Puberty is a complex biologically driven process that has an impact on emotional and behavioural well-being, resulting in a period with increased risk of developing emotional disorders and risk-taking behaviour. The brain undergoes cognitive maturation via synaptic remodelling well into the 20s. The limbic system, responsible for governing reward processing, appetite and pleasure seeking, matures before the prefrontal cortex, which is responsible for executive functioning such as problem-solving, planning, emotional regulation and multitasking. This difference in cortical maturity is hypothesised to create a developmental imbalance, making teens vulnerable to behavioural and mental health problems, such as depression(Reference Hawton, Saunders and O’Connor1).
An episode of major depressive disorder (MDD) during adolescence is a major personal and public health problem across the world(2). The disorder has many acute and long-term adverse consequences on adolescents’ education and occupational success, relationships and family life and on their future physical and mental health(Reference Clayborne, Varin and Colman3). Each year about 7·5 % of adolescents aged 13–18 years experience an episode of MDD(Reference Avenonoli, Swendsen and Jian-Ping4–Reference Jane Costello, Erkanli and Angold6). Symptoms of MDD are distressing and include sleep and cognitive problems, low mood, irritability, feelings of worthlessness and lack of pleasure(7). Sub-clinical MDD is even more common: recent surveys in the UK suggest that about 25 % of young people report elevated symptoms of depression in any given year(Reference Avenonoli, Swendsen and Jian-Ping4,8) , including depressive symptoms that are not sufficient in number or severe enough to meet diagnostic criteria. Sub-clinical symptoms have a major impact on daily functioning and are associated with increased risk of developing the disorder(Reference Avenonoli, Swendsen and Jian-Ping4).
Treatment for MDD in this age group includes psychological therapies and anti-depressant medication; however, these are only moderately effective and are often inaccessible to young people due to limited public health service resources(8). A recent meta-analysis of psychological treatments for children and young people with mental health problems found that the effect size of treatment for depression was small (d = 0·29) and was lower than effects of treatment for other common mental health problems(Reference Brent, Gibbons and Wilkinson9). Many young people with MDD do not receive an evidence-based treatment, and the prevention of adolescent depression is, therefore, a highly valued goal(10). One potential way to prevent the onset of MDD and sub-clinical depression is through diet. Diet and depression symptoms are significantly associated in adults, although this relationship is complex and potentially bidirectional, that is, unhealthy diet leading to low mood and viceversa(Reference Murakami and Sasaki11). A recent systematic review of the association between depression symptoms and diet in adolescents found that ‘healthy’ diets (i.e. consumption of fruits and vegetables) were associated with lower depression symptoms, whilst ‘unhealthy’ diets (i.e. consumption of junk foods and saturated fats) were associated with higher depression symptoms(Reference Khalid, Williams and Reynolds12). A large well-controlled epidemiological study examining associations between habitual intakes of dietary flavonoids and depression risk showed that individuals consuming diets higher in flavonoids presented a lower depression risk, particularly amongst older women(Reference Chang, Cassidy and Willett13). A similar study assessed symptoms of depression and the total habitual intake of polyphenols among the participants and found that higher dietary intake of flavonoids was inversely associated with depressive symptoms(Reference Godos, Castellano and Ray14). Thus, diets rich in fruits and vegetables are associated with low depression symptoms. Dietary flavonoids are present in substantial concentrations in commonly consumed fruits and vegetables and may be a potential mediator for the anti-depressant action of diets rich in fruits and vegetables.
The hypothesis that there is a causal relationship between diet and depression symptoms and the onset of MDD has recently been strengthened by number of intervention studies. Acute purple grape juice intervention resulted in increase in self-reported ratings of ‘calm’ in healthy young adults(Reference Haskell-Ramsay, Stuart and Okello15). Similarly, acute consumption of flavonoid-rich wild blueberry (WBB) improved short-term positive mood in children aged 7–10 years and in young adults aged 18–25 years(Reference Khalid, Barfoot and May16). In a recent randomised controlled trial with sixty-seven depressed adults(Reference Jacka, O’Neil and Opie17), participants randomised to an intervention promoting a healthy diet with at least nine portions of fruit and vegetables each day reported significantly less depression symptoms at 12 weeks than those randomised to receive social support. Anti-depressive effects of flavonoid-rich plants and their extracts have also been investigated. Hypercium perforatum (also known as Saint Johnʼs wort, derived from a flowering plant in the Hypericaceae family) extract intervention studies show its effectiveness as treatment for mild/moderate depression when compared with placebo and have similar effects to pharmacological treatments(Reference Brattström18–Reference Mannel, Kuhn and Schmidt21). Similarly, saffron (Crocus sativus, derived from the saffron spice of the flowering plant of Crocus genus) extract consumption had equivalent effect as pharmacological treatment for depression and was significantly more effective than the matched placebo(Reference Moshiri, Basti and Noorbala22–Reference Shahmansouri, Farokhnia and Abbasi24).
The specific effects of sustained WBB flavonoid consumption on symptoms of depression in adolescents have not yet been tested. Here, we designed a double-blind, placebo-controlled experiment to test the effect of consuming a flavonoid-rich WBB intervention for 4 weeks on symptoms of depression, anxiety and transient affect in healthy adolescents. Participants were randomly assigned to a WBB or a matched placebo drink with transient affect, and symptoms of depression and anxiety assessed before and after the 4-week intervention period.
Method
Ethics
This research was reviewed and given a favourable ethical opinion for conduct by the University of Reading Research Ethics Committee (UREC 16/55). The study was registered at clinicaltrials.gov NCT03119597.
Participants
An a priori power analysis (using G Power 3.1.9.2) based on data from a previous study(Reference Khalid, Barfoot and May16) revealed that twenty-four participants per group were required to achieve a power of 0·8 with α set at 0·5 level. Students aged 11–17 years of varying ethnicity, from four schools in Reading Berkshire, UK, were invited to take part in this study. All parents or legal guardians provided informed written consent for young people under the age of 16 years. Participants under the age of 16 years provided written assent and those over 16 years gave written consent. All participants were screened for any health conditions (including mental health), any treatment they were receiving and food-related allergies that would exclude them from the study. We screened eighty-two young people, of whom eighteen dropped out after the first screening session. Sixty-four participants were randomly assigned to either a WBB drink or a matched placebo drink. The randomised allocation of participants to treatment was generated using excel. The groups were coded A and B, and the sequence was saved in a password-protected spreadsheet. Both the researchers and the participant were blind to treatment group, and participants were told the study was investigating effects of different fruit drinks so were not aware of the study hypothesis.
Interventions
Both interventions (WBB and placebo) were measured and packaged into silver opaque sachets at the University of Reading. Sachets were identical for the WBB and the placebo drink, and neither the researchers nor the participants knew what their sachets contained. Wild Blueberry Association of North America provided the blueberry powder, whilst the matched sugars and vitamin C (placebo) was obtained from Bulk Powders. The packets of WBB contained 13 g of freeze-dried WBB powder (containing about 253 mg anthocyanins). Placebo packets were matched to the WBB for sugars (4·52 g glucose and 4·79 g fructose) and vitamin C (4 mg). Each participant was given 14-d supply of their requisite intervention, along with written and video instructions for their parents/guardians on how to prepare the intervention. Each intervention was prepared daily, by adding 30 ml of low-flavonoid ‘Rockʼs Organic Orange Squash’ and 170 ml of water and the contents of the sachet to the opaque cup provided. Each participant was given a checklist to record the dates and times when they consumed the drink each day and the name of the person who prepared the drinks. Participants were also asked to bring back their used sachets after 2 weeks as a measure of compliance. The remaining 14-d supply of each intervention was given to the participants 2 weeks into the intervention period. The true aim of the study was not disclosed to the participants; they were informed that it was a fruit drink study, to avoid revealing the contents of the drink.
Measures
The Mood and Feelings Questionnaire (MFQ) was used to measure symptoms of depression(Reference Angold, Costello and Messer25). The MFQ is considered to be the ‘gold standard’ self-report measure for depression in young people (NICE, 2015). It is a standardised and well-validated thirty-three-item self-report measure of the severity of depression symptoms in adolescents. Each item relates to a symptom or experience associated with depression. Participants are asked to rate each item in relation to their symptoms in the past 2 weeks on a three-point Likert scale (not true = 0, sometimes = 1, true = 2). Total MFQ scores range from 0 to 66 where higher scores indicate greater risk of depression. The clinical cut-off for the MFQ is 27, with scores above 27 indicating significant risk of a diagnosis of MDD(Reference Angold, Costello and Messer25).
Anxiety symptoms were assessed using the anxiety sub-scale of the Revised Child Anxiety and Depression Scale (RCADS)(Reference Chorpita, Yim and Moffitt26), a standardised and validated measure of anxiety symptoms in young people used routinely in UK NHS mental health services. The anxiety sub-scale of RCADS consists of thirty-seven items, each rated on a four-point Likert scale (never = 1, sometimes = 2, often = 3, always = 4). Total scores range from 37 to 148 with higher scores indicating increased risk of an anxiety disorder. Again, participants were asked to rate the items keeping the past 2 weeks in mind.
Current mood (i.e. transient affect) was assessed using the Positive and Negative Affect Schedule-NOW (PANAS-NOW) at screening, and at 2 and 4 weeks. As the term suggests, this is a measure of transient mood. The PANAS is a valid and reliable twenty self-report measure of positive affect (PA – ten items) and negative affect (NA – ten items) that can be used on multiple test occasions(Reference Watson, Clark and Tellegen27,Reference Crawford and Henry28) . Participants rated the degree to which they were currently experiencing each item on a five-point Likert scale ranging from ‘very slightly’ to ‘extremely’. Ratings of positive and negative items were summed to calculate an overall PA and overall NA score, each ranging from 10 to 50 where lower scores indicate lower levels of PA or NA.
Habitual fruit and vegetable consumption was assessed using EPIC-Norfolk FFQ, a semi-quantitative paper-based questionnaire, which includes 130 food items, each rated on nine-point Likert scale (never or less than a month (1) to 6+ per d (9)). FETA software was used to analyse the data collected to calculate forty-six nutrient and fourteen food group values including average daily fruit and vegetable intake(Reference Angela, Robert and Amit29).
Other measures, that is, working memory, verbal fluency, cognitive accuracy and reaction time were assessed and are reported elsewhere(Reference Khalid30).
Procedure
As outlined in Fig. 1, participants were seen by the researchers four times across a 5-week period. All participants did not attend all assessment – the number of participants assessed at each time point is indicated in Fig. 1. Research sessions took place either at the University of Reading or at the participantʼs school. Sessions were scheduled at the same time of day for each participant. The first two sessions, scheduled 48 h apart, were screening sessions where participants completed a battery of questionnaires: MFQ, RCADS, (screening session 1) PANAS, EPIC-Norfolk FFQ and a questionnaire about their health status (screening session 2). Screening sessions were limited to 30 min to fit with the school timetable and to maintain high levels of participant engagement in both sessions. Parents were also asked to complete a demographic questionnaire. Participants started the intervention the day after the second screening session was completed. After 2 weeks, they returned their used drink sachets, were given a new checklist and completed the PANAS (test session 1). Participants were also asked if they were experiencing any adverse effects of the drink and feedback on its palatability. They then returned 2 weeks later (test session 2), returned their drink sachets, completed the PANAS, MFQ and RCADS and were debriefed. For each test session, participants were instructed not to consume their allocated intervention before the test session to ensure that chronic, not acute, effects of the intervention were being measured.

Fig. 1. Schematic of the study procedure. MFQ, Mood and Feelings Questionnaire; RCADS, Revised Child Anxiety and Depression Scale; PANAS, Positive and Negative Affect Schedule.
Statistical analysis
Statistical analyses were conducted using IMB SPSS version 22. We used t tests to investigate differences in symptoms of depression, anxiety and fruit and vegetable intake between the two groups at baseline. Effects of intervention on transient affect were analysed using linear mixed modelling using an unstructured covariance matrix to model successive repeat test sessions, with subjects included as random effects. Data from 2 to 4 weeks measures of the PANAS and treatment group were included as fixed factors, with baseline PANAS scores included as a covariate. Linear mixed modelling deals with data that is missing at random and with multiple measurement points, giving unbiased estimates of each of the means. To test the effects of the intervention on anxiety and depressive symptoms at 4 weeks, data were analysed using ANCOVA with drink (placebo, WBB) as an independent variable and MFQ and RCADS scores at 4 weeks as a dependent variable. Baseline measures of depression and anxiety were used as covariates, and Bonferroni-corrected t tests were used to investigate all fixed effects and interactions.
Results
Sample characteristics
Sixty-four participants were randomised (thirty-five females, twenty-nine males) aged 12–17 years (mean 14·20 (sd 1·71) years). Thirty-five participants were randomly allocated to receive the placebo drink and twenty-nine to the WBB intervention. Participants’ demographic data, baseline mood scores and habitual fruit and vegetable intakes are reported in Table 1. There were no significant differences between groups in the amount of daily fruit t(51) = 0·14, P = 0·89 or vegetables t(51) = 1·45, P = 0·15 consumed. One-sample t test revealed that the mean fruit and vegetable consumption by the participants was significantly lower than 400 g/d as recommended by WHO (fruit: t(52) = 11·20, P < 0·005; vegetables: t(52) = 7·12, P < 0·005).
Table 1. Demographic details, fruit and vegetable intake and depression and anxiety scores at baseline for both intervention groups
(Mean values and standard deviations; percentages)

MFQ, Mood and Feelings Questionnaire; RCADS, Revised Child Anxiety and Depression Scale.
At baseline, mean depression and anxiety scores were 12·35 (sd 9·31) and 23·19 (sd 13·80), respectively, both below the clinical threshold. There was no significant group difference in symptoms at baseline; MFQ t(60) = 0·60, P = 0·55, RCADS t(40) = 0·45, P = 0·66 and no group difference in mean PA and NA; t(62) = 1·40, P = 0·17 and t(62) = 0·80, P = 0·98, respectively. A minority of participants (9·38 %) reported depression symptoms above the clinical cut-off of 27 on the MFQ (11·4 % in the placebo group, 3·4 % in the intervention group). No participants reported anxiety symptoms above the clinical threshold. No participants reported a diagnosis of depression or anxiety or that they were receiving treatment for these disorders.
Hypothesis testing
At 4 weeks, fifty-nine participants provided self-report data on anxiety (RCADS) and depression (MFQ) symptoms: twenty-six from the intervention group and thirty-three from the placebo group. As shown in Fig. 2(a), after 4 weeks of the intervention, the mean MFQ score for participants who consumed WBB was significantly lower than the mean MFQ score for participants who consumed the placebo drink. This was significant F (1,57) = 5·52, P = 0·02, 95 % CI –6·71, –5·35 with a medium effect size (d = 0·65). The change in the depression scores for each participant including regression line for both treatments is shown in Fig. 3. There was no significant effect of WBB on symptoms of anxiety (Fig. 2(b)) after 4 weeks of supplementation F (1,34) = 2·1, P = 0·16; mean RCADS score for participants in the WBB group was 13·90 (sd 8·39), and the mean RCADS for the placebo group was 19·3 (sd 11·31).
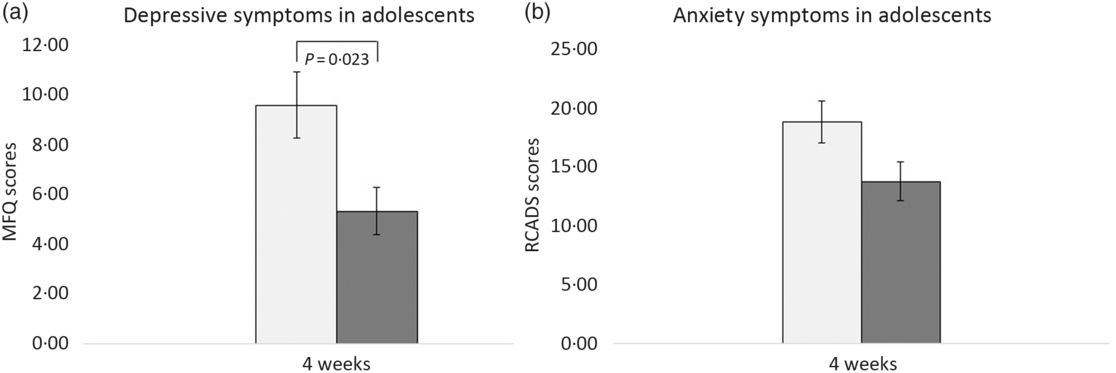
Fig. 2. Mean scores in adolescents aged 11–17 years. (a) Mean Mood and Feelings Questionnaire (MFQ) scores after 4 weeks’ consumption of placebo and intervention drinks. (b) Mean Revised Child Anxiety and Depression Scale (RCADS) scores after 4 weeks’ consumption of placebo and intervention drinks.  , Placebo;
, Placebo;  , wild blueberry.
, wild blueberry.

Fig. 3. Scatterplot showing the Mood and Feelings Questionnaire (MFQ) scores at baseline and 4-week post-intervention.  , Placebo;
, Placebo;  , wild blueberry (WBB);
, wild blueberry (WBB);  , linear (placebo);
, linear (placebo);  , linear (WBB).
, linear (WBB).
We also examined the effect of intervention on PANAS after 2 and 4 weeks (see Fig. 4). There was no significant effect of Drink, F (1,64·33) = 0·26, P = 0·62, Repeated trial, F (1,62·22) = 2·95, P = 0·09, or any Drink × Repeated trial interaction F (1,62·22) = 3·686, P = 0·06 on transient PA. Fig. 4(a) shows the mean PA scores following intervention of WBB and placebo at 2 and 4 weeks. There was also no significant effect of the intervention on NA; Repeated trial, F (1,59·3) = 0·66, P = 0·42, Drink, F (1,63·79) = 0·24, P = 0·63 or Repeated trial × Drink interaction, F (1,59·30) = 1·17, P = 0·28. As shown in Fig. 4(b), NA was not significantly different after consuming the WBB drink or the placebo drink.

Fig. 4. Mean Positive and Negative Affect Schedule-NOW (PANAS-NOW) mood scores in adolescents aged 11–17 years. (a) Mean positive affect scores 2 and 4 weeks’ post-consumption of placebo and intervention drinks. (b) Mean negative affect scores 2 and 4 weeks post-consumption of placebo and intervention drinks.  , Placebo;
, Placebo;  , wild blueberry.
, wild blueberry.
Discussion
This randomised, placebo-controlled, double-blinded trial investigated the effects of 4 weeks consumption of a flavonoid-rich WBB drink on symptoms of depression and anxiety and on transient affect in a community sample of healthy 12–17-year old. The results demonstrated that after 4 weeks of daily WBB intervention, there was a between-group difference in self-reported depressive symptoms; participants randomised to the WBB intervention reported significantly lower scores on the measure of depression symptoms than participants who were randomised to the placebo drink. There was no significant effect of the intervention on anxiety symptoms or on PA or NA (i.e. transient affect). The data suggest that flavonoid supplementation may be beneficial in reducing depressive symptoms in healthy adolescents.
This is, to our knowledge, the first randomised double-blinded study to show the effects of chronic WBB flavonoids on depression symptoms in teenagers. The participants in the study were healthy but at baseline assessment were consuming sub-optimal habitual levels of flavonoids, that is, their daily consumption of fruit (44·87 %) and vegetables (57·46 %) was well below the WHO recommended amount of 400 g/d(31,Reference Vereecken, Pedersen and Ojala32) . This is consistent with the typical diet of young people in the UK, where only 18 % of adolescents meet the recommended daily requirement, and the average daily consumption within this age group is 256 g (3·5 portions) of fruit and vegetables(33). Levels of depression and anxiety were similar to community norms on ‘gold standard’ self-report measures. Importantly, because the effects of the intervention were observed in a community sample, these effects cannot necessarily be generalised to adolescents with more severe symptoms of depression or a diagnosis of depression.
Within this community sample, the effect size of the flavonoid intervention compared with the control group on the measure of depression symptoms, the MFQ, was d = 0·65, a medium effect size. To put this into context, two recent meta-analyses have examined the effects of psychological treatments for depression and the prevention of depression. Eckshtain etal.(Reference Eckshtain, Kuppens and Ugueto34) concluded that the treatment effect size of psychological treatments for adolescents with depression was d = 0·36. In a review of interventions to prevent depression Ssegonja etal.(Reference Ssegonja, Nystrand and Feldman35) reported an effect size of d = 0·22. In relation to the specific measure of depression used in this study, the reduction of the 4 points on mean MFQ scores in the intervention group indicates complete amelioration of two items on the scale or a reduction (from 2 to 1, or 1 to 0) of four items. Because each item reflects a symptom or adverse effect of depression, clinically this would be likely to reflect a meaningful reduction in the impact of depression on the young person(Reference McCarty, Violette and Duong36).
Previously, the effects of flavonoids from different sources such as apples, cocoa and grape juice showed no effects on depression in healthy adults(Reference Khan, Perviz and Sureda37–Reference Scholey, Haskell and French40). However, our results are consistent with previous animal and epidemiological studies that suggest anti-depressive effects of a flavonoid-rich diet(Reference Chang, Cassidy and Willett13,Reference Brattström18,Reference Mihrshahi, Dobson and Mishra41–Reference Bouayed43) . They also are in keeping with experimental data on the acute effects of WBB on positive mood in children and young adults(Reference Haskell-Ramsay, Stuart and Okello15,Reference Khalid, Barfoot and May16) , and the acute effect of grape juice on mood in healthy adults(Reference Haskell, Stuart and Okello44). Unlike a previous acute intervention study, we did not observe a significant effect of WBB on momentary mood (i.e. transitory affect). However, the interval between consuming the WBB drink and assessing NA and PA was variable, unlike the standard 2-h interval used in previous studies. In addition, the 4-week assessment (our end point) was conducted during the first week of school after the summer holidays. Unlike symptoms of depression (and anxiety) which were measured over a minimum 2-week period and which are conceptualised as relatively stable, PA and NA are conceived as short-lived events that have rapid decay after elicitation(Reference Qiao-Tasserit, Garcia Quesada and Antico45). It is therefore possible that this external event (returning to school) had a measurable impact on participants’ momentary affect.
Although anxiety and depression are frequently co-morbid in young people and share some symptoms (e.g. fatigue, low concentration and sleep disturbances), the results of this intervention study suggest that flavonoids may reduce symptoms that are more prominent in depression than anxiety, for example, low mood, anhedonia, feelings of guilt and worthlessness and do not reduce symptoms that are specific to anxiety. However, it is also possible that the effect of flavonoids on anxiety is smaller than the effect on depression and that a larger sample, with greater power, might result in a significant effect.
Some authors have proposed that flavonoids increase cerebral blood flow to the dorsolateral prefrontal cortex, a site that is highly associated with cognitive and emotional regulation, including rumination, a cognitive process of repetitive thinking that may exacerbate feelings of guilt and worthlessness(Reference Vauzour, Vafeiadou and Rodriguez-Mateos46–Reference Schore48). This suggests that there may be an indirect pathway between flavonoid consumption and depression whereby flavonoid consumption enhances cerebral blood flow, which boosts executive functioning; in turn improved executive functioning helps to enhance cognitive control, inhibits rumination and thus reduces depression. Adolescents with depression have impaired executive function compared with non-depressed and anxious young people(Reference Fisk, Ellis and Reynolds49), and therefore, the benefits of flavonoid consumption may be more prominent in these young people. However, potentially any positive effects of flavonoid consumption on executive function would have benefits for more young people because executive function is critical for academic achievement(Reference St Clair-Thompson and Gathercole50).
A plausible direct pathway between flavonoid consumption and mood is the effects of flavonoids on monoamine oxidase(Reference Watson, Haskell-Ramsay and Kennedy51). Monoamine oxidase inhibitors have been used to treat mood disorders, and flavonoids may mimic their effects(Reference Watson, Haskell-Ramsay and Kennedy51,Reference Carradori, Gidaro and Petzer52) . A recent study showed that consuming fruits high in flavonoids, that is, blackcurrants significantly reduces monoamine oxidase activity and increases the circulating monoamines and thereby elevates mood(Reference Watson, Haskell-Ramsay and Kennedy51). Another possible mechanism by which flavonoids may affect mood is by mimicking anxiolytic-like effects by binding to benzodiazepine receptors, enhancing the effect of GABA via GABAA receptors(Reference Eckshtain, Kuppens and Ugueto34,Reference Hanrahan, Chebib and Johnston53,Reference Wasowski and Marder54) . However, in line with a previous study(Reference Khalid, Barfoot and May16) that showed no changes in NA (an indicator of anxiety) after acute flavonoid intervention, here there was no significance of flavonoid consumption on anxiety.
Although the mechanisms of action require further investigation, there is accumulating evidence of a causal relationship between flavonoid consumption and depression symptoms. This evidence has been published by independent research groups using different research designs, including epidemiology, clinical trials and experiments. However, the research is preliminary and requires robust replication and extension, with larger samples, longer time scales and careful tests of mechanisms of action. Our study examined the effects of flavonoids on healthy young people, some of whom had elevated symptoms of depression. We did not have adequate power to conduct sub-group analysis, but clearly it is important to identify if the change in depression symptoms is driven by improvements in those with relatively elevated symptoms, or if the effects are similar across all levels of baseline depression. This distinction is important because flavonoids may have the potential to prevent depression in those at risk (i.e. those with elevated symptoms) or may have a more general effect. The former would suggest that dietary interventions could be used for early intervention in those exhibiting symptoms of depression; the latter that dietary interventions could have a broader benefit to public mental health.
Conclusion
This randomised double-blind study demonstrated the chronic effects of WBB flavonoid consumption on reducing symptoms of depression in a community sample of adolescents. Dietary flavonoid interventions may have potential to reduce symptoms of depression in adolescents. This study requires replication, not only in healthy participants but also in clinically referred samples to assess the potential of flavonoids to be used as a practical and cost-effective intervention. In addition to this, studies focused on investigating biochemical changes and investigating the mechanistic pathways in which flavonoids decrease depressive symptoms in humans are essential.
Acknowledgements
We are grateful to the Wild Blueberry Association of North America who provided the freeze-dried wild blueberry powder used for this study. We would also like to acknowledge the contribution of the staff and participants of the EPIC-Norfolk Study.
EPIC-Norfolk has been supported by the Medical Research Council programme grants (G9502233, G0401527 and G1000143) and Cancer Research UK programme grants (SP2024/0201, SP2024/0204, C865/A2883, C864/A8257 and C864/A14136).
All the authors were involved in the design of the experiments; S. K. and J. F. performed the experiments and analysed the data. S. K., J. F., C. M. W. and S. A. R. were involved in the writing and revisions of the manuscript.
The authors declare that there are no conflicts of interest.








