Inflammation is becoming increasingly acknowledged as having a major role in the pathogenesis of CHD(Reference Hanson1) and certain cancers(Reference Marx2). Atherosclerosis may involve subclinical thrombosis and inflammation that can result in the activation of the haemostatic process(Reference Hanson1, Reference Tedgui and Mallat3). Many dietary components(Reference Lichtenstein, Appel and Brands4) have been shown to reduce the risk of CHD by mechanisms involving the lowering of blood lipids. One such component is nuts(Reference Kris-Etherton, Hu and Ros5) that are now being recommended as part of heart-healthy diets(Reference Lichtenstein, Appel and Brands4). Clinical studies in healthy and hypercholesterolaemic adults(Reference Mukuddem-Petersen, Oosthuizen and Jerling6, Reference Griel and Kris-Ehterton7) have shown that nuts lower LDL-cholesterol while improving the overall blood lipid profile. In these studies, the cholesterol-lowering effects alone do not fully explain the percent decrease in CHD risk. Mechanisms beyond blood lipid lowering may also be contributing to the overall CHD risk reduction. Frequent nut and seed consumption is associated with lower levels of inflammatory markers such as C-reactive protein (CRP), IL-6 and fibrinogen, even after adjusting for confounding factors(Reference Jiang, Jacobs and Mayer-Davis8), suggesting yet another mechanism by which nuts may reduce risk of CHD.
Nuts are very high in unsaturated fat and contain non-lipid components such as antioxidant vitamins (vitamin E), plant protein rich in arginine, Mg, fibre and several phytochemicals(Reference Ros9). While the role of nuts high in monounsaturated fat such as almonds has not been previously studied in relation to inflammation, some of the components of nuts such as arginine, Mg, fibre and vitamin E have demonstrated anti-inflammatory properties(Reference Kris-Etherton, Hu and Ros5, Reference Blomhoff, Carlsen and Frost10, Reference Wells, Mainous and Everett11). Recently, a portfolio diet consisting of cholesterol-lowering foods, including almonds, has showed a similar reduction in CRP as observed with statin therapy(Reference Jenkins, Kendall and Marchie12). Similarly, a Mediterranean diet with nuts was effective in lowering IL-6 levels compared with a control diet without nuts(Reference Estruch, Martinez-González and Corella13). In both these studies, nuts were one of the many components of the intervention diet, making it difficult to tease out the actual effect of nuts on inflammation. However, based on the nutrient and non-nutrient composition of nuts, one can hypothesise a favourable role for nuts in reducing inflammation.
The aim of the present study was to determine the influence, if any, of selected markers of inflammation in healthy adults. We also looked at a possible dose–response effect of almonds on these markers.
Experimental methods
Study design
The present study was part of an intervention trial(Reference Sabaté, Haddad and Tanzman14) designed to evaluate the effect of different doses of almonds on CHD risk factors. This was a randomised, single-blind, crossover, controlled feeding study. All subjects completed a 2-week run-in period during which they consumed a typical Western diet (34 % energy from fat). Following this, they were randomised to a sequence of three isoenergetic treatment diets for 4 weeks each: a cholesterol-lowering control diet without nuts ( < 30 % energy from fat), a low-almond diet and a high-almond diet. The almond diets were the same as the control diet, but had 10 or 20 % of the total energy replaced with almonds. This accounted for 34 g/8368 kJ (2000 kcal) diet for the low-almond diet and 68 g/8368 kJ (2000 kcal) diet for the high-almond diet.
All meals were prepared and served in the Nutrition Department Research Kitchen at Loma Linda University. Sunday to Friday, breakfast and dinner were served on site, while lunch and all Saturday meals were packed to go. Nine menus were used in rotation to provide variety and the meals consisted of foods commonly consumed and represented all the food groups. Almonds were served on their own or incorporated into certain hot (e.g. pizza) or cold foods (e.g. salads). Menus were developed for seven levels of energy intake ranging from 7533 to 15 066 kJ (1800–3600 kcal)/d. Subjects were weighed every day during the run-in phase to establish their baseline body weight and then twice a week thereafter throughout the treatment phases, and their energy intake was adjusted as needed to maintain stable body weight throughout the study.
To ensure compliance to the treatments, the almonds were served either during breakfast or dinner and were eaten under the supervision of a senior investigator. In addition, subjects maintained a diary in which they recorded any deviations from the study diet protocols and this was reviewed on a weekly basis by a senior investigator. To ensure that the actual diet composition matched that of the planned, a random sampling of the diets was analysed for macronutrient composition (Covance Laboratory, Madison, WI, USA). The present study was carried out with the highest degree of quality control with compliance of subjects to diet and study protocol close to 100 %. A more detailed description of the diet protocol is provided elsewhere(Reference Sabaté, Haddad and Tanzman14).
Subjects
Subjects were included in the study if they were between the ages of 20 and 60 (mean age 40·9 (se 12·8)) years, healthy, and if their habitual diet before the study was close to the typical American diet. Subjects were excluded if they had any previous history of hypertension, arteriosclerosis or metabolic diseases, had any weight change 6 months before the study, had BMI >30 kg/m2, fasting serum cholesterol < 15th or >90th percentile for age, sex and race, fasting TAG >2·26 mmol/l (>200 mg/dl), were chronically ill or taking medications, consumed nuts frequently (>2 times/week), had erratic exercise habits or were athletes that had rigorous exercise routine, were smokers or drank alcohol >2 times a week. Women with irregular menses or hormone use within the past 5 years (either hormone replacement for postmenopausal women or women on birth control pills) were excluded from the study as well. Subjects were asked to maintain the same exercise pattern throughout the study that was established at the start. Subjects were asked to record in a daily diary any deviations from the exercise routine that was established at baseline and an investigator reviewed this diary on a weekly basis to ensure compliance to the exercise routine.
Out of the twenty-seven subjects that started, twenty-five completed the trial. The sample size of twenty-five subjects had 90 % power to detect differences of 10 % in E-selectin, at a significance level of P < 0·05. Subjects were screened using a detailed medical and general questionnaire, one-on-one interviews and group meetings. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of Loma Linda University. Written informed consent was obtained from all study subjects.
Data collection and analyses
Twelve hour fasting blood samples were collected at the end of the run-in period and at the end of each dietary period on two alternate days by standard venepuncture. Samples were drawn at the same time in the morning on each occasion (between 06.30 and 08.30 hours). Blood was collected into two vacutainers: one coated with citrate and the other without any anticoagulants. The blood was kept on ice for 15 min and then centrifuged at 1500 g for 15 min at 4°C. The plasma and serum samples were separated, aliquoted and stored at − 80°C, and all analyses were completed within 1 year of completing the study. The erythrocytes from the citrate vacutainer were separated and washed three times with 150 mmol/l NaCl at 800 g (15 min at 4°C) and the buffy coat was removed. The red cells were mixed with saline (50 % packed cell volume) and stored at − 80°C until analysed for fatty acid composition.
Serum CRP, E-selectin, IL-6, plasma fibrinogen and tissue plasminogen activator (tPA) antigen were analysed at the end of the study (Department of Pathology, University of Vermont, Burlington, VT, USA). These makers were selected from the many different markers available because they have been previously shown to be influenced by nuts or dietary fatty acids. Fibrinogen was measured in an automated clot-rate assay(Reference Clauss15) using an ST4 instrument (Diagnostica Stago, Parsipanny, NJ, USA). Plasma tPA antigen was determined by a two-site immunoassay utilising three different monoclonal antibodies(Reference Holvoet, Cleemput and Collen16). This assay is sensitive to both free tPA and tPA in complex with its inhibitors. E-selectin and IL-6 were measured by ultra-sensitive ELISA (R&D Systems, Minneapolis, MN, USA). A high-sensitivity colorimetric competitive immunoassay was used to determine CRP levels(Reference Macy, Hayes and Tracy17). Serum and erythrocyte samples were also analysed for fatty acid composition (Lipomics, West Sacramento, CA, USA) using methods previously described in the present original study(Reference Sabaté, Haddad and Tanzman14).
Statistical analysis
Statistical analysis was performed with the Statistical Analysis System, version 8.0 (SAS Institute Inc., Cary, NC, USA). Duplicate measurements of the outcome variables were averaged and used in further analysis. Changes in inflammatory and haemostatic factors in response to dietary treatment were determined by ANOVA using mixed linear models that included a random-effect term for subjects and fixed-effect term for diet and period. Tests for trend were conducted by ANOVA by replacing the diet variables in the above model with continuous variable representing the percent total energy in the diet contributed by almonds. Results with a P value of ≤ 0·05 were considered statistically significant. Results are reported as the least-squares means with their standard error. Fibrinogen, IL-6 and CRP were found to violate the assumption of homogeneity of variance. Therefore, the log-transformed values were used for statistical analysis.
Results
Out of the twenty-seven subjects that started the study, two dropped out for lack of adherence to the study protocol and twenty-five completed all the requirements. Of those that completed eleven were women and fourteen were men. The mean age of the subjects was 41 (se 13) years (range 22–53 years). Subjects were apparently healthy with normal baseline cholesterol and TAG levels (mean), baseline serum cholesterol 5·61 (se 0·14) mmol/l, LDL cholesterol 3·86 (se 0·15) mmol/l and TAG 1·37 (se 0·13) mmol/l. The mean baseline body weight of study subjects was 71 (se 2·7) kg. Body weight remained constant throughout the study (control diet 71 (se 2·7) kg; low-almond diet 71·2 (se 2·7) kg; high-almond diet 70·7 (se 2·7) kg).
Table 1 shows the analysed diet composition of the three diets. As expected, the percent energy from fat was higher in the low- (35 %) and high-almond diets (39 %) compared with the control diet (30 %). The incorporation of almonds in the diets resulted in small decreases in saturated fat, small increases in polyunsaturated fat and a substantial increase in monounsaturated fat.
Table 1 Macronutrient composition of the three diets*

* Values obtained during chemical analysis of samples from the study diets.
† The values for carbohydrate intake were calculated by subtracting the values for fat and protein intake from those of total energy intake.
The changes in the fatty acid composition of plasma TAG and erythrocyte membrane phospholipids during the treatment diets are reported in Table 2. The percentage of total monounsaturated fat increased with increasing amounts of almonds in the diet both in the erythrocyte membrane and serum TAG lipid fraction. This trend is similar to those observed for monounsaturated fat content of the diets (Table 1), and validates the consumption of almonds and close adherence to the diets by the subjects.
Table 2 Distribution of fatty acids in serum TAG and erythrocyte membrane phospholipids (mole %)
(Mean values with their standard errors)

The results of the almond diets on inflammatory markers are presented in Table 3. Differences between diet treatments showed that the high-almond diet had significantly lower E-selectin than the control (7·8 % decrease) and low-almond (6·3 % lower) diets, but there were no differences between low-almond and control diets. For each 1 % increase in energy from almonds, there was an estimated decrease of 0·18 μg/l in E-selectin. Serum E-selectin decreased with progressively higher amounts of almonds in the diet (P < 0·0001), but there was no clear dose response.
Table 3 Inflammatory markers after the three treatment diets
(Mean values with their standard errors and standard deviations)
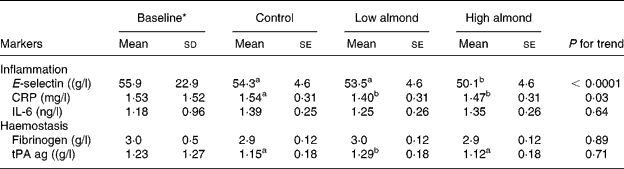
CRP, C-reactive protein; tPA ag, tissue plasminogen activator antigen.
a,b Mean values with different superscript letters were significantly different by ANOVA (P < 0·05).
* Baseline values were measured at end of the run-in phase.
Both the almond-enriched diets lowered serum CRP compared with the control, diet but there was no dose response. For serum IL-6 (P = 0·64) and fibrinogen (P = 0·89), there were no treatment effects. For tPA, low-almond diet had significantly higher values than high-almond and control diets (P < 0·01).
Discussion
Frequent consumption of nuts is associated with a reduced risk of CHD(Reference Kris-Etherton, Hu and Ros5). One of the mechanisms by which they may bring about their cardioprotective effects is by lowering blood lipids. We had previously shown that 68 g almonds for 8386 kJ (2000 kcal) diet per day lowers serum total and LDL-cholesterol in healthy adults(Reference Sabaté, Haddad and Tanzman14). In the present study, we further explored the effects of an almond-enriched diet on markers of inflammation. We showed that consuming a high-almond diet (68 g/d per 8386 kJ) for 4 weeks significantly decreased serum E-selectin compared with the isoenergetic control diet in healthy men and women. Increasing the percent energy from almonds and thus monounsaturated fat content in the diet progressively lowered E-selectin, but no clear dose response was observed. We showed that both the almond diets were able to lower serum CRP compared with the control diet but not in a dose-dependent manner. Other markers of inflammation and haemostasis that we studied were not influenced by the almond-enriched diets.
Circulating levels of adhesion molecules such as E-selectin are critical to leucocyte and platelet recruitment and are indicative of endothelial dysfunction(Reference Bonetti, Lerman and Lerman18). A decrease in E-selectin could lower the inflammatory response and thus lower the risk of CHD. Our data suggest that the decrease in serum E-selectin and CRP in subjects during the high-almond diet could be explained by the change in MUFA concentration in the diets. This is in fact verified by a significant increase in both erythrocyte membrane and plasma TAG MUFA content in subjects during the high-almond diet compared with the control diet. It is possible that the effects of dietary fatty acids on E-selectin may be mediated by alteration in gene expression of these adhesion molecules(Reference Bellido, López-Miranda and Pérez-Martinez19).
A study similar to ours, but using olive oil as the source of MUFA(Reference Perez-Jimenez, Castro and Lopez-Miranda20), reported no effect of a high-MUFA diet on plasma E-selectin levels. Although this was also a feeding study with the same duration of intervention as ours, there were a few differences that may have contributed to the discordant outcomes. The baseline plasma E-selectin concentration reported in their study was almost half that observed in ours, suggesting that the effect of MUFA is perhaps seen only at higher concentrations of E-selectin. Also, subjects in their study were men less than 30 years, while the present study had both men and women from 20 to 60 years. The sources of MUFA in the two studies were also different suggesting that other non-lipid bioactive components in almonds may have influenced E-selectin. Dietary polyphenols have been shown to modulate signal transduction pathways causing the repression of pro-inflammatory genes(Reference Rahman, Biswas and Kirkham21). Almonds, especially eaten with their skin intact, have a significant amount of flavonoids that seem to help protect LDL from oxidation(Reference Chen, Milbury and Chung22), and whether it has a role in suppressing gene expression of adhesion molecules remains to be determined.
CRP is a sensitive marker of inflammation and high levels are strongly linked with coronary events, stroke and peripheral vascular disease(Reference Tedgui and Mallat3). There are only two other studies that have looked at the effects of almonds on serum CRP, one of which showed no change in CRP levels(Reference Jenkins, Kendall and Marchie23) and the other showed a decrease in serum CRP levels with a portfolio diet that contained many cholesterol-lowering foods including almonds(Reference Jenkins, Kendall and Marchie12). Because multiple dietary changes were made in the portfolio diet study, one cannot tease out the effects of almonds. Although the present study showed a decrease in serum CRP of about 4·5–9 % on almond diets, the mean individual values were in the moderate risk estimate (1–3 mg/l) at baseline and at the end of the diet period. Almonds are rich in Mg, which have been inversely related to serum CRP levels(Reference Song, Li and van Dam24), and may be a potential mechanism that might explain the effects of almonds on CRP warranting further investigation. Also other bioactive components in almonds including arginine, α-tocopherol and phytonutrients(Reference Ros9, Reference Blomhoff, Carlsen and Frost10, Reference Huynh and Chin-Dusting25, Reference Jambazian, Haddad and Rajaram26) may have a role in modifying some of the inflammatory mediators, but more research is needed to fully understand their role.
While serum CRP levels were influenced by almonds, no significant change was observed for serum IL-6 although the direction of change was the same as for CRP. Serum IL-6 tends to be lower in the morning and higher at night, while CRP does not appear to go through similar diurnal variations(Reference Meier-Ewert, Ridker and Rifai27). Therefore, CRP tends to be better able to predict CHD in comparison with IL-6 on morning-extracted blood samples. The haemostasis factors examined in the present study were also not influenced by the almond-enriched diets. Plasma tPA antigen was significantly lower on the control and high-almond diets in comparison with the low-almond diet. However, the concentrations were at the lower end of the normal range, and the observed differences do not appear to be biologically significant. We found no effect of almonds on plasma fibrinogen and others have made similar observations(Reference Temme, Mensink and Hornstra28) with dietary fatty acids.
Several things may explain the lack of an effect on these haemostatic factors including a small sample size, baseline values and duration of intervention. While a sample size of 25 was adequate to detect a 10 % difference in inflammatory markers at 90 % power, thirty-six subjects would be required for similar statistical power for haemostatic variables. Also, 4 weeks of intervention although sufficient to see changes in blood lipids(Reference Kris-Etherton and Dietschy29) and some selected inflammatory markers, it may not be adequate to measure changes in haemostatic factors. Finally, it is likely that neither MUFA nor other bioactive components in almonds influence haemostasis factors especially in an apparently healthy population with baseline values near lower end of normal. Studies suggest that in participants that already have elevated inflammatory status like in obesity, metabolic syndrome or cancer do respond more favourably to bioactive components in food(Reference Browning, Krebs and Moore30–Reference Kim, Young and Bobe32). The role of almonds and other nuts in reducing inflammation in such clinical populations needs to be explored in the future.
Tree nuts and peanuts are fatty food containing mostly unsaturated fatty acids. Of the different nuts, almonds, peanuts, pistachio, macadamia and pecans are some of the monounsaturated fat-rich nuts, while walnuts are rich in polyunsaturated fat(Reference Ros9). The role of nuts in decreasing inflammation has received more attention recently with the Multi-Ethnic Study of Atherosclerosis first showing an inverse association between frequent consumption of nuts and seeds and serum CRP, IL-6 and fibrinogen levels(Reference Jiang, Jacobs and Mayer-Davis8). Two other studies showed that a Mediterranean diet pattern, which includes nuts, is associated with anti-inflammatory markers more strongly than diets with no nuts(Reference Montzoros, Williams and Manson33, Reference Salas-Salvado, Garcia-Arellano and Estruch34). While nuts share a similar fatty acid profile, they do constitute different bioactive components that make up their non-lipid matrix(Reference Ros9). Therefore, whether or not all nuts produce similar changes in markers of inflammation needs to be explored in future studies. Studies to date have mainly looked at walnuts(Reference Ros, Nunez and Perez-Heras35, Reference Mukuddem-Petersen, Stonehouse Oosthuizen and Jerling36) and almonds(Reference Jenkins, Kendall and Marchie12, Reference Jenkins, Kendall and Marchie23) with respect to inflammation.
The present study shows that incorporating about 68 g almonds per 8368 kJ (2000 kcal) cholesterol-lowering diet decreases serum E-selectin and CRP in healthy men and women, but does not influence other markers like IL-6 and haemostasis factors. There is some evidence to suggest that even small changes in CRP observed within the normal range are predictive of vascular events in healthy individuals(Reference Hackam and Anand37). While the absolute change observed in some of the markers may be small, the additive effect of different markers on CHD risk reduction may be more meaningful clinically. Further studies are therefore needed to substantiate the present findings, elucidate mechanisms of action and determine the biological and clinical significance of these diet-induced changes to the overall disease process. While the evidence supporting the inclusion of nuts in heart-healthy diets is well established, it is being increasingly recognised that nuts provide cardiovascular protection that go beyond cholesterol lowering.
Acknowledgements
The funding for the present research was provided by the Center for Health and Nutrition Research at the University of California, Davis, and by the Almond Board of California, Fresno, CA. We acknowledge the support provided by Dr Russell Tracy, University of Vermont, Burlington, VT, USA for analysing many of the serum markers in the present study. The authors have no conflicts of interest.
S. R. and J. S. designed the study. S. R. coordinated the study. S. R. and K. M. C. were responsible for data collection and analyses and quality control. K. M. C. and J. S. were involved in statistical analyses. K. M. C. was the dietitian and was responsible for all diet analyses. All authors contributed to the interpretation of data. S. R. and K. M. C. wrote the first draft of the manuscript and all authors critically reviewed and revised the manuscript. S. R. obtained the funding for the present study.





