There is overwhelming evidence to show that folic acid supplements during pregnancy are efficacious in reducing the risk of neural tube defects (NTD) in the fetus. However, numerous studies of folate levels have failed to establish a connection between folic acid metabolism in the fetus and the risk of NTDReference Butterworth and Bendich1, Reference Stover2. Moreover, the frequency of NTD is not increased following the inactivation of enzymes essential for folate metabolism in the fetusReference Copp, Greene and Murdoch3. An adequate intake of folic acid during the weeks just before and after conception also improves the overall outcomeReference Tamura and Picciano4, notably, increasing infant birth weightReference Relton, Pearce and Parker5 and reducing the incidence of spontaneous abortion and pre-term deliveryReference Scholl and Johnson6. However, as with NTD, there is no clear evidence to link impaired fetal growth directly to folate deficiency in fetal tissues. These observations suggest that some of the broad benefits of improved folate status could be the indirect consequences of improvements in maternal metabolism.
Derivatives of folic acid play an important role in one-carbon transfer reactions, supplying methyl groups to complete the methionine cycle. Under some circumstances choline and methionine also donate methyl groups and their availability in the diet modifies the requirement for folic acid in one-carbon transfers. Before conception, the main consumer of methyl groups supplied by the methionine cycle is believed to be the hepatic enzyme phosphatidyl ethanolamine methyl transferase (PEMT)Reference Stead, Brosnan, Brosnan, Vance and Jacobs7. Therefore, folate deficiency during pregnancy may alter hepatic function and disturb maternal metabolic homeostasis, indirectly affecting fetal development. In the present study, a combination of two approaches has been used to examine the liver of pregnant rats fed one of two experimental diets; either a diet deficient in folic acid (–F), or one deficient in all three key methyl donors, folic acid, choline and methionine (–FLMLC). First, we analysed the soluble protein fraction from the maternal liver on day 21 of gestation (term is 22·5 d in the rat) by two-dimensional electrophoresis. Since the cytosol is in communication with many of the intracellular compartments, this fraction gives an overall picture of the changes. Second, we carried out a targeted analysis of hepatic mRNA coding for the enzymes that control the synthesis of fatty acids (acetyl CoA carboxylase-1), fatty acid oxidation (carnitine palmitoyl transferase-1) and the transcriptional regulators that control them (PPAR, CCAAT enhancer binding protein and sterol response element-binding protein-1c (SREBP-1c)). We have also investigated the expression of gadd153 and p57 to evaluate the extent of metabolic stress.
Methods
Experimental diets
The experimental diets were based on the AIN-76 formula8 and contained 90 g casein/kg supplemented with a mixture of synthetic amino acids equivalent to those found in a further 90 g/kg casein as described previouslyReference Maloney, Hay and Rees9. Folic acid was omitted from the –F and –FLMLC diets. No additional methionine was added to the –FLMLC diet (total methionine concentration 2·3 mg methionine per kg diet compared with 5·6 mg methionine per kg diet in the control) and choline chloride was reduced to 0·1 % w/w (compared with 0·2 % w/w in the control).
Animals
All experimental procedures were approved by the ethical review committee of the Rowett Research Institute and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986. Female rats of the Rowett Hooded strain bred in the institute were allocated to three groups of eight animals. The animals were approximately 8 to 10 weeks of age at the start of the experiment with a mean body weight of 208 g. There were no significant differences between the weights of animals in the different treatment groups when the experiment commenced. Animals were housed on sawdust bedding and no steps were taken to prevent coprophagy. Three groups of animals were fed control, –F or –FLMLC diets. The animals were offered the experimental diets for a 2-week adaptation period and then were mated with males of the same strain. The day on which a vaginal plug was detected was denoted day 0. The female rats were maintained on their corresponding diets until day 21 of gestation, when they were anaesthetised by halothane inhalation and killed by exsanguination. The fetuses were rapidly removed, weighed and killed by decapitation. Samples of the maternal liver were frozen in liquid N as soon as possible and subsequently stored at − 80°C until required. Plasma was prepared from samples of trunk blood and alanine aminotransferase and aspartate aminotransferase were analysed using a Konelab selective chemistry analyser (Labmedics Ltd, Salford, Manchester, UK).
Proteomics
Pieces of frozen maternal liver from six pregnant animals chosen randomly from each group were homogenised in buffer containing protease inhibitors as described previouslyReference de Roos, Duivenvoorden, Rucklidge, Reid, Ross, Lamers, Voshol, Havekes and Teusink10. The homogenate was centrifuged for 30 min at 100 000 g at 4°C (Beckman TL-100 centrifuge; Beckman Coulter Ltd, High Wycombe, Bucks, UK). Proteins in the supernatant (300 μg from each extract) were separated in the first dimension on BioRad immobilized pH gradient strips (pI 3–10; BioRad, Hemel Hempstead, UK)Reference Drew, Padidar, Horgan, Duthie, Russell, Reid, Duncan and Rucklidge11. Following equilibration with the second dimension buffer, which contained 135 mm-iodoacetamide, proteins were separated on an 18 × 18 cm SDS polyacrylamide gel at 200 V for 9·5 hReference Drew, Padidar, Horgan, Duthie, Russell, Reid, Duncan and Rucklidge11. Molecular weights were determined with a Bio-Rad Precision Plus Protein Mr standard. Gels were stained with colloidal coomassie brilliant blue, dried and scanned as previously describedReference Drew, Padidar, Horgan, Duthie, Russell, Reid, Duncan and Rucklidge11. The gel images were analysed (PDQuest v7 BioRad) and spots of interest were excised from the gels using a robotic spot cutter (BioRad). Protein identities were determined by MALDI-TOF (Voyager-DE PRO; Applied Biosystems, Warrington, Cheshire, UK) or by liquid chromatography/MS/MS (Q-trap; Applied Biosystems) MS of trypsin-digested protein spots as previously describedReference Drew, Padidar, Horgan, Duthie, Russell, Reid, Duncan and Rucklidge11. The mass spectra of spots that were significantly different were corrected for isotope abundance and the resulting peptide mass list profiles were analysed using the Matrix Science ‘Mascot’ web tool (http://www.matrixscience.com). The Mascot database search criteria allowed one missed cleavage, carbamidomethyl modification of cysteine; partial oxidation of methionine, a charged state of MH+ and positive identities of at least 20 % matched peptides covering at least 10 % of the protein sequence. Positive matches with proteins in the database were assigned at a probability value of P < 0·05.
TAG analysis
Approximately 0·2 g tissue was homogenised in 1 ml ice cold 0·145 m-NaCl. The homogenate was extracted with 10 ml chloroform:methanol (2:1). The chloroform phase was transferred to a clean tube and the residue was extracted with a further 3 ml chloroform. The extracts were combined, the chloroform was removed by evaporation and the lipids were dissolved in 5 ml absolute ethanol. TAG in 10 μl samples of the ethanol suspension were measured by adding 0·25 ml reagent (ThemoElectron Triglyceride Kit; Lab Medics Ltd), incubating for 20 min at room temperature and measuring the absorbance at 510 nm. TAG concentrations in the samples were determined from a standard curve.
Real Time PCR
Total RNA was extracted using the Trizol reagent (Sigma, Poole, Dorset, UK) as described previouslyReference Maloney, Lilley, Cruickshank, McKinnon, Hay and Rees12. Samples of 50 ng total RNA were reverse transcribed using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems) primed with random hexamers. The levels of cDNA relative to the 18S ribosomal RNA were measured using the SYBR Green real time PCR kit (Applied Biosystems). The primer sequences used are described in Table 1. The identity of the products was confirmed by sequencing and the response was linear over the range measured. Relative target quantity was calculated from the standard curve and the results expressed as the ratio of the product relative to the product from the 18S rRNA.
Table 1 Primers used for PCR analysis*

* For details of procedures, see Methods.
Acc, acetyl CoA carboxylase; L-CPT-1, carnitine palmitoyl transferase; SREBP-1c, sterol response element-binding protein 1c; C/EBP-α, CCAAT enhancer binding protein α.
Statistical analysis
Data are presented as means with their standard errors of the mean. Scans of the proteomic gels (six animals per treatment) were normalised using PDQuest software to determine the mean corrected spot density. The PDQuest statistical analysis (Student's t test) was performed by pairwise comparisons of each spot on the control gels with either the –F or the –FLMLC gels. The spot densities of proteins identified in this preliminary screen were subsequently analysed by one-way ANOVA followed by Fischer's multiple comparison test (Genstat 7 statistical package; Lawes Agricultural Trust, Rothamsted Experimental Station, Harpenden, Herts., UK). The gene expression data were analysed by one-way ANOVA followed by Fischer's multiple comparison test (Genstat).
Results
Maternal growth
The growth of the animals has been described previouslyReference Maloney, Hay and Rees9. Briefly, the weight gain of animals fed the –F diet during gestation was not different from the control group, whereas the animals fed the –FLMLC diet gained approximately 15 % less than animals in the control group. Food intake during the last week of gestation did not differ significantly between the groups. The number of fetuses was not affected by the diets. Fetuses of dams fed the –F diet were approximately 18 % heavier (4·84 (sem 0·07) g) than the controls (4·10 (sem 0·03) g), while those from dams fed the –FLMLC diet were approximately 10 % smaller than the controls (3·71 (sem 0·07) g). There were no gross abnormalities in either restricted group. The livers of dams in the –F group (10·1 (sem 0·6) g) and –FLMLC group (9·1 (sem 0·4) g) were significantly smaller (P = 0·002) than those of animals in the control group (11·8 (sem 1·2) g). The metabolic changes observed in these animals have been described previouslyReference Maloney, Hay and Rees9. Hepatic folate was reduced by approximately 80 % in animals fed –F or –FLMLC diets. Hepatic phosphocholine stores were reduced by approximately 30 % (–F) and 60 % (–FLMLC) when compared with the control. Plasma homocysteine, glycine, serine and threonine concentrations in the maternal plasma of animals fed the –F diet were higher than the control and this was further exacerbated by the –FLMLC dietReference Maloney, Hay and Rees9.
Proteomic analysis of soluble proteins
Two-dimensional gel electrophoresis separated approximately 900 proteins in the soluble fraction of the female rat liver at day 21 of gestation. Comparisons of the relative abundance of proteins from the liver of animals fed the control diet with those fed the –F diet showed that eighteen proteins were up regulated and sixteen were down regulated (Student's t test P < 0·05). Comparisons of the patterns from animals fed the –FLMLC diet with the control showed that twenty-six proteins were up regulated and sixteen proteins were down regulated (P < 0·05). A further thirteen proteins were identified as changed (P < 0·05) in a comparison of the soluble hepatic proteins in livers from animals fed –F and –F M LC diets.
All of the proteins identified in the initial analysis were sequenced by MS and their identities are shown in Table 2. The proteins were allocated to different groups depending on their functions. Proteins associated with metabolic functions are shown in Table 3. They include proteins associated with folate/methionine metabolism, protein and amino acid metabolism, tyrosine metabolism, energy metabolism and lipid metabolism. There were also a number of proteins associated with cellular functions and these are shown in Table 4. These proteins could be allocated into groups associated with the endoplasmic reticulum, bile production and oxidative stress. There were also a small number of proteins that could not be allocated to specific functions.
Table 2 Sizes and identities of differentially abundant proteins†

* Indicates identification from Matrix-assisted laser desorption/ionisation–time of flight (MALDI–TOF) spectra and confirmed using liquid chromatography/MS/MS (LCMSMS).
† MALDI and LCMSMS probabilities and the corresponding Entrez code for differentially expressed proteins. The predicted mass/pI is the mass/pI is taken from the Mascot database entry. The mass/pI of the spot is that estimated from the two-dimensional gel.
Table 3 Differentially abundant proteins involved in metabolic processes* (Data are mean pixel density with their standard errors of the mean for six rats per group)

Data analysed by one-way ANOVA (Fpr) followed by Fischer's unprotected test. Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
–F, diet deficient in folic acid; –FLMLC, diet deficient in all three key methyl donors, folic acid, choline and methionine.
* For details of diets and procedures, see Methods.
Table 4 Differentially abundant structural proteins classified by function (Data are mean pixel density with their standard errors of the mean for six rats per group)

Data analysed by one-way ANOVA (Fpr) followed by Fischer's unprotected test. Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
–F, diet deficient in folic acid; –FLMLC, diet deficient in all three key methyl donors, folic acid, choline and methionine; ER, endoplasmic reticulum.
* For details of diets and procedures, see Methods.
TAG in the maternal liver
There were no differences in maternal serum TAG concentrations (Fig. 1). The TAG content of the maternal liver of animals fed –F diets was not significantly different from the controls. In contrast, the hepatic TAG content of dams fed the –FLMLC diet was increased by three-fold compared with the controls. The serum alanine aminotransferase and aspartate aminotransferase activities were not different from the controls in either –F or –FLMLC diet groups (data not shown).

Fig. 1 TAG concentrations in maternal serum (a) and maternal liver (b). Values are means with their standard errors of the mean. Control n 8; diet deficient in folic acid (–F) n 6; diet deficient in all three key methyl donors, folic acid, choline and methionine (–FLMLC) n 7. Data analysed by one-way ANOVA followed by Fischer's unprotected test. Columns with unlike superscript letters are significantly different (P < 0·05). For details of diets and procedures, see Methods.
Gene expression in the maternal liver
The relative expression of a number of mRNA associated with lipid metabolism in the maternal liver is shown in Table 5. The hepatic acetyl CoA carboxylase-1 mRNA levels was decreased by approximately 25 % in the livers of animals fed the –F and –FLMLC diets when compared with the controls. At the same time, there was an increase of approximately 50 % in the relative expression of carnitine palmitoyl transferase-1 in animals fed the –FLMLC diet. The mRNA for PPARα and PPARγ was increased by approximately 25 % in the livers of animals fed the –FLMLC diet. The mRNA for SREBP-1c was reduced by approximately 50 % in the animals fed –F and –FLMLC diets. There was no change in the relative expression of CCAAT enhancer binding protein-α, gadd153 or p57.
Table 5 Relative mRNA levels in maternal liver*
(Values are means with their standard errors of the mean for six rats per group)
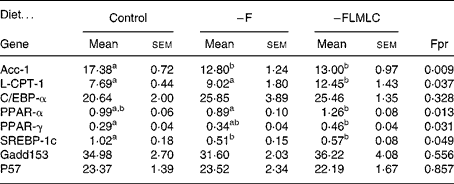
All data are expressed as relative expression corrected for 18S rRNA. Data analysed by one-way ANOVA (Fpr) followed by Fischer's unprotected test. Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
–F, diet deficient in folic acid; –FLMLC, diet deficient in all three key methyl donors, folic acid, choline and methionine; Acc, acetyl CoA carboxylase; L-CPT-1, carnitine palmitoyl transferase; C/EBP-α, CCAAT enhancer binding protein α; SREBP-1c, sterol response element-binding protein 1c.
* For details of diets and procedures, see Methods.
Discussion
Although numerous hypotheses have been put forward to explain the importance of folic acid in reproduction, the mechanism that underlies its action remains unclear. The present study shows that there are widespread changes in both the metabolism and function of the maternal liver when the diet of pregnant rats is deficient in folic acid and the related methyl donors, methionine and choline. These findings suggest a putative mechanism related to changes in the maternal liver and lipid metabolism in particular.
It is apparent from the differentially abundant proteins involved in metabolic processes (Table 3) that proteins associated with folate metabolism and the methionine cycle change in response to the deficient diets. Two of the major folate binding proteins, 10-formyltetrahydrofolate dehydrogenase and dimethyl glycine dehydrogenase are lower in both the –F and –FLMLC diet groups (Table 3), a result that is consistent with the reduced hepatic folate stores in these animalsReference Maloney, Hay and Rees9. There are also differences in the abundance of proteins associated with the methionine cycle in both groups. There is an increase in methionine adenosyl transferase and a tendency for an increase in adenosyl homocysteinease in the animals fed the –F diet, suggesting that methionine and choline compensate for the reduced availability of folates when they are available. Glycine and serine are important donors of methyl groups, which enter the methionine cycle via derivatives of folic acid. Plasma concentrations of both amino acids increase especially in the animals fed –FLMLC dietsReference Maloney, Hay and Rees9 and the increase in the abundance of the glycyl- and seryl-t-RNA synthetases is probably a response to these changes.
Although there is no direct involvement of folic acid or its derivatives in tyrosine metabolism, a number of differentially abundant proteins are involved in the metabolism of this amino acid. Plasma tyrosine concentrations increase relative to the complete diet by 45 % in animals fed the –F diet and decrease by 12 % in animals fed the –FLMLC dietsReference Maloney, Hay and Rees9, suggesting that these changes are having differential effects on tyrosine metabolism. Tyrosine is produced from phenylalanine via the enzyme phenylalanine hydroxylase, which requires tetrahydrobiopterin as an essential cofactor in the reaction. There are several reports of a link between folate and biopterin metabolism, possibly related to the structural and functional similarities of the folate and biopterin coenzymesReference Lucock, Yates, Hall, Leeming, Rylance, MacDonald and Green13. The present results add further circumstantial evidence to suggest that folate deficiency has an effect on tetrahydrobiopterin metabolism. As tetrahydrobiopterin is thought to be required for successful neural tube closureReference Nachmany, Gold, Tsur, Arad and Weil14, this may be a possible mechanism for an indirect effect of folate deficiency on fetal development.
Beyond the changes directly associated with the metabolism of folates and the methionine cycle, the relative abundance of proteins associated with hepatic protein, glucose and lipid metabolism is also influenced by diets deficient in folic acid and the related methyl donors (Table 3). The changes in protein metabolism appear to be rather non-specific and probably reflect differences in growth as the livers of animals fed the –FLMLC diet are some 22 % smaller than those of animals fed the complete diet. This suggests that there is a change in protein turnover that may be related to a limitation in sulphur amino acids in the animals fed the –FLMLC diet. In contrast, the changes in carbohydrate and lipid metabolism suggest more specific alterations, albeit to a diverse range of processes including gluconeogenesis, glycogen synthesis, cholesterol metabolism, the malate-aspartate shuttle and mitochondrial β-oxidation. In general, the changes are greater and more widespread in the animals fed the –FLMLC diet, suggesting that the multiple deficiency is exacerbating the impact on energy and lipid metabolism. However, it is difficult to make specific predictions on the metabolic effects of these changes, since, in addition to transcriptional control, the activities of several of these enzymes are tightly regulated by a combination of allosteric effectors and phosphorylation.
The increase in hepatic TAG that occurs in the animals fed the –FLMLC diets is clearly associated with the changes in energy metabolism. Excessive synthesis and reduced oxidation of fat appear to cause steatosis in both alcoholic liver injury and non-alcoholic fatty liver diseaseReference Reddy and Sambasiva Rao15. However, there is no evidence for this in the case of folate deficiency. The hepatic mRNA for the key enzyme regulating fatty acid synthesis (acetyl CoA carboxylase-1) is reduced in animals from both the –F and –FLMLC groups. At the same time, the mRNA for carnitine palmitoyl transferase-1 (Table 5) the key regulator of lipid oxidation is increased in the livers of animals fed –FLMLC diets but not in those fed the –F diet. As the mRNA levels reflect the enzyme activitiesReference Kim and Freake16, these findings suggest a reduction in the synthesis and an increase in the oxidation of fatty acids, opposing the accumulation of TAG in the –FLMLC group. It appears that the responses to the –F and –FLMLC diets differ because the mRNA for PPAR-α and PPAR-γ, which regulate fatty acid oxidation, are only increased in the animals fed the –FLMLC diets, whereas SREBP-1c, an important regulator of hepatic lipogenesis and cholesterol synthesisReference Brown and Goldstein17, is decreased by both –F and –FLMLC diets. The PPAR and SREBP-1c also regulate other key metabolic enzymes and the differential abundance of several other proteins is probably due to these transcription factors. For example the abundance of pyruvate kinase, which is regulated by PPAR-αReference Xu, Christian and Jump18, is also increased in animals fed the –FLMLC diet.
PPAR-α has also been implicated in other models of hepatic steatosis, such as alcoholic fatty liverReference You and Crabb19 or diets deficient in methionine and cholineReference Ip, Farrell, Robertson, Hall, Kirsch and Leclercq20. The steatosis caused by folate deficiency during pregnancy appears to differ from other forms of steatosis, in that there is no increase in markers of liver damage such as elevated serum aspartate aminotransferase and alanine aminotransferase or an increase in the expression of gadd153, p57 and CCAAT enhancer binding protein-α. This suggests that although there is an increase in the hepatic TAG content, it is not sufficient to induce cell damage. The metabolic changes initiated by activation of SREBP and PPAR appear to be sufficient to minimise the accumulation of lipid and this may in turn prevent the damage to cells that is responsible for initiating the pathological changes seen in models of alcoholic liver injuryReference Kaplowitz and Ji21.
Since production and oxidation of fatty acids does not account for the accumulation of TAG, the hepatic steatosis in animals fed the –FLMLC diet is probably caused by a reduced rate of TAG export. Evidence for this comes from the differential abundance of a number of proteins associated with the secretion of LDL, including fatty acid binding proteins, calreticulin and the apoB precursor. All of these proteins are associated with intracellular lipid handling and export via the endoplasmic reticulum and Golgi apparatus. There is also a change in the abundance of the transitional endoplasmic reticulum ATPase (down regulated in both –F and –FLMLC), which transports vesicles within the cell via the cytoskeletonReference Fisher and Ginsberg22. All of these changes would be consistent with a reduced rate of TAG export leading to steatosis in the animals fed the –FLMLC diet. Furthermore, the steatosis caused by methyl-deficient diets is similar to that found in animals with a deletion of the PEMT geneReference Noga and Vance23. As phosphatidyl choline produced by PEMT is used for the production of lipoproteins, it is a strong candidate for the underlying target of folate deficiency. The PEMT enzyme is localised in a subfraction of endoplasmic reticulum membranes, known as mitochondria-associated membranesReference Cui, Vance, Chen, Voelker and Vance24, where it is a major user of S-adenosyl methionineReference Noga and Vance23, Reference Shields, Lehner, Agellon and Vance25. The change in the abundance of the dnaK type molecular chaperone glucose regulated protein 75 precursor is evidence for a change in these membranes, as it has been suggested that this protein is associated with mitochondrial import, antioxidant defence and lipid metabolismReference Wadhwa, Taira and Kaul26.
In addition to lipoprotein production, significant amounts of the phosphocholine produced de novo by PEMT are used for the production and secretion of phosphatidic acid, lyso-phosphatidyl choline and taurocholic acid found in bileReference Sehayek, Wang and Ono27. The down regulation of vinculin, a cytoskeletal protein specifically associated with bile canniculusReference Roman and Hubbard28, and two proteins associated with bile production, 3-α hydroxysteroid dehydrogenase and hydroxysteroid sulphotransferaseReference Tsukada, Ackerley and Phillips29, suggest that bile production is reduced in animals fed the –F and –FLMLC diets. Reduced bile production or secretion may have widespread effects, particularly on the digestion of lipids and the bile acid cycle. Interestingly, in addition to the production of bile acids, 3-α hydroxysteroid dehydrogenase and hydroxysteroid sulphotransferase also have a critical role in the clearance of steroid hormones by the liverReference Falany30–Reference Penning, Jin, Steckelbroeck, Lanisnik Rizner and Lewis32. It is therefore possible that folate deficiency may have an indirect effect on systemic steroid hormone levels as a result of a reduced clearance rate.
Comparison of the soluble hepatic proteome of the pregnant female with the male rat exposed to folate-deficient dietsReference Chanson, Sayd, Rock, Chambon, Sante-Lhoutellier, Potier de Courcy and Brachet33 shows a number of common features. These include 3-α hydroxysteroid dehydrogenase, endoplasmic reticulum-associated proteins (glucose regulated protein 75) as well as changes in the antioxidant capacity. Previous studies have shown that oxidative stress is increased in folate-deficient animals and is strongly correlated to the accompanying elevation in plasma homocysteineReference Chanson, Sayd, Rock, Chambon, Sante-Lhoutellier, Potier de Courcy and Brachet33, Reference Huang, Hsu, Lin and Yang34. The up regulation of γ-glutamylcysteine synthetase and glutathione S-transferase is greater in the livers of animals fed the –FLMLC diet despite the fact that plasma homocysteine concentrations are similar in animals fed –F and –FLMLC dietsReference Maloney, Hay and Rees9. Since both groups are exposed to similar levels of homocysteine, these results suggest that the response to oxidative damage is more likely to be induced by the development of steatosis rather than a direct effect of elevated homocysteine. A similar observation of increased lipid peroxidation has been made in animals that develop a fatty liver due to a defect in VLDL secretionReference Lin, Chen, Yue, Averna, Ostlund, Watson and Schonfeld35. In both cases, it appears that the fatty liver due to a defect in TAG export is associated with increased oxidative stress caused by the accumulation of TAG.
The present study has shown that changes in the flux through the methionine cycle cause widespread changes in lipid metabolism and export from the maternal liver, suggesting that lipid metabolism may be a common factor linking folate status, BMI and NTD occurrence. During pregnancy, fatty acids derived from lipoprotein-associated TAG provide an important source of lipid for the developing fetusReference Lindegaard, Olivecrona, Christoffersen, Kratky, Hannibal, Petersen, Zechner, Damm and Nielsen36. By interfering with the export of lipoprotein, folate deficiency may disrupt this supply. It is striking that NTD and other developmental anomalies are more common in infants born to obese womenReference King37. A BMI >30 doubles the risk of having a child with a NTD compared with normal-weight women and, critically, this increased risk does not appear to be modified by folic acid supplementationReference Anthony38. Obese individuals frequently have steatotic livers, suggesting a possible link between hepatic lipid metabolism and NTD. The close interrelationship between folates, the methionine cycle and fat metabolism suggest that high-fat, low-folate diets, typical of those found in Western Europe, may be delivering a double blow to the delivery of essential lipids to the developing fetus.
Acknowledgements
This work was supported by the Scottish Executive Environment and Rural Affairs Department as part of the core funds of the Rowett Research Institute and by the European Union Sixth Framework programme EARNEST (CT-2005-007036). Christopher Maloney is supported by a cooperative agreement from the NIH (U01 HD044638) as a component of the NICHD Cooperative Program on Female Health and Egg Quality. We express our thanks to staff from Bioresources Unit for animal care and to Dr C. Mayer (Biomathematics and Statistics, Scotland) for advice on the statistical analysis.








