Obesity affects more than 500 million individuals and is currently the fifth leading risk of death worldwide( 1 ). The clinical importance of this disease stems from its associated complications including liver diseases, metabolic diseases, cardiovascular disease, gastrointestinal problems and musculoskeletal disorders( Reference Hubert, Feinleib and McNamara 2 – Reference Madan, Chen and Goodman 6 ). Therefore, overweight and obese patients are on numerous medications, many of which are cleared through hepatobiliary mechanisms. However, it is plausible that obesity and its related complications may alter the hepatobiliary clearance of endogenous and exogenous compounds. There is a scarcity of information regarding the effect of obesity on hepatic transport and/or metabolic pathways. Previous studies have reported obesity-related alterations in the pharmacokinetics of clinically important drugs such as verapamil, vancomycin and β-lactam antibiotics( Reference Boullata 7 ), and studies have indicated that anti-retroviral drugs may be less effective in obese patients( Reference Crum-Cianflone, Roediger and Eberly 8 ). This could be attributed to alterations in the distribution, metabolism and/or transport of the drug. Indeed, increased cytochrome P450 2E1 (CYP2E1) activity leading to the increased clearance of chlorzoxazone has been reported in obese patients( Reference Jain, Chung and Jain 9 ). Studies in genetic rodent models of obesity have reported altered expression of hepatic drug transporters and metabolic enzymes( Reference Pizarro, Balasubramaniyan and Solis 10 – Reference Martin and Minkenberg 13 ). Studies in human subjects, as well as in diet-induced obesity rodent models, have shown that obesity, insulin resistance and type 2 diabetes are associated with a tendency towards increased cholesterol synthesis and decreased absorption, which is associated with an increase in the expression of the cholesterol efflux transporters Abcg5/Abcg58 (ATP-binding cassette subfamily G member 5/8) and Abca1 (ATP-binding cassette subfamily A member 1)( Reference Sabeva, Liu and Graf 14 – Reference Attia, Fournier and Vedie 17 ).
In addition to genetic factors, an imbalance between energy intake and expenditure highly contributes to the development of obesity. In particular, the intake of high-fat diets (HFD) seems to be a principal factor( Reference Satia-Abouta, Patterson and Schiller 18 ). Several models of diet-induced obesity using a HFD have been developed in rodents( Reference Angela, Gajda and Mathew 19 ). Besides increased weight gain, high fat feeding results in a surplus of fatty acids, which contributes to hepatic lipogenesis in the form of triglycerides (TAG). It is believed that the liver has a limited capacity to store excess TAG, after which steatosis accompanied by cell damage occurs( Reference Trauner, Arrese and Wagner 20 ). Several inflammatory mediators released during hepatocellular damage, particularly IL-6 and TNF-α, seem to act as key players( Reference Gregor and Hotamisligil 21 ), and have previously been shown to regulate drug disposition pathways. One of the mechanisms by which this may occur is through alterations in the expression and translocation of nuclear receptor proteins( Reference Teng and Piquette-Miller 22 ).
Many nuclear receptors act as key transcriptional regulators of genes that are involved in transport, metabolism and detoxification pathways (Table 1)( Reference Teng and Piquette-Miller 22 ). Pregnane X receptor (PXR/NR1I2 (nuclear receptor subfamily 1, group I, member 2)), which serves as the main xenobiotic sensor, regulates the expression of the most abundantly expressed microsomal enzyme, CYP3A4 (cytochrome P450 3A4; Cyp3a2 (cytochrome P450 3A2) in rats)( Reference Urquhart, Tirona and Kim 23 ), as well as a plethora of drug transporters including key members of the ABC family such as P-glycoprotein (Pgp) (ABCB1, ATP-binding cassette subfamily B member 1) and the multi-drug resistance-associated proteins (ABCC, ATP-binding cassette subfamily C). The constitutive androstane receptor (CAR/NR1I3 (nuclear receptor subfamily 1, group I, member 3)) is another xenobiotic-sensing nuclear receptor, which regulates drug detoxification pathways through the induction of an overlapping set of genes, including Pgp, ABCC2, ABCC3 and CYP2B6 (cytochrome P450 2B6)( Reference Urquhart, Tirona and Kim 23 ).
Table 1 Nuclear receptors and their target genes

PXR, pregnane X receptor; NR1I2, nuclear receptor subfamily 1, group I, member 2; Abcb1, ATP-binding cassette subfamily B member 1; Abcc2, ATP-binding cassette subfamily C member 2; Abcc3, ATP-binding cassette subfamily C member 3; Abcg2, ATP-binding cassette subfamily G member 2; Slco1a4, solute carrier organic anion transporter family, member 1a4; Cyp3a, cytochrome P450 3A; Cyp2b, cytochrome P450 2B; Cyp2c, cytochrome P450 2C; CAR, constitutive androstane receptor; NR1I3, nuclear receptor subfamily 1, group I, member 3; Abcc1, ATP-binding cassette subfamily C member 1; Abcc4, ATP-binding cassette subfamily C member 4; Abcc5, ATP-binding cassette subfamily C member 5; Cyp1a, cytochrome P450 1A; NR1H4, nuclear receptor subfamily 1, group H, member 4; FXR, farnesoid X receptor; Abcb11, ATP-binding cassette subfamily B member 11; Abcb4, ATP-binding cassette subfamily B member 4; Cyp7a, cytochrome P450 7A; SHP, small heterodimer partner; Slco10a1, solute carrier organic anion transporter family, member 10A1; LXRα, liver X receptor α; NR1H3, nuclear receptor subfamily 1, group H, member 3; Abca1, ATP-binding cassette subfamily A member 1; Abcg5/Abcg8, ATP-binding cassette subfamily G member 5/8; Srebp-1c, sterol regulatory element-binding protein 1.
Liver X receptor (LXRα/β/NR1H3/2 (nuclear receptor subfamily 1, group H, member 3/2)) is a main regulator of cholesterol metabolism, and has recently been shown to play a role in the pathogenesis of inflammation( Reference Im and Osborne 24 ). Oxysterols, the oxygenated derivatives of cholesterol, activate LXR, thereby promoting the clearance of cholesterol through the induction of several transporters including ABCA1, ABCG1 (ATP-binding cassette subfamily G member 1), ABCG5/ABCG8 as well as the sterol regulatory element-binding protein 1 that promotes TAG synthesis( Reference Sonoda, Pei and Evans 25 ). In rodents, LXR also induces cytochrome P450 7A1 (Cyp7a1), which metabolises cholesterol to bile acids. Farnesoid X receptor (FXR/NR1H4 (nuclear receptor subfamily 1, group H, member 4)) is a master regulator of bile acid homeostasis, responding to intracellular bile acid concentrations by promoting bile acid and phospholipid biliary secretion through the induction of the bile salt export pump (ABCB11 (ATP-binding cassette subfamily B member 11)) and ABCB4 (ATP-binding cassette subfamily B member 4), respectively. FXR activation also indirectly suppresses the Na-dependent taurochlorate co-transporting protein (SLC10A1 (solute carrier family 10 (Na/bile acid co-transporter), member 1))-mediated uptake of bile into the liver through activation of the small heterodimer partner (SHP/NR0B2 (nuclear receptor subfamily 0, group B, member 2))( Reference Kalaany and Mangelsdorf 26 ).
As the involvement of nuclear receptors in regulating drug transporters and enzyme activity in diet-induced obesity is currently unclear, the objective of the present study was to investigate the effect of a HFD on the hepatic expression of the nuclear receptors PXR, CAR, LXR and FXR and several of their target genes (Table 1). We hypothesised that high fat feeding would alter the hepatic gene expression of major transporters through a dysregulation of the expression of the nuclear receptors.
Experimental methods
Animals
Female Sprague–Dawley rats aged 6 weeks were purchased from Charles River. The rats were individually caged in a temperature- and humidity-controlled 12 h light–12 h dark cycle, and allowed free access to food and water. The experiments were conducted in accordance with the Canadian Council on Animal Care. Female rats were randomised into two groups, either fed a HFD or a standard diet (SD) for a period of 13 weeks. The HFD consisted of 42·8 % energy from fat, 38·5 % energy from carbohydrate and 18·7 % energy from protein in the form of a pellet (TD 06 092; Harlan Teklad). The SD consisted of 12·5 % energy from fat, 63·2 % energy from carbohydrate and 24·3 % energy from protein in the form of a pellet of standard rat chow (5075; Charles River). Weight and food intake were monitored three times per week.
Blood and tissue collection
On week 13, the rats were killed between 09.00 and 12.00 hours, depending on the time at which food was removed. All food was removed from the cages at least 2–3 h before sacrificing. After complete anaesthesia with isoflurane, the abdominal cavity was opened and approximately 5 ml of blood were withdrawn from the abdominal vena cava into 15 % EDTA pre-treated tubes. To collect plasma samples, whole blood was then centrifuged at 3000 rpm for 10 min at 4°C (Allegra 6R Centrifuge; Beckman Coulter) and then immediately stored at − 80°C. The liver median lobe was weighed, freeze-clamped and stored at − 80°C for further use. Fat depots were collected from the urogenital, retroperitoneal and subcutaneous regions.
Blood chemistry
Plasma levels of free fatty acid (non-esterified fatty acid, NEFA), insulin, glucose, cholesterol, leptin, TAG and glycerol were measured as described previously( Reference Gauthier, Couturier and Latour 27 , Reference Collin, Chapados and Dufresne 28 ). Briefly, plasma insulin and leptin concentrations were determined with RIA test kits distributed by LINCO Research, plasma glucose concentrations were measured using the Autokit Glucose enzymatic colorimetric assay (Wako Diagnostics), and plasma NEFA concentration was determined using an enzymatic colorimetric assay (Roche Diagnostics). Plasma total cholesterol concentration was determined using a commercial kit (Wako Diagnostics and Chemicals USA) according to the manufacturer's instructions. Commercial kits (Sigma) were used to determine glycerol and TAG levels in plasma and hydrolysed liver homogenate samples by colorimetric methods. The analysis of plasma bile acid concentration was performed by IDEXX Laboratories. Total hepatic bile acid concentrations were determined in 100 mg liver samples by homogenising in 75 % ethanol followed by incubation for 2 h at 50°C. The samples were centrifuged at 6000 g and the supernatant was used for the determination of total bile acid concentrations according to the manufacturer's protocol (Crystal Chem, Inc.).
PCR
Total RNA was extracted from 100 mg of frozen liver tissue using TRIzol reagent (Invitrogen), according to the manufacturer's instructions, and absorbance was measured at 260 and 280 nm (NanoDrop; Thermo Fisher Scientific). Total RNA (2 μg) was treated with deoxyribonuclease I (Invitrogen) and reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Primer sets were synthesised at the Hospital for Sick Children (DNA Synthesis Center; Table 2). Real-time quantitative PCR was performed using LightCycler® technology with SYBR Green I fluorescence detection (Roche Diagnostics). A sample without the reverse transcription enzyme and a no-template control was used to detect genomic or DNA contamination. An efficiency-corrected ΔC t method was used to calculate the relative amounts of RNA, and amplification efficiency was calculated using the equation E= 10( − 1/slope). mRNA levels were normalised to those of β-actin, and results are presented as a percentage of control values.
Table 2 Quantitative PCR primers

Abcg5, ATP-binding cassette subfamily G member 5; Abcb4, ATP-binding cassette subfamily B member 4; Abca1, ATP-binding cassette subfamily A member 1; Abcb1a, ATP-binding cassette subfamily B member 1a; Abcc2, ATP-binding cassette subfamily C member 2; Abcc3, ATP-binding cassette subfamily C member 3; Abcb11, ATP-binding cassette subfamily B member 11; PXR, pregnane X receptor; NR1I2, nuclear receptor subfamily 1, group I, member 2; Cyp7a1, cytochrome P450 7A1; LXRα, liver X receptor α; NR1H3, nuclear receptor subfamily 1, group H, member 3; LXRβ, liver X receptor β; NR1H2, nuclear receptor subfamily 1, group H, member 2; FXR, farnesoid X receptor; NR1H4, nuclear receptor subfamily 1, group H, member 4; SHP, small heterodimer partner; NR0B2, nuclear receptor subfamily 0, group B, member 2; CRP, C-reactive protein.
Western blot analysis
Protein extraction was performed by methods as described previously( Reference Anger, Cressman and Piquette-Miller 29 , Reference De Souza, Zahedi and Badame 30 ). Briefly, 300 mg of liver tissue were homogenised in 1 × RIPA (radioimmunoprecipitation assay) lysis buffer (Cell Signaling Technology) with freshly added 0·5 mm-phenylmethylsulfonyl fluoride (BioShop) and 4 μl/ml protease inhibitor (P8340; Sigma-Aldrich). Protein concentrations were quantified by the Bradford assay. A total of 20 μg of membrane protein or 60 μg of whole cell lysate in Laemmli sample buffer (Bio-Rad) for Pgp and Cyp3a2, respectively, were separated by 10 % SDS–PAGE and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories Canada Limited). The membranes were blocked for 1 h in 5 % (w/v) skimmed milk powder in Tris-buffered saline containing 0·05 % (v/v) Tween-20, and then incubated at 4°C overnight with mouse anti-Pgp antibody (C-219, 1:500, 1 mg/ml; Abcam, Inc.), rabbit anti-Cyp3a2 (ab78279, 1:1000; Abcam, Inc.) or goat anti-PXR.1 (A-20, 1:100, 0·2 mg/ml; Santa Cruz Biotechnology, Inc.). After a series of washes with Tris-buffered saline, the membranes were incubated for 2 h with secondary antibodies from Jackson ImmunoResearch Laboratories (goat anti-mouse at 1:3000 for Pgp, goat anti-rabbit at 1:5000 for Cyp3a2 and donkey anti-goat at 1:2000 for PXR.1). The membranes were visualised with ECL Plus (GE Healthcare) using a FluorChem imaging system (Alpha Innotech), and the optical density of each band was determined with the AlphaEaseFC software (Alpha Innotech). β-Actin (AC-15, 1:40 000, 2 mg/ml; Sigma-Aldrich) was used as a housekeeping gene to correct for variability in protein loading.
Data and statistical analysis
Data were analysed with GraphPad Prism version 5 (GraphPad Software, Inc.). A two-tailed Student' t test was used to compare the results between the HFD and SD rats. Significance was set at P< 0·05. Data are presented as means with their standard errors of the mean. Correlation was performed using Pearson's correlation test.
Results
Body composition and metabolic characteristics
Compared with the SD group, rats fed the HFD gained significantly more weight after 6 weeks of commencing the diet until the end of the study (P< 0·05; Fig. 1(a)). Differences in weight gain between the two groups increased with time. The initial weight of the rats was 210·3 (sem 1·8) g in the SD group and 209·0 (sem 1·9) g in the HFD group, while the final weight of the rats was 348·4 (sem 5·9) g in the SD group and 372·1 (sem 7·3) g in the HFD group. A similar intake of energy was observed between the two groups (Fig. 1(b)). A significant gain in intra-abdominal and subcutaneous fat mass was observed in the HFD group (P< 0·05) (Table 3).

Fig. 1 (a) Average weight gain (g) and (b) weekly food intake (kcal/d) of rats. Female rats were fed a high-fat diet ((a) –○– and (b) –■–) or a standard diet (–●–) for 13 weeks as described in the ‘Experimental methods’ section. Values are means (n 7), with their standard errors represented by vertical bars. Mean value was significantly different from that of the SD group: * P< 0·05, ** P< 0·01. To convert food intake in kcal/d to kJ/d, multiply by 4·184.
Table 3 Body composition and metabolic characteristics of female rats fed a high-fat diet (HFD) or a standard diet (SD) for 13 weeks (Mean values with their standard errors)
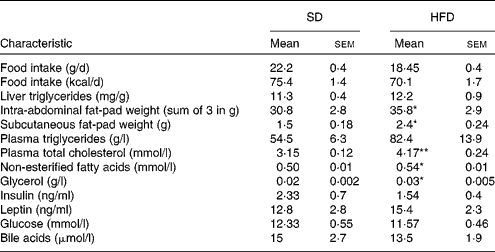
Mean value was significantly different from that of the SD group: * P< 0·05, ** P< 0·01.
Blood chemistry of the HFD group showed a trend towards mild hyperlipidaemia with significantly higher levels of cholesterol, NEFA and glycerol in the HFD group compared with the SD group. Plasma TAG levels were also somewhat elevated in the HFD group, but did not reach significance (Table 3). Total hepatic bile acid concentrations were not different between the HFD (0·42 (sem 0·04) μmol/g) and SD (0·45 (sem 0·08) μmol/g) groups.
Effect of the high-fat diet on cytokine expression
Hepatic mRNA levels of IL-1β and IL-6 were not significantly different between the HFD and SD groups; however, mRNA levels of C-reactive protein (CRP), which is a biomarker for IL-6 activity and systemic inflammation, were significantly higher in the HFD group (Fig. 2).
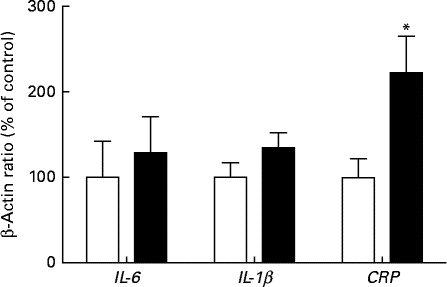
Fig. 2 Hepatic mRNA expression of pro-inflammatory cytokines in female rats fed a high-fat diet (■) or a standard diet (□) for 13 weeks, as determined by real-time quantitative PCR. Values are expressed as a percentage of the control value (100 %). Values are means (n 7), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the SD group: (P< 0·05). CRP, C-reactive protein.
Effect of the high-fat diet on nuclear receptor expression
We observed significant differences in the hepatic expression of key nuclear receptors in rats fed the HFD (Fig. 3). Compared with the SD group, the rats fed the HFD had a 2-fold increase in the expression of PXR and FXR (P< 0·05). The expression levels of both LXRα and LXR were slightly but significantly increased in rats fed the HFD (P< 0·05). However, the expression level of CAR was not significantly affected.
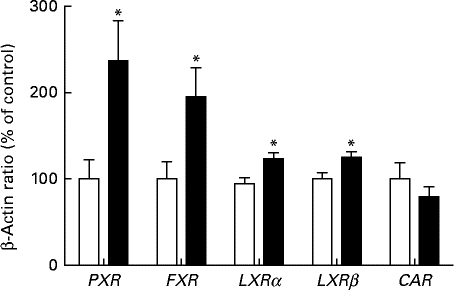
Fig. 3 Hepatic mRNA expression of nuclear receptors in female rats fed a high-fat diet (■) or a standard diet (□) for 13 weeks, as determined by real-time quantitative PCR. Values are expressed as a percentage of the control value (100 %). Values are means (n 7), with their standard errors represented by vertical bars. * Mean value was significantly different from that of the SD group: (P< 0·05). PXR, pregnane X receptor; FXR, farnesoid X receptor; LXRα, liver X receptor α; LXRβ, liver X receptor β; CAR, constitutive androstane receptor.
Target genes of pregnane X receptor
The HFD had a pronounced effect on the expression of several target genes of PXR (Fig. 4(a)). Compared with the SD group, a 2- and 5-fold increase in the hepatic expression of the canalicular efflux transporters Abcc2 (ATP-binding cassette subfamily C member 2) and Abcb1a (ATP-binding cassette subfamily B member 1a) was observed in rats fed the HFD. While the mRNA expression of the apical uptake transporter Slco1a4 (solute carrier organic anion transporter family, member 1a4) was significantly higher in the HFD group, the expression levels of Abcc3 (ATP-binding cassette subfamily C member 3) were significantly decreased (P< 0·05). The mRNA expression level of Cyp3a2 was 2·5-fold higher in the HFD group than in the SD group. The increased expression levels of these genes were strongly correlated with the increased expression levels of PXR (Pearson's r>0·67; Abcb1a (P= 0·0002), Abcc2 (P= 0·002), Slco1a4 (P= 0·009) and Cyp3a2 (P= 0·012)). Changes in the expression levels of Cyp3a2 and Pgp were further confirmed at the protein level (Fig. 4(b) and (c)).

Fig. 4 (a) Hepatic mRNA expression of the target genes of pregnane X receptor in rats fed a high-fat diet (HFD, ■) and a standard diet (SD, □), as determined by real-time quantitative PCR. Values are expressed as a percentage of the control value (100 %). Values are means (n 7), with their standard errors represented by vertical bars. Mean value was significantly different from that of the SD group: * P< 0·05, ** P< 0·01. Hepatic protein expression of (b) P-glycoprotein (Pgp) and (c) Cyp3a2 (cytochrome P450 3A2) in rats fed a HFD and SD. Protein levels were determined by Western blot analysis and normalised to those of β-actin. Abcb1a, ATP-binding cassette subfamily B member 1a; Abcc2, ATP-binding cassette subfamily C member 2; Abcc3, ATP-binding cassette subfamily C member 3; Slco1a4, solute carrier organic anion transporter family, member 1a4.
Target genes of farnesoid X receptor
As the expression levels of FXR were significantly increased in the HFD group, the expression levels of several target genes of FXR involved in bile acid homeostasis were examined (Fig. 5). Compared with the SD group, an approximately 2- and 3-fold increase in the mRNA levels of Abcb11 and Abcb4, respectively, was observed in rats fed the HFD. The increased expression levels of these genes were strongly correlated with the expression levels of FXR in both groups (Pearson's r>0·83; Abcb11 (P< 0·0001) and Abcb4 (P= 0·0001)). The expression level of the small heterodimer partner (SHP), which is transcriptionally increased as a result of FXR activation, was found to be 2·5-fold higher in the HFD group than in the SD group (P< 0·05). Surprisingly, the expression level of Slc10a1, which is negatively regulated by FXR through SHP mediated repression, was found to be significantly increased in the HFD group. In contrast, the expression level of Cyp7a1, a metabolic enzyme involved in cholesterol synthesis, was not significantly different between the groups.
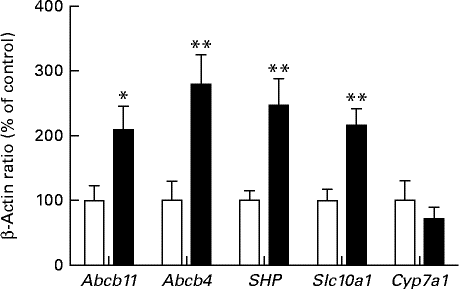
Fig. 5 Hepatic mRNA expression of the target genes of farnesoid X receptor in rats fed a high-fat diet (■) or a standard diet (□), as determined by real-time quantitative PCR. Values are expressed as a percentage of the control value (100 %). Values are means (n 7), with their standard errors represented by vertical bars. Mean value was significantly different from that of the SD group: * P< 0·05, ** P< 0·01. Abcb11, ATP-binding cassette subfamily B member 11; Abcb4, ATP-binding cassette subfamily B member 3; SHP, small heterodimer partner; Slc10a1, solute carrier family 10 (Na/bile acid co-transporter), member 1; Cyp7a1, cytochrome P450 7A1.
Target genes of liver X receptor
The effect of the HFD on the expression of LXR target genes is shown in Fig. 6. The canalicular ABC half-transporters Abcg5 and Abcg8 act in unison to efflux cholesterol into the bile. Although a pronounced increase in the expression level of Abcg5 was observed in the HFD group compared with the SD group, the expression levels of Abcg8 were not significantly affected. The HFD also significantly increased the mRNA levels of the cholesterol efflux regulatory protein (CERP/Abca1). In conjunction with ApoAI, CERP/Abca1 is involved in the transfer of cholesterol from macrophages into hepatocytes.
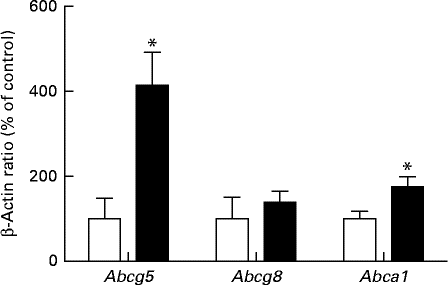
Fig. 6 Hepatic mRNA expression of the target genes of liver X receptor in rats fed a high-fat diet (■) or a standard diet (□), as determined by real-time quantitative PCR. Values are expressed as a percentage of the control value (100 %). Values are means (n 7), with their standard errors represented by vertical bars. Mean value was significantly different from that of the SD group: * P< 0·05. Abcg5, ATP-binding cassette subfamily G member 5; Abcg8, ATP-binding cassette subfamily G member 8; Abca1, ATP-binding cassette subfamily A member 1.
Discussion
Similar to our previous findings( Reference De Souza, Zahedi and Badame 30 ), we found that rats fed a HFD (42 % energy from fat) for a period of 13 weeks had a higher weight gain and larger intra-abdominal fat mass compared with those fed a SD; however, the total lipid content in the liver did not change, as determined by biochemical and histological analysis of TAG levels in the liver( Reference Ngo Sock, Cote and Mentor 31 ). This result is in agreement with previous studies showing that liver TAG levels increase during the first weeks of high fat feeding, but then gradually return to normal levels( Reference Collin, Chapados and Dufresne 28 , Reference Ngo Sock, Cote and Mentor 31 ), which suggests that there are mechanisms by which the liver adapts to increased dietary lipids( Reference Ngo Sock, Cote and Mentor 31 ).
Altered hepatobiliary transport of endogenous and exogenous compounds has previously been reported in transgenic and dietary rodent models of obesity( Reference Pizarro, Balasubramaniyan and Solis 10 , Reference Gylling, Hallikainen and Kolehmainen 15 , Reference Sugioka, Haraya and Fukushima 32 – Reference Khemawoot, Yokogawa and Shimada 34 ). The HFD, most commonly referred to as the Western-style diet, is currently the leading cause of obesity and its associated co-morbidities( 35 – Reference Buettner, Scholmerich and Bollheimer 37 ). The main findings of the present study were that high fat feeding in female rats, which was associated with increased total weight and intra-abdominal fat, was accompanied by an increase in the gene expression levels of several nuclear receptors. HFD-induced changes in nuclear receptors were further associated with an increase in the expression levels of their target genes, including numerous hepatobiliary transporters. While the lack of a universal definition of diet-induced obesity in rodents might allow the HFD rats to be considered obese, in relation to human obesity and other diet-induced obesity models in rats, and the difference in body weight observed between the two groups in the present study (11·3 %), the HFD rats should be described as mildly obese or overweight.
The present study demonstrated that the HFD induced a significant increase in the expression levels of the nuclear receptor PXR and its target genes. A strong correlation was also observed between the expression levels of these genes. The mRNA and protein expression levels of Pgp and Cyp3a2, which are key target genes of PXR( Reference Moore, Kato and Xie 38 ), were significantly increased in the HFD group. While PXR activation has been reported in the ob/ob mouse model of obesity( Reference Martin and Minkenberg 13 ), the present study was the first to observe PXR activation and induction of target genes such as Pgp and Cyp3a2 in mildly obese rats. Sugioka et al. ( Reference Sugioka, Haraya and Fukushima 32 ) reported that diet-induced obesity imposed a decrease in the protein expression of Pgp and Cyp3a2 in male rats. However, they utilised a high-fat/high-cholesterol diet containing cholic acid, which is known to be associated with hepatic steatosis( Reference Angela, Gajda and Mathew 19 , Reference Cote, Ngo Sock and Levy 39 , Reference Bhathena, Kulamarva and Martoni 40 ). We did not find any evidence for hepatic steatosis in our HFD model. Thus, discrepancies between the present results and previous findings could result from the differences in the models used to induce obesity and the time points of the investigation. PXR and CAR play a key role in energy metabolism in the liver and could possibly act as a link between energy homeostasis and drug metabolism( Reference Gao and Xie 41 ). It has previously been suggested that accumulation of dietary fatty acids in the liver triggers the activation of PXR, leading to the induction of cytochrome P450 enzymes( Reference Finn, Henderson and Scott 42 , Reference Hernandez, Mota and Baldwin 43 ). It is possible that the increased levels of fatty acids observed in our HFD rats could have contributed to PXR activation and up-regulation of their target genes. High fat feeding was also associated with a substantial increase in the plasma concentrations of cholesterol, which probably contributes to PXR activation as recent studies have shown that PXR is activated by oxysterols, which are the oxidised derivatives of cholesterol( Reference Sonoda, Chong and Downes 44 , Reference Anger and Piquette-Miller 45 ). With regard to the clinical implications of these findings, Cyp3a2, which corresponds to CYP3A in humans( Reference Nelson, Kamataki and Waxman 46 ), is responsible for the metabolism of the majority of drugs currently on the market( Reference Takagi, Nakajima and Mohri 47 ) Pgp also plays a key role in the hepatobiliary clearance of many structurally diverse compounds including many anticancer, antiviral and anti-arrythmic drugs( Reference Klaassen and Aleksunes 48 ). Thus, it is plausible that PXR activation, resulting in the induction of CYP3A and Pgp, could increase the clearance of numerous drugs.
The present study demonstrated a significant elevation in the expression of the nuclear receptor FXR, the principal regulator of bile acid homeostasis. This correlated with the increased expression levels of its target genes Abcb4, Abcb11 and Slco10a1 in response to the HFD. Slco10a1 and Abcb11 are the principal uptake and efflux transporters of bile acids. In contrast, Abcb4 is responsible for the efflux of phospholipids into bile, making it more lipophilic and less damaging to biliary cells( Reference Klaassen and Aleksunes 48 , Reference Jyrki, Eloranta, Kullak-Ublick, You and Morris 49 ). The HFD was also associated with an increased expression level of Abcc2, which is regulated by FXR as well as by PXR and CAR( Reference Kast, Goodwin and Tarr 50 ). The efflux transporter Abcc2 is involved in the transport of drugs, bilirubin, glucouronide and glutathione drug conjugates in addition to bile constituents. The underlying cause of FXR induction and activation in our overweight rats is not clear. The principal activator of FXR is bile acids( Reference Jyrki, Eloranta, Kullak-Ublick, You and Morris 49 ). While several changes were observed in the expression of bile acid transporters, we did not observe any significant changes in the liver or plasma concentrations of bile acids in the HFD group. Nevertheless, it is possible that HFD-induced changes could have resulted in bile acid retention at an earlier time point as FXR activation leads to negative feedback inhibition to normalise bile acid levels by increasing the efflux of bile acids from hepatocytes and decreasing de novo synthesis of bile from cholesterol( Reference Calkin and Tontonoz 51 , Reference Lefebvre, Cariou and Lien 52 ). This could explain our findings of FXR-mediated induction of Abcb11, Abcc2 and Abcb4, which would lead to an increased efflux of bile and bile acids from hepatocytes. The up-regulation of Slco10a1 could increase hepatobiliary clearance and normalise serum bile acid level. Likewise, More & Slitt( Reference More and Slitt 33 ) reported an increase in the hepatic expression levels of Abcc2 as well as Slco1a4 in a diet-induced murine model of obesity. In addition, Martin et al. ( Reference Martin and Minkenberg 13 ) reported an increase in the mRNA levels of Abcc2, Abcb11 and FXR in an ob/ob mouse model.
The results from the present study show that total cholesterol levels were significantly elevated in the HFD group, as well as an increased expression level of the cholesterol transporters Abca1 and Abcg5. The nuclear receptor LXR, which is activated by cholesterol derivatives such as oxysterols( Reference Calkin and Tontonoz 51 ), is involved in the induction of the expression levels of Abca1, Abcg5, Abcg8 and Cyp7a1, leading to increased serum cholesterol levels. The expression of Cyp7a1, which was not significantly affected by the HFD in the present study, is regulated by both LXR and FXR( Reference Handschin and Meyer 53 , Reference Chow, Maeng and Liu 54 ). While LXR activation causes the induction of Cyp7a1, FXR activation suppresses its expression. Our findings demonstrating an increased expression level of both FXR and LXRα/LXRβ probably contribute to the overall effect of the HFD on the expression level of Cyp7a1.
In conclusion, the present study demonstrates that the HFD increases the hepatic expression levels of PXR, LXRα, LXRβ and FXR as well as their activation, as illustrated by the induction of their key target genes. The HFD was also associated with changes in plasma lipid profiles, as well as with the hepatic expression levels of several uptake and efflux transporters along with the metabolic enzyme Cyp3a2. This suggests that mild obesity may trigger changes in the hepatobiliary transport and clearance of both endogenous and exogenous compounds. The results from the present study and those from different models of obesity indicate that obesity is a complex condition with several co-morbidities that might affect hepatobiliary disposition pathways. If these findings in rodent models translate to humans with mild obesity, the distribution and clearance of many clinically important therapeutics could be affected in patients.
Acknowledgements
The present study was funded by an operating grant from the Natural Sciences and Engineering Research Council of Canada (7594) and from the Canadian Institutes of Health Research (M. P.-M.: MOP 57688; J.-M. L.: T 0602 145·02). R. H. G. was a recipient of King Abdulaziz University Scholarship for Postgraduate studies.
The authors' contributions are as follows: R. H. G., E. T. N. S., M. P.-M. and J.-M. L. contributed to the experimental design; R. H. G. and E. T. N. S. performed the experiment and data analysis; R. H. G., J.-M. L. and M. P.-M. wrote the paper.
There are no conflicts of interest to declare.












