Fortification of white wheat flour, and other select grains, with folic acid and periconceptional supplementation guidelines are designed to improve folate status, and have been associated with a reduced risk of neural tube birth defects (NTD). The USA and Canada were among the first countries to fully implement mandatory policies specific to the fortification of certain grain products with folic acid in 1998( 1 , 2 ). The US Food and Drug Administration requires folic acid fortification of all enriched cereal grain products – including wheat flour, maize grits, maize meal, rice and pasta – at levels of 0·95–309 mg/100 g, based on a fortification level of 0·14 mg/100 g of the cereal grain product. These ranges are intended, in part, to account for cooking losses in some products. In Canada, white wheat flour and enriched maize meal are fortified with folic acid at a level of 0·15 mg/100 g, and enriched pasta is fortified at a level of 0·20 mg/100 g( 2 ). Discretionary fortification of ready-to-eat breakfast cereals with folic acid allows up to 0·40 mg/serving in the USA, and up to 0·06 mg/serving in Canada. Monitoring the effectiveness and safety of an intervention should accompany any change in population-level policy to assess the need for modification. Folic acid intake in pregnancy has been associated with a significant reduction in NTD( Reference Berry, Mulinare, Hamner and Bailey 3 – 5 ). Although folic acid policies are somewhat different between Canada and the USA, these have been credited with a reduction in NTD rates since the programmes were implemented( Reference De Wals, Tairou and Van Allen 6 – 8 ). Currently, more than fifty countries have reported a requirement to add folic acid to flour; however, regulations may not be implemented in all countries( 9 , Reference Crider, Bailey and Berry 10 ).
In addition to public policy regarding food fortification, the USA has adopted the Institute of Medicine guidelines recommending that women of childbearing age consume 0·40 mg/d of folic acid from fortified foods, supplements or both in addition to natural food sources of folate to reduce the risk of NTD( Reference Berry, Mulinare, Hamner and Bailey 3 , 11 ). In Canada, this amount is recommended in the form of a multivitamin supplement, in addition to folate-rich foods( 12 ).
Erythrocyte folate concentrations have been monitored regularly in the USA through the National Health and Nutrition Examination Survey (NHANES) since 1976( Reference Yetley and Johnson 13 ); however, erythrocyte folate concentrations of Canadians at a national level have only recently been examined through the Canadian Health Measures Survey (CHMS)( Reference Colapinto, O'Connor and Tremblay 14 ). Virtually no clinical folate deficiency (erythrocyte folate concentration < 305 nmol/l) has been reported in these population-level surveys in either country since folic acid fortification of the food supply, though concerns have now shifted towards the higher erythrocyte folate concentrations that have been observed( Reference Colapinto, O'Connor and Tremblay 14 , Reference Pfeiffer, Hughes and Lacher 15 ). Still, some women of childbearing age may not be achieving erythrocyte folate concentrations considered optimal for NTD risk reduction( Reference Colapinto, O'Connor and Tremblay 14 , Reference Dietrich, Brown and Block 16 ). Furthermore, there has never been a national-level comparison of folate concentrations between Canada and the USA. Such a comparison is complicated as two different assay methods were used for measuring erythrocyte folate concentration – the 2007–9 CHMS used the Immulite 2000 immunoassay (Siemens Canada Limited) and the 2007–8 NHANES used a microbiological assay. It is well known that blood folate concentrations vary depending on the laboratory in which they are conducted and the assay employed( Reference Gunter, Bowman and Caudill 17 – Reference Pfeiffer, Zhang and Lacher 20 ). In one study in the literature, the Immulite 2000 immunoassay (Siemens Canada Limited) was found to yield higher concentrations of erythrocyte folate than the microbiological assay( Reference Icke, Dennis and Sjollema 21 ). Whether the direction of this difference was specific to this laboratory or, in fact, reflects an assay-dependent bias is not clear. Considering both the similarities and important differences in Canadian and American folic acid fortification and supplementation policies, the aim of the present study was to compare erythrocyte folate concentrations in the Canadian and American populations. This comparison was facilitated by generating a conversion equation through a method-comparison experiment.
Methods
The analyses included data from the 2007–9 CHMS and the 2007–8 NHANES. The methodology for each survey is described briefly herein, and has been explained in greater detail elsewhere( Reference Bryan, St-Denis and Wojtas 22 , 23 ).
Sampling and survey methods
Canadian Health Measures Survey
The CHMS used a complex, multi-stage, cluster sampling protocol to achieve a nationally representative cross-sectional sample. The final sample included 5604 Canadians aged 6–79 years balanced by sex in each of the following age groups: 6–11, 12–19, 20–39, 40–59 and 60–79 years. This sample was representative of approximately 96 % of the Canadian population. An interviewer administered a detailed health questionnaire, in each participant's home, which included questions on sociodemographic characteristics and dietary supplement use. A certified phlebotomist collected blood samples 1 d to 6 weeks later in a Mobile Examination Centre to measure a variety of analytes, including erythrocyte folate( Reference Bryan, St-Denis and Wojtas 22 , Reference Day, Langlois and Tremblay 24 ). The Health Canada Research Ethics Board approved the CHMS protocol and the Children's Hospital of Eastern Ontario and University of Ottawa Research Ethics Boards approved the secondary data analysis.
National Health and Nutrition Examination Survey
The 2007–8 NHANES is a 2-year cycle release from the continuous NHANES survey. This survey employed a complex, multi-stage design and is representative of the civilian, non-institutionalised US population( 23 ). This research included a final sample of 6- to 79-years-olds (n 7996), from a total sample of 10 149 participants, to match the age range for the CHMS. Similar to the CHMS, participants completed an in-home interviewer-administered survey, which included information on sociodemographic characteristics and dietary supplement use, followed by a physical examination and blood collection in a Mobile Examination Centre 1–2 weeks later. The NHANES protocol was reviewed and approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board.
Erythrocyte folate concentrations
A total of 5248 CHMS participants and 7070 NHANES participants had erythrocyte folate data available.
Erythrocyte folate allows for an estimate of tissue folate stores and is therefore considered a long-term indicator of status( Reference McNulty, Pentieva and Marshall 25 ). The CHMS and NHANES collected venepuncture samples in EDTA-treated vacutainers that were immediately processed on-site. The NHANES also collected regular red-capped vacutainers for serum collection. After haematocrit measurement, aliquots of whole blood were frozen, stored at − 20°C and shipped weekly on dry ice to the Health Canada Nutrition Laboratory (CHMS) or the Centers for Disease Control and Prevention Laboratory (NHANES)( Reference Bryan, St-Denis and Wojtas 22 , 26 ). The NHANES diluted whole blood (1:11) with 10 g/l of ascorbic acid solution before freezing. The NHANES also generated serum stabilised with 0·5 % (w/v) sodium ascorbate, for later determination of folate content.
Canadian Health Measures Survey immunoassay
Researchers thawed and then diluted whole-blood samples (1:26) with 5 g/l of ascorbic acid solution, allowed them to incubate for 180 min at room temperature, and then analysed the samples for folate using the Immulite 2000 immunoassay (Siemens Canada Limited)( 27 ). Researchers calculated erythrocyte folate concentration from the measured whole-blood folate concentration, adjusting for erythrocyte volume but without correction for serum folate concentration. The CHMS laboratory assessed the accuracy and reproducibility of the whole-blood folate assay using the manufacturers’ serum controls (Con6: Tri-level multi-constituent control) and whole-blood controls (Bio-Rad Lyphochek Trilevel; Bio-Rad Laboratories). These controls had an inter-assay CV of 6–8·5 % for serum and 8 % for whole blood. All the analysed controls (serum and whole blood) were within 10 % of target values.
National Health and Nutrition Examination Survey microbiological assay
The NHANES laboratory thawed and then prepared samples by diluting serum (1:100) and whole-blood lysates (1:140) with 5 g/l of sodium ascorbate( Reference Pfeiffer, Zhang and Lacher 20 , 26 ). These dilution factors are appropriate for a population consuming folic acid-fortified foods. Researchers added diluted samples to a ninety-six-well plate together with assay medium containing chloramphenicol-resistant Lactobacillus rhamnosus (American Type Culture Collection no. 27773; National Collection of Industrial Bacteria 10463) and all of the nutrients necessary for the growth of L. rhamnosus, with the exception of folate, and then incubated for 45 h at 37°C. The calibration curve for this assay was generated using 5-methyltetrahydrofolate from Merck & Cie( Reference Pfeiffer, Zhang and Lacher 20 , 26 ). The total folate concentration was assessed by measuring the turbidity of the inoculated medium at 590 nm in a PowerWave microplate reader (Bio-Tek Instrument). Researchers calculated erythrocyte folate concentration from the measured whole-blood folate concentration, adjusting for erythrocyte volume and correcting for serum folate concentration( 28 ). Long-term CV from three whole-blood lysate quality-control pools analysed in every run were 8·0–14 % at 402–1570 nmol/l. The National Institute for Biological Standards and Control WHO reference material (lyophilised) for whole-blood folate (95/528) was analysed several times a year to verify comparability of the results over time. The mean value obtained was 11·5 (sd 1·15) ng/ampoule compared with an assigned value of 13 ng/ampoule.
Cut-offs
The only formally accepted cut-offs associated with erythrocyte folate status are those associated with deficiency, which are based on haematological indicators (e.g. macrocytic anaemia) as well as metabolic indicators (e.g. folate concentration below which plasma homocysteine concentrations start to rise)( 11 , Reference Selhub, Jacques and Dallal 29 ). We used the classical definition of erythrocyte folate deficiency < 305 nmol/l ( < 140 ng/ml). This cut-off was defined by the Institute of Medicine based on several studies demonstrating the appearance of hypersegmented neutrophils, a characteristic of megaloblastic anaemia, at erythrocyte folate concentration below this level( 11 ).
Selected sociodemographic factors of interest
Both surveys used self-reported sociodemographic characteristics. For the present study, age groups of the CHMS sample were used. We examined socio-economic status by per-person household income equivalents (which grouped respondents into quintiles after adjusting for family size and composition) for each country separately. We defined race/ethnicity as non-Hispanic white or ‘other’ and assigned missing responses for race/ethnicity to the largest parameter (non-Hispanic white) since the percentage of missing responses was small ( < 3 %).
Supplement use
CHMS interviewers collected drug identification and natural health product numbers from containers shared by participants at the household visit. Researchers verified this information at the clinic visit and collected information about any changes in drug and supplement usage at this time( 30 ). We determined supplemental folic acid use – whether consumed alone or as a multivitamin – in the 30 d before the clinic visit by matching drug identification and natural health product numbers with product information extracted from the Health Canada Drug Product and Licensed Natural Health Product databases( 31 , 32 ). We used the derived variable in the NHANES public data file for use of folic acid-containing supplements in the last 30 d. NHANES participants showed the supplement container at the household interview, or if this was unavailable, named the supplement verbally. Trained nutritionists reviewed these data and matched entries with the in-house NCHS Product Label Database. If no match was available, the product was added or a product of similar nutrient composition was imputed.
Cross-over equation sub-study
We conducted an erythrocyte folate method-comparison study to generate a conversion equation between the immunoassay employed in the CHMS and the microbiological assay used by the NHANES. A convenience sample of 152 healthy men and women, aged 16–79 years, was recruited from The Hospital for Sick Children (SickKids) and the greater Toronto area. Participants attended the Clinical Investigation Unit at SickKids where written informed consent was secured and then blood was drawn. Following determination of haematocrit, aliquots of the whole-blood sample were immediately frozen (Immulite 2000 immunoassay; Siemens Canada Limited) or diluted with a 1:11 solution of 10 g ascorbic acid/l (microbiological assay). Folate concentrations were determined by the Immulite 2000 immunoassay (Siemens Canada Limited) at the Health Canada CHMS laboratory using the methods described above and by the microbiological assay at SickKids. The manufacturer's kits used for the immunoassay were the same as those used for the CHMS, though the short shelf-life of the kits led to the use of several different lots. The manufacturer did not release any notices regarding changes to the kits during the time frame of the CHMS cycle 1 and the cross-over study analyses. The microbiological assay used at SickKids was specifically modified to mimic that of the NHANES laboratory – using the chloramphenicol-resistant micro-organism (L. rhamnosus) with 5-methyltetrahydrofolate as the calibrator( 26 ). A mean of 12·7 (sd 1·7) ng/ampoule (expected 13 ng/ampoule) and a CV of 13 % were obtained when analysing the 95/528 National Institute for Biological Standards and Control WHO whole-blood reference standard over 4 months (n 35 runs). To further verify that the microbiological procedures at SickKids were comparable to those of the NHANES, six quality-control whole-blood haemolysates were analysed blinded at the SickKids laboratory. On average, calculated values were 9 % lower in Toronto compared with the NHANES, with all values within ± 20 %.
Generation of the cross-over equation
Erythrocyte folate concentrations determined by the microbiological assay and immunoassay were analysed using Analyse-it, version 2.26 Excel 12+ software (Analyse-it Software Limited). Values were deemed outliers and removed (n 6) from the dataset where the absolute difference in erythrocyte folate concentrations between the two methods for a given blood sample (immunoassay–microbiological assay) was greater than 2 sd. Plotting the two sets of the remaining values against each other resulted in a Pearson correlation coefficient (r) of 0·67. We examined the scatter plot of the residuals (standard residual by estimated ‘true’ value) and determined that curvilinearity was not present. Since the r value was < 0·80, we used Deming regression methods to generate the cross-over equation, as opposed to ordinary least-squares regression which is appropriate when the r value is ≥ 0·80. At an r value of < 0·80, ordinary least-squares regression is known to underestimate the actual slope of the data( Reference Good and Hardin 33 ). Furthermore, Deming regression accounts for the random error present in both the values of X and Y, whereas ordinary least-squares regression assumes random error for only the Y values, which rarely occurs in method-comparison studies( Reference Ludbrook 34 , Reference Linnet 35 ). A weighted Deming regression was performed to account for proportional error, or the progressive increase in the scatter of the sample residuals (see online supplementary Fig. S1)( Reference Linnet 36 ). Bland–Altman analysis demonstrated a bias of 24 % (95 % limits of agreement − 26 to 75 %) between the immunoassay and microbiological assay methods (see online supplementary Fig. S2). The final weighted Deming regression equation was as follows:
We assessed the goodness-of-fit by estimating the mean absolute percentage error (MAPE) resulting from the model. This statistic,
indicated that there was 23 % error in the model, where k is the dummy index or the range for a quantity from k up to, and including, n; y is the actual value for microbiological assay; pred_y is the predicted value for microbiological assay; and n is the number of fitted points. To validate the model, the study population was randomly split into two groups, one for estimation (n 76) and the other for validation (n 76). The estimation sample was used to fit the model, and the validation sample was used to obtain the MAPE. A similar MAPE was obtained, indicating robustness in the precision of the model's predicted values.
As a final examination of the model, we conducted a boundary assessment by generating two additional equations using the lower and upper 95 % CI values of the intercept from the conversion equation. Since the slope was robust, this was held constant. The two additional equations are as follows:
We applied these equations to the CHMS data to estimate the cumulative distributions at the lower and upper boundaries of the conversion equation.
Since the CHMS immunoassay does not correct for serum in its calculation of erythrocyte folate, and the NHANES microbiological assay does, we also adjusted CHMS whole-blood folate concentrations for comparison with the NHANES whole-blood folate concentrations.
Ethical approval for the cross-over study was obtained from the Children's Hospital of Eastern Ontario, the Hospital for Sick Children, the University of Ottawa and the Health Canada Research Ethics Boards.
Statistical analysis
Where relevant, each of the analyses was conducted using the unadjusted Canadian erythrocyte folate data first, and then repeated following the application of the cross-over equation to the Canadian erythrocyte folate data to derive a method-adjusted measure for comparison with the NHANES erythrocyte folate data. We stacked the CHMS and NHANES datasets based on regional strata and primary sampling units. Descriptive statistics (frequencies, medians and percentiles) were used to characterise the population. Differences between the countries for erythrocyte folate concentrations and supplement intake by age and race/ethnicity were examined using t tests. Differences between the countries for whole-blood folate concentrations were also examined using t tests. All estimates were based on weighted data to represent the population. Variance estimation (95 % CI) and significance testing (t tests and logistic regression) were based on Taylor linearisation( Reference Rust and Rao 37 ). Significance was defined as a P value of < 0·05. Analyses were conducted in SAS 9.1.3 (SAS Institute, Inc.) and SUDAAN version 10.0 (RTI International, Research Triangle Park).
Results
A summary of the demographics of the study populations and folic acid supplement use is given in Table 1. It shows that 25 % of the general population in Canada reported folic acid supplement use, whereas in the USA this was 32 %.
Table 1 Summary description of population demographics and folic acid supplement use by country (Percentages and 95 % confidence intervals)

* Interpret with caution (high sampling variability; CV ≥ 16·6 and < 33·3).
Unadjusted erythrocyte folate
Less than 1 % of the population in Canada or the USA had clinical folate deficiency (erythrocyte folate concentration < 305 nmol/l). The median (50th percentile) of the unadjusted Canadian erythrocyte folate data (1250 nmol/l) was higher than the American median of 1100 nmol/l (Table 2). Median erythrocyte folate concentrations were significantly higher for Canadians compared with Americans among folic acid supplement users and non-users, for a race/ethnicity other than non-Hispanic white, and for individuals aged 12–59 years (Table 3).
Table 2 Selected percentiles for erythrocyte folate concentrations by country† (Percentiles and 95 % confidence intervals)

* P< 0·05 for tests comparing the unadjusted and method-adjusted Canadian concentrations at the 50th percentile with the US distribution.
† Tests were not conducted at the 5th, 25th, 75th, 95th and 97th percentiles.
Table 3 Erythrocyte folate for country by age, race/ethnicity and folic acid supplement use for the general population (Medians and 95 % confidence intervals)
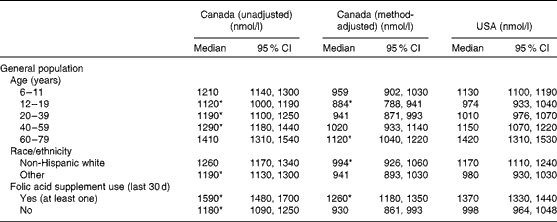
* P< 0·05 for tests comparing the unadjusted and method-adjusted Canadian concentrations at the 50th percentile with the US distribution.
Method-adjusted erythrocyte folate
Consistent with the unadjusted analyses, less than 1 % of the population in Canada or the USA were folate deficient following adjustment of CHMS erythrocyte folate values (Fig. 1). In contrast to the unadjusted analyses, the method-adjusted median erythrocyte folate concentration of Canadians was significantly lower than the median value for Americans (988 v. 1100 nmol/l, respectively; Table 2). This significant difference persisted for the youngest age groups (6–19 years) and the oldest age group (60–79 years), as well as for folic acid supplement users and non-Hispanic whites (Table 3). Comparing the method-adjusted Canadian whole-blood folate concentration in comparison to the American whole-blood folate distribution (see online supplementary Table S1) – to understand the impact of correcting erythrocyte folate for the contribution of serum folate, which the NHANES does, but the CHMS does not – demonstrated similar parallel distributions across both countries as observed with the erythrocyte folate distributions (Table 2), suggesting that the lack of correction for the contribution of serum folate in the CHMS was not a likely reason for the observed differences between the two countries.

Fig. 1 Cumulative percentile distributions of erythrocyte folate concentrations by country from the 2007–9 Canadian Health Measures Survey (adjusted (![]() ) for microbiological assay comparison and unadjusted (
) for microbiological assay comparison and unadjusted (![]() )) and the 2007–8 National Health and Nutrition Examination Survey (USA,
)) and the 2007–8 National Health and Nutrition Examination Survey (USA, ![]() ). Values exceeding 3500 nmol/l are not shown (n 32).
). Values exceeding 3500 nmol/l are not shown (n 32).
Boundary assessment
The examination of the extreme boundaries of the conversion equation demonstrated that the cumulative distribution of erythrocyte folate concentrations in the upper boundary for the method-adjusted Canadian data did not approach the unadjusted Canadian values, except at the 5th percentile (see online supplementary Fig. S3). When comparing the boundaries for the method-adjusted Canadian data with the USA, the cumulative distributions overlapped at the upper boundary, while the lower boundary was below the US distribution.
Discussion
The present study presents the first national-level comparison of folate status based on erythrocyte concentrations between Canada and the USA. To facilitate these analyses, we generated a conversion equation to account for the different analytical methods employed to measure erythrocyte folate concentration at the national level in the two countries. The fact that erythrocyte folate concentrations were either higher or lower in Canadians compared with Americans, depending on whether an adjustment was made to account for assay differences, suggests that caution must be exercised in evaluating erythrocyte folate data from different countries because analytical methods are not readily comparable. Furthermore, we cannot unequivocally conclude that there are true differences in erythrocyte folate concentrations between the Canadian and American populations in the post-fortification era.
Comparing the distribution of erythrocyte folate concentrations for Canadians and Americans is of interest from the perspective of population-level surveillance. Assessing these distributions, particularly in countries where folic acid fortification and supplementation policies are in place, provides information on the success of these interventions and indicates where there may be a need for modification. The virtual absence of clinical deficiency demonstrates the success of folic acid-related public health efforts in both countries, despite certain differences. It is notable that both countries demonstrate median folate concentrations that are much higher than the deficiency cut-off of 305 nmol/l. We observed an apparent difference in median erythrocyte folate status between Canada and the USA; however, the upper boundary of the method-adjusted CHMS distribution overlapped that of the NHANES distribution, and the lower boundary was considerably below that of the NHANES distribution. Further investigation into the presence of a disparity between countries, and factors driving a potential difference, is needed. In the present study, a smaller proportion of Canadians reported taking folic acid supplements, a primary predictor of folate status, than Americans( Reference Colapinto, O'Connor and Dubois 38 ). The proportions of folic acid supplement consumption for each country in the present study are congruent with those reported in past examinations of nationally representative Canadian and American data( Reference Shakur, Garriguet and Corey 39 , Reference Tinker, Cogswell and Devine 40 ). Dietary intake of folic acid may also be a factor since fortification policies differ in some aspects between the two countries. Most significantly, the USA mandates a greater number of foods to be fortified and Canada allows lower fortification levels for breakfast cereals( 1 , 2 ). The limited CHMS dietary data precluded our ability to examine dietary intake as a factor in the discrepancy between countries.
There is growing support for harmonisation of biochemical measurement methods in national-level studies. With the change in the NHANES erythrocyte folate concentration measurement method from the Bio-Rad radioassay (before 2006) to the microbiological assay (2006–10), there came the need to formulate regression equations to allow for accurate comparisons of erythrocyte folate data across survey periods( Reference Pfeiffer, Hughes and Durazo-Arvizu 41 ). Adjustment of CHMS erythrocyte folate concentrations using our calibration equation resulted in different conclusions regarding median erythrocyte folate concentrations between the two countries. The median unadjusted Canadian erythrocyte folate concentration of 1250 nmol/l was higher than the American median of 1100 nmol/l, whereas the method-adjusted Canadian value of 988 nmol/l was lower. Vitamin D is an example of a nutrient currently undergoing methodological scrutiny as numerous and varying measurement methods have impeded the ability to formulate evidence-based guidelines and surveillance efforts( Reference Sempos, Vesper and Phinney 42 ). It has been suggested that standardised measures are needed to determine appropriate levels in the population that are comparable to other countries. Similarly, harmonising folate measurements between Canada and the USA would allow for effective monitoring and assessment of the longitudinal trajectory of elevated levels to determine the need for modification to current programmes. While harmonisation of survey data may be achievable through well-designed comparison studies that generate a conversion equation between two methods, standardisation can only be pursued when standard reference materials and higher-order reference measurement procedures are available. This is not yet the case for erythrocyte folate.
The present study is the first comparison of erythrocyte folate concentrations between two nationally representative North American datasets using harmonised data. Although it is imperative to harmonise methods before making comparisons, method-comparison studies are not without limitations. The CHMS data are limited as the manufacturer's serum and whole-blood controls were used to assess the accuracy and reproducibility of the assay, rather than an external standard reference material as per the NHANES methodology. A certain level of error is evident in our conversion equation, as indicated by the relatively poor correlation and wide CI demonstrated in the boundary assessment. Our examination of method-adjusted whole-blood folate concentration provides assurance that not correcting for serum folate concentrations in the CHMS immunoassay – where the NHANES microbiological assay does – had no influence on the direction or magnitude of our findings. Since the microbiological assay for the CHMS method comparison was not conducted at the NHANES laboratory, we examined the agreement between the SickKids laboratory and NHANES microbiological assays based on a small set of six blood samples, and found that the results were within ± 20 % of each other, which is fairly common for an assay that has an imprecision of approximately 10 %. On average, the SickKids laboratory microbiological assay was 9 % lower than the NHANES microbiological assay, thus the study may be limited by a potential underestimation of the adjusted CHMS erythrocyte folate values. Considering the low correlation, and potential interlaboratory and inter-assay variation in erythrocyte folate measurement, caution should be used in applying the conversion equation outside of the current context.
Comparing erythrocyte folate data across time and countries is an important step in evaluating the success of current interventions, and will advance future dialogue and research on folic acid fortification and supplementation policies. The present study highlights that caution must be exercised in comparing erythrocyte folate data from different countries since the use of analytical methods that have not been harmonised may lead to incorrect data interpretation, and, furthermore, we do not recommend that the conversion equation be applied outside of the current context. Adjusting laboratory data allows for a comparison of erythrocyte folate data; however, it is important to consider the limitations of the method-comparison study. Harmonisation of folate measurement methods in future surveillance efforts would facilitate comparisons and inform policy directions.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514002906
Acknowledgements
The authors thank the Health Analysis Division at Statistics Canada, in particular Julie Bernier for her support during the generation of the conversion equation, and the Physical Health Measures Division at Statistics Canada for preparing and granting special access to data. The authors also thank Steve Brooks and Penny Jee for the CHMS laboratory blood analysis in the conversion equation sub-study and Trevor Stewart for his review and feedback on the manuscript. The authors thank all participants in the conversion equation sub-study, the CHMS and the NHANES. This work was supported by Canadian Institutes of Health Research (CIHR) Health Professionals Fellowship in the Area of Public Health (funding reference no. 180375) to C.K.C. and a CIHR Operating Grant (funding reference no. 218776) to M. S. T., D. L. O. and C. K. C.
The authors' contributions to the study were as follows: C. K. C., M. S. T. and D. L. O. conceptualised and designed the study and crossover equation sub-study. S. A. and D. L. O. conducted the data collection and blood analysis for the sub-study. C. K. C. generated the crossover equation with the assistance of T. B., M. S. T., C. M. P. and D. L. O. C. K. C. conducted all analyses and T. B., M. S. T., C. M. P. and D. L. O. assisted in the interpretation of the results. C. K. C. drafted the manuscript. All of the authors read and approved the final manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry.






