotic activity of ACN is attributable to its B benzoyl ring structure (delphinins and cyanidins), the hydroxylation, methoxylation and the O-diphenyl structure of the B-ring have been ascribed to the blunting of αIIbβ3 integrin activation and blocking of the ADP receptor (P2Y1/P2Y12) platelet activation pathway( Reference Yang, Shi and Reheman 32 ). The ability of the B-ring structure to attenuate these pathways has been exhibited in this study via reduction of ADP-induced platelet aggregation and platelet degranulation and therefore allowing ACNs to target similar pathways as currently used anti-platelet drugs( Reference Fernandes, Faria and Calhau 36 ).
This study showed no significant reduction in collagen or arachidonic acid induced platelet aggregation, indicating ACN supplementation in this study did not blunt the GPVI or COX-1 pathway of platelet activation in the sedentary population. An in vivo study evaluating the effects of ACNs on platelet function also demonstrated no reactivity to collagen-induced platelet activation( Reference Rechner and Kroner 37 ). Alternatively, in a recently conducted in vivo study by our group on overweight and obese participants, 4-week ACN supplementation showed inhibition of collagen and arachidonic acid stimulated platelet aggregation( Reference Thompson and Pederick 38 ). Furthermore, ACN-rich food supplements have exhibited a greater arachidonic acid platelet aggregation inhibitory effect during a simulated physiological oxidative stress environment( 26 ). It is believed that ACN intervention has the potential to inhibit prothrombotic pathways triggered by collagen and arachidonic acid (COX-pathway) greater under oxidative stress environments such as in obese/overweight or diabetic population( Reference Thompson and Pederick 38 ). Although platelet aggregation has been an effective measure of thrombotic risk in humans, reproducibility and the need for repetitive standardisation should be taken into consideration. Nevertheless, there was minimal variability (tests performed in duplicates) observed in this study and in previous studies performed by our group( Reference Thompson and Pederick 38 ).
Conclusion
It was observed that ACN supplementation for 4 weeks reduces ADP-induced platelet activation, aggregation and biomarkers of thrombogenesis such as monocyte-platelet aggregate formation and PECAM-1 expression. Owing to a similar platelet activation receptor blunting effect as exhibited by current antiplatelet therapy, it is suggested that ACN could potentially act as a natural complementary therapeutic in pro-thrombotic sedentary population. Further mechanistic studies comparing intervention of clopidogrel v. ACN in pro-thrombotic population is warranted.
Acknowledgements
The authors would like to sincerely thank QML Pathology and Central Queensland University for their resources. A special mention to Mr Mitchell Thorpe, Ms Alyssa Tyrrell and Mrs Judy Couper for their technical assistance.
This clinical trial was financially supported by the Central Queensland University New Staff Grant, Queensland, Australia.
K. T. performed experimental analysis and prepared the manuscript. H. H. performed experimental analysis. W. P. and I. S. assisted in experimental design and manuscript critical review. A. B. S. designed the study, analysed data and critically reviewed the manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517002124









