Oxidative stress appears to be centrally involved in the pathogenesis of Alzheimer's disease (AD) and many of the pathological processes involved in its development and progression are either caused by or lead to an augmented generation of reactive oxygen and nitrogen species(Reference Christen1). Therefore, the importance of vitamin E (VE) for the maintenance of neuronal integrity and brain function is becoming increasingly recognised.
Epidemiological studies indicate that a high intake of VE from food sources, but not from dietary supplements, may contribute to the prevention of age-related neurodegenerative disorders such as AD(Reference Engelhart, Geerlings and Ruitenberg2–Reference Morris, Evans and Tangney4). Morris et al. evaluated the contribution of the different VE congeners towards the prevention of AD. While a higher dietary intake of both α-tocopherol (αT) and γ-tocopherol (γT) was associated with a decreased risk of developing AD, their data suggested that the combination of the different VE isoforms may be more effective in preventing AD than the intake of αT alone(Reference Morris, Evans and Tangney4). In line with this, in some intervention studies, supplementation of high doses of αT, the major lipid-soluble antioxidant in human plasma and the VE congener with the highest biological potency(5), failed to delay the onset of dementia(Reference Fillenbaum, Kuchibhatla and Hanlon6) and to slow down the progression of AD(Reference Petersen, Thomas and Grundman7). Other clinical intervention studies employing high dosage αT supplements, on the other hand, found a significant reduction in AD progression(Reference Sano, Ernesto and Thomas8, Reference Onofrj, Thomas and Luciano9).
Little is known regarding the long-term effects of dietary VE on the expression of genes involved in the development or prevention of AD and on the antioxidant status in the healthy brain. The aim of the present study was therefore to investigate (i) whether suboptimal supply of dietary VE may cause unfavourable physiological changes with regard to the parameters of oxidative stress and the expression of genes relevant in the pathogenesis of AD in the healthy brain, and (ii) whether supplementation of a high dose of αT on top of an already sufficient dietary supply of VE may induce AD-preventive effects.
Materials and methods
Experimental diets
The tocopherol-rich rapeseed variety S-NAP 944 (a kind gift from Norddeutsche Pflanzenzucht Hans-Georg Lembke KG, Holtsee, Germany) was cold pressed under a nitrogen atmosphere. The resulting oil was vacuum filtered and contained 341 mg αT, 386 mg γT and 11 mg δ-tocopherol per kg oil. A part of the native rapeseed oil was stripped of tocopherols by column chromatography as described by Lampi et al. (Reference Lampi, Hopia and Ekholm10). The fatty acid composition of the oil (oleic acid, 49·1 % of total fatty acids; linoleic acid, 19·7 %; linolenic acid, 10·8 %; erucic acid, 7·2 %; gadoleic acid, 4·9 %; palmitic acid, 4·5 %) was not altered by the removal of antioxidants (data not shown). The tocopherol-stripped oil was used as follows: (i) unmodified or (ii) blended at a ratio of 1:1 with the native oil, (iii) the native oil was used unaltered or (iv) fortified with 4 g natural RRR-α-tocopherol (a kind gift of Cognis, Monheim am Rhein, Germany) per kg oil. Butylated hydroxytoluene (BHT 200 mg/kg; Carl Roth GmbH, Karlsruhe, Germany) was added to all oils as a preservative to protect the tocopherols from oxidative decay.
These oils were used to prepare diets (ssniff Spezialdiaeten GmbH, Soest, Germany) with VE concentrations that, according to the Nutrient Requirements of Laboratory Rats (11), can be classified as VE deficient (dVE), marginal (mVE), sufficient (sVE) and fortified (fVE).
The composition of the four semi-synthetic experimental diets was as follows (g/kg diet): casein, 240; maize starch, modified, 480; glucose, 110; cellulose, 50; VE-free vitamin premix (E15313-2), 10; mineral premix (E15000), 60; rapeseed oil, 50. All VE in the experimental diets originated from the rapeseed oil and were as follows (as analysed by HPLC and given in mg/kg diet). dVE: αT, < 1; γT, < 1; mVE: αT, 6; γT, 11; sVE: αT, 12; γT, 24; fVE: αT, 140; γT, 24.
Experimental animals and study design
The animal experiment was conducted in accordance with the German regulations on animal care and with the permission of the responsible authority. Thirty-two male Fisher 344 rats (Charles River Laboratories, Sulzfeld, Germany) with a mean initial body weight of 51 (sd 5) g were randomly divided into four groups of eight animals each and fed the experimental diets for 6 months. The rats were housed in Makrolon III cages in a conditioned room (22 ± 2°C, 55 % relative humidity, 12 h light–dark cycle). The animals had free access to tap water and the experimental diets throughout the experiment. Food consumption and animal weight were recorded daily and weekly, respectively. At the end of the experiment, the rats were fasted for 12 h before CO2–anaesthesia and decapitation. Blood samples were collected in tubes containing heparin as anticoagulant and plasma was obtained by centrifugation (4°C, 3000 g, 10 min) and stored at − 80°C until analysed. The brain was excised and dissected; one part of the cortex and the hippocampus were stored in RNAlater (Qiagen, Hilden, Germany). The remaining brain was snap frozen in liquid nitrogen and stored at − 80°C.
Quantification of tocopherols
Tissues (100 mg) were weighed into test tubes with screw caps on ice. Ethanol (2 ml) containing 1 % (by weight) ascorbic acid, 700 μl H2O and 300 μl saturated aqueous KOH were added. The samples were saponified at 70°C in a shaking water-bath for 30 min and then cooled on ice. Fifty microlitres of BHT (1 mg/ml) and 2 ml of n-hexane were added, and the samples were centrifuged (167 g, 4°C, 5 min) to aid phase separation. One millilitre of the supernatant was transferred to a clean tube and dried under a stream of nitrogen gas. The residue was dissolved in 250 μl mobile phase (methanol–water (98:2, v/v)) and injected into the HPLC system. Plasma samples (100 μl) were processed as described previously, but without saponification. Tocopherols were analysed using a Jasco (Gross-Umstadt, Germany) HPLC system (pump PU2080Plus, autosampler AS2057Plus, detector FP2020Plus). The separation of tocopherols was performed on a Waters Spherisorb ODS-2 column (100 mm × 4·6 mm, 3 μm). The fluorescence detector was set to an excitation wavelength of 296 nm and emission wavelength of 325 nm. The concentrations of αT and γT were quantified by the use of authentic tocopherols (Calbiochem, Schwalbach, Germany) as external standards (limit of quantification, ≈ 50 pg). Analyses were performed in duplicate. Porcine plasma with known concentrations of αT and γT was analysed in parallel as quality control. Plasma VE concentrations were normalised for plasma cholesterol concentration, which was determined spectrophotometrically using a commercial kit (Fluitest® CHOL 4241, Biocon Diagnostik, Voehl/Marienhagen, Germany), according to the manufacturer's protocol.
Determination of rat cortex concentrations of F2-isoprostanes
Cortex samples were homogenised using Dounce homogeniser to yield 10 % homogenates in 180 mm-KCl, 10 mm-EDTA and 0·1 mm-BHT (pH 7·4). To hydrolyse esterified F2-isoprostanes, the cortex homogenates (100 μl) were treated with 4 m-KOH at 45°C for 30 min and neutralised by the addition of 4 m-HCl (pH adjusted to 2·0 with 0·1 m-HCl). 9α,11α-PGF2α-d 4 (0·5 ng; Cayman Chem. Co., Ann Arbor, MI, USA) was used as an internal standard. Fatty acids, including isoprostanes, were extracted two times by ethyl acetate. The samples were centrifuged for phase separation and the upper phases were applied onto NH2 cartridges. Solid phase extraction and derivatisation steps were performed as described previously(Reference Wiswedel, Hirsch and Kropf12). F2-isoprostanes were separated and measured by GC/negative-ion chemical ionisation MS (DSQ/Trace GC Ultra; Thermo Fisher Scientific, Dreieich, Germany) with ammonia as reagent gas using selected ion monitoring of the carboxylate anion [M-181] at m/z 569 and 573 for F2-isoprostanes and the deuterated internal standard. The F2-isoprostane peak co-eluted with authentic 8-iso-PGF2α, but it is known that other F2-isoprostane isomers occur at the same retention time(Reference Morrow, Zackert, Van der Ende, E and L13). All analyses were performed in triplicate for each tissue sample. The protein content was determined using the method of Bradford(Reference Bradford14).
Antioxidant enzyme activities and glutathione concentration in the cortex
Cortex tissue (100 mg) was diluted 1:10 (by weight) in ice-cold PBS and homogenised. The homogenate was centrifuged (3000 g, 4°C, 10 min), the supernatant collected and used for the measurements after appropriate dilution in PBS. Superoxide dismutase enzyme activity was measured according to the method of Marklund & Marklund(Reference Marklund and Marklund15). Se-dependent glutathione peroxidase activity was measured according to the method of Lawrence & Burk(Reference Lawrence and Burk16) using bovine GPx (Sigma Aldrich, Darmstadt, Germany) to generate the external standard curve. GSH concentrations were measured in homogenates according to Tietze(Reference Tietze17) with modifications(Reference Dringen and Hamprecht18). All measurements were carried out on a DU 800 spectrophotometer (Beckman Coulter, Krefeld, Germany). Results for antioxidant enzyme activities and GSH concentrations were adjusted for protein content as assessed using a commercial kit (BCA Protein Assay; Pierce, Rockford, IL, USA).
RNA isolation and real-time qRT-PCR
Total RNA was extracted according to the RNeasy® Lipid Tissue Protocol (Qiagen). DNA digestion was performed with the RNase-Free DNase Set (Qiagen). The concentration of isolated RNA was spectrophotometrically determined (Beckmann Instruments, Munich, Germany) by measuring the absorbance at 260 nm; the purity was determined by the ratio of 260 and 280 nm and a ratio of 1·6–1·9 was considered as acceptable. RNA aliquots were stored at − 80°C until PCR analysis. Primer sequences for real-time RT-PCR experiments were designed with Primer3 software (version 0.4.0; http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). Primer pairs (Table 1) were obtained from MWG Biotech AG (Ebersberg, Germany). The Bcl-2 primer was obtained from Qiagen. One-step quantitative RT PCR was carried out with the QuantiTect™ SYBR® Green RT-PCR kit (Qiagen). Real-time PCR amplification was performed in a Rotor-Gene 3000 thermocycler (Corbett Research, Sydney, Australia). Relative mRNA concentrations of genes were quantified by the use of a standard curve. Target gene mRNA concentration was normalised to the mRNA concentration of the housekeeping gene β-actin.
Table 1 Nucleotide sequences of primers used for the real-time qRT-PCR experiments
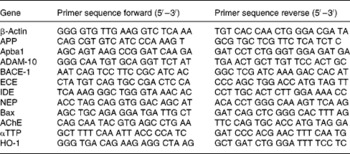
APP, amyloid β-(A4) precursor protein; Apba1, APP-binding family member 1; ADAM-10, a disintegrin and metalloprotease domain 10; BACE-1, β-site amyloid β-(A4) precursor protein-cleaving enzyme 1; ECE, endothelin-converting enzyme; IDE, insulin-degrading enzyme; NEP, neprilysin; AChE, acetylcholinesterase; αTTP, α-tocopherol transfer protein; HO-1, haeme oxygenase 1.
Western blot experiments in the cortex homogenates
Cortex tissue (100 mg) was homogenised in RIPA buffer (NaCl, 150 mm; sodium deoxycholate, 0·5 %; SDS, 0·1 %; NP-40, 1 %; EDTA, 20 mm; 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)–HCl, 50 mm (pH 7·4); dithiothreitol, 1 mm; protease inhibitor cocktail). Lysates were purified by centrifugation (14 000 g, 4°C, 30 min). Total protein concentrations in each lysate were quantified using a BCA Protein Assay kit (Pierce). Total proteins of the lysate (40 μg per lane) were separated by SDS gel electrophoresis followed by transferring the proteins to a PVDF membrane, which was then blocked for 1·5 h in blocking buffer (5 % non-fat milk in Tris-buffered saline). Primary antibodies were diluted in 5 % non-fat milk (Bax, Bcl-2, 1:200; β-actin, 1:1000) and the blots were incubated overnight at 4°C. The blots were washed and incubated with secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA; anti-mouse horseradish peroxidase) in blocking buffer (Bax, Bcl-2, 1:5000; β-actin, 1:1000) with gentle agitation for 1 h at room temperature. The blots were washed and exposed to Immun-Star Western Chemiluminescent kit (Bio-Rad Laboratories, Hercules, CA, USA) and scanned with a ChemiDoc (Bio-Rad). Digital images were captured and quantified using the Quantity-One system (Bio-Rad). Relative concentration of the proteins were quantified as the ratio between the amount of target protein and the amount of the housekeeping protein β-actin.
Statistical analysis
Statistical calculations were conducted with SPSS Version 13.0 (SPSS, Munich, Germany). For the comparison of group means, one-way ANOVA was performed with the Scheffé test (normal distribution, homogeneity of variances) or the Games–Howell test (normal distribution, heterogeneity of variances) as post hoc tests. When data were not normally distributed, the Mann–Whitney U test was performed. Significance was accepted at P < 0·05.
Results
Feed intake and body weight gain
Total feed intake (data not shown) and final body weights (means dVE, 362·8 (sd 11·5) g; mVE, 366·6 (sd 18·3) g; sVE, 372·1 (sd 21·2) g; fVE, 349·9 (sd 21·0) g) did not differ between the dietary groups.
Plasma and tissue concentrations of α- and γ-tocopherols
Plasma concentrations of total cholesterol were significantly lower in the dVE group than in the mVE (P < 0·05) and fVE (P < 0·001) groups (means dVE, 2·23 (sd 0·22) mmol/l; mVE, 2·6 (sd 0·24) mmol/l; sVE, 2·51 (sd 0·17) mmol/l; fVE, 2·80 (sd 0·24) mmol/l; one-way ANOVA with Scheffé post hoc test) and were used to normalise plasma tocopherol concentrations. Consumption of the experimental diets with graded concentrations of VE for 6 months significantly and dose dependently affected αT concentrations in the plasma, lung, muscle, cortex and hippocampus of male Fisher 344 rats (Table 2).
Table 2 Tocopherol concentrations in the plasma, lung, muscle, cortex and hippocampus of rats fed diets deficient (dVE), marginal (mVE) or sufficient (sVE) in vitamin E or fortified (fVE) with α-tocopherol (αT) for 6 months
(Mean values with their standard errors, n 8 except where indicated differently)

γT, γ tocopherol.
a,b,c Mean values within a row with unlike superscript letters are statistically different at P.
F2-isoprostanes and antioxidant enzymes in the cortex
The concentrations of F2-isoprostanes in the cortex homogenates were not significantly different between the dietary groups (Fig. 1).

Fig. 1 F2-isoprostane concentrations (ng/mg protein) in the cortex of Fisher 344 rats fed diets deficient (dVE), marginal (mVE) or sufficient (sVE) in vitamin E (VE) or fortified (fVE) with α-tocopherol for 6 months. Values are represented as means with their standard errors (n 8). No statistical differences were observed.
Superoxide dismutase and glutathione peroxidase activities as well as glutathione concentrations in the cortices of the rats were similar in all groups (Table 3).
Table 3 Antioxidant enzyme activities and glutathione concentrations in the cortex of rats fed diets deficient (dVE), marginal (mVE) or sufficient (sVE) in vitamin E or fortified (fVE) with α-tocopherol for 6 months*
(Mean values with their standard errors, n 8)

SOD, superoxide dismutase; SeGPx, Se-dependent glutathione peroxidase.
* No statistical differences were observed.
Gene expression in cortex and hippocampus
In the cortex and hippocampus, the differences in dietary supply with VE did not result in significant changes in relative mRNA concentrations of amyloid β-(A4) precursor protein (APP), APP-binding family member 1, a disintegrin and metalloprotease domain 10, β-site APP-cleaving enzyme 1, endothelin-converting enzyme, insulin-degrading enzyme, neprilysin, Bax (Bcl-2-associated X protein), Bcl-2 (B-cell leukaemia/lymphoma 2), acetylcholinesterase, αTTP (α-tocopherol transfer protein) and HO-1 (haeme oxygenase 1) (Table 4).
Table 4 Relative mRNA expression (corrected for the housekeeping gene β-actin) of Alzheimer's disease-relevant genes in the cortex and hippocampus of rats fed diets deficient (dVE), marginal (mVE) or sufficient (sVE) in vitamin E or fortified (fVE) with α-tocopherol for 6 months*
(Mean values with their standard errors, n 8)

APP, amyloid β-(A4) precursor protein; Apba1, APP-binding family member 1; ADAM-10, a disintegrin and metalloprotease domain 10; BACE-1, β-site amyloid β-(A4) precursor protein-cleaving enzyme 1; ECE, endothelin-converting enzyme; IDE, insulin-degrading enzyme; αTTP, α-tocopherol transfer protein; HO-1, haeme oxygenase 1; AChE, acetylcholinesterase.
* No statistical differences were observed.
Protein concentrations of apoptosis markers in the cortex homogenates
Protein concentrations of Bcl-2 and Bax in the cortex did not differ between the groups (Fig. 2). Protein concentrations of activated caspases 3 and 8 were undetectable (data not shown).

Fig. 2 (A) B-cell leukaemia/lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and β-actin protein concentrations in the cortex homogenates and (B) relative concentration of Bcl-2 (![]() ) and Bax (
) and Bax (![]() ) of rats fed diets deficient (dVE), marginal (mVE) or sufficient (sVE) in vitamin E (VE) or fortified (fVE) with α-tocopherol for 6 months. β-Actin served as endogenous (loading) control. Values are represented as means with their standard errors (n 6).
) of rats fed diets deficient (dVE), marginal (mVE) or sufficient (sVE) in vitamin E (VE) or fortified (fVE) with α-tocopherol for 6 months. β-Actin served as endogenous (loading) control. Values are represented as means with their standard errors (n 6).
Discussion
Epidemiological as well as clinical intervention studies indicate that a high intake of VE may be important for the prevention of age-related neurodegenerative disorders such as AD(Reference Grundman, Grundman and Delaney19). Of the different tocopherols present in foods, RRR-α-tocopherol is thought to be the most important to human health and has the highest VE activity in human and animal systems(5). Plant oils are the major dietary source of tocopherols and rapeseed oil, due to its high concentrations of tocopherols and n-3 fatty acids(Reference Grusak and DellaPenna20), is considered of high value for human nutrition. Therefore, in the present study, we investigated the effects of graded dietary concentrations of VE from rapeseed oil on the antioxidant status and the regulation of genes relevant in the pathogenesis of AD in the brains of Fisher 344 rats during growth and maturation. During the 6-month feeding period of the present experiment, no differences in feed intake and life weight gain were observed between the dietary groups, which is in accordance with our previous findings(Reference Barella, Muller and Schlachter21).
Vitamin E in plasma and tissues
In correspondence with the intake of αT, we observed dose-dependent changes in the concentrations of αT in the brain (cortex and hippocampus), plasma and other tissues in our rats (Fig. 1). In accordance with the data from the previously published rat experiments(Reference Vatassery, Brin and Fahn22, Reference Martin, Janigian and Shukitt-Hale23), the changes in αT concentrations in the brain, however, were less pronounced than the changes observed in all other tissues. Similarly, in Parkinson's disease patients who, over a period of 5 months, consumed high amounts of αT (from 400 IU/d (364 mg all-rac-α-tocopherol/d) during the first month to 4000 IU/d (3636 mg all-rac-α-tocopherol/d) during the last month of supplementation), Pappert et al. (Reference Pappert, Tangney and Goetz24) observed large increases in αT concentrations in plasma (from 19 to 111 μmol/l), while its concentrations in ventricular cerebrospinal fluid remained unchanged (in a range of 0·114–0·164 μmol/l).
In our rats, a deficient supply of αT ( < 1 mg/kg diet) resulted in an almost complete depletion of αT in the plasma (0·15 (sd 0·002) mmol/mol cholesterol), while again changes in the brain were less pronounced (Table 2). In the cortex and hippocampus, 3·7 (sd 0·4) and 2·0 (sd 0·4) nmol αT/g tissue, respectively, were retained even after 6 months of deficient VE intake. These data are in agreement with the results of Clement et al. (Reference Clement, Dinh and Bourre25), who fed rats from VE-deficient mothers a VE-deficient diet for 8 weeks, and observed a marked reduction in the concentrations of αT in the serum, liver and muscle, while significant concentrations of αT were preserved in all analysed nervous tissues. Vatassery et al. (Reference Vatassery, Brin and Fahn22) observed that even though other tissues could be almost completely depleted of αT by feeding of a VE-deficient diet for 4 months, in the brain low levels of αT were retained. Together, these and the present results suggest that the brain is especially efficient at maintaining αT within a certain physiological range, preventing both excessive αT accumulation and its depletion.
In the present study, the dVE, mVE and sVE diets provided increasing concentrations of αT and γT. The fVE diet, on the other hand, was prepared by the addition of αT to the natural rapeseed oil and thus provided the same amount of γT as the sVE diet but about 10 × more αT. With decreasing γT concentrations in the dVE, mVE and sVE diets, we observed dose-dependent decreases in the concentrations of γT in the lung, muscle and cortex tissue, but not in the plasma. Interestingly, the fVE-fed rats consuming additional αT also had significantly reduced plasma and tissue γT concentrations compared with the sVE-fed rats despite a similar γT intake (Table 2). In the human system, the phenomenon of reduced γT concentrations caused by a high intake of αT was first described by Handelman and co-workers. In eighty-six subjects (>50 years of age, mean age 71·8 (sd 6·4)), they observed an inverse relationship between αT and γT concentrations in the plasma. Supplementation of eight volunteers (30–60 years of age) with 1200 IU (1091 mg) all-rac-αT per day for 8 weeks resulted in a significant increase in plasma αT and a significant decrease in γT(Reference Handelman, Machlin and Fitch26). These findings were later confirmed in human subjects by other researchers(Reference Melchert and Pabel27, Reference Huang and Appel28) and are thought to be the result of an induction of cytochrome P450 enzymes, which are involved in VE metabolism and preferentially degrade the desmethyl vitamers(Reference Sontag and Parker29).
F2-isoprostanes
With increasing age, lipid peroxidation of membrane fatty acids in the brain appears to augment(Reference Floyd and Hensley30) and oxidative stress in the brain is thought to be an important factor in the pathology of AD(Reference Grundman, Grundman and Delaney19). Accordingly, the concentrations of F2-isoprostanes, which are non-enzymatic free-radical-induced peroxidation products of arachidonic acid(Reference Morrow, Hill and Burk31), have been found to be significantly higher in the cerebrospinal fluid or brains of AD patients than those of matched healthy controls(Reference Montine, Quinn and Zhang32). We investigated the effects of increasing doses of dietary VE on F2-isoprostane concentrations in the cortex of male Fisher 344 rats and found a non-significant trend towards higher F2-isoprostane concentrations in the group fed the dVE diet, but overall no significant differences were observed between the dietary groups (Fig. 1). Similar to our findings, Cuddihy et al. did not observe significant differences between the F2-isoprostane concentrations in the cortices of mice fed dVE or sVE diets for 40 weeks. Surprisingly, cortex concentrations of F4-neuroprostanes, which are oxidation products of DHA (22:6), an abundant fatty acid in the brain, were positively correlated with αT concentrations(Reference Cuddihy, Ali and Musiek33).
The present results indicate that the low concentrations of αT retained in the brain may have been sufficient to protect the cortex from lipid peroxidation.
Another aim of the present study was to investigate whether or not the intake of different amounts of VE may influence the activity of antioxidative enzymes and modulate cellular glutathione levels. Activities of total superoxide dismutase and Se-dependent glutathione peroxidase as well as concentrations of total GSH in the cortices of our rats did not differ between the experimental groups. It appears likely that the lack of oxidative stress in our animals may be responsible for the absence of the differences in the activities and concentrations, respectively, of antioxidant enzymes. Supplementation with αT (fVE group) did not result in enhanced enzyme activities or concentrations, either. However, there is no consistent opinion regarding this in the literature: while some groups report no changes in antioxidative enzyme activities or concentrations(Reference Kolosova, Shcheglova and Sergeeva34), others report increases in the activities or concentrations of antioxidant enzymes caused by VE depletion(Reference De and Darad35), as well as supplementation(Reference Devi and Kiran36).
Gene expression
The accumulation and aggregation of misfolded proteins (particularly amyloid-β), the loss of neurons due to programmed cell death (e.g. apoptosis) and impaired neuronal signal transduction in specific parts of the brain are major pathological features of AD(Reference Christen1). Therefore, we measured mRNA concentrations of genes involved in the formation (APP-binding family member 1, a disintegrin and metalloprotease domain 10, β-site APP-cleaving enzyme 1) or degradation (neprilysin, insulin-degrading enzyme, endothelin-converting enzyme) of amyloid-β, programmed cell death (Bcl-2, Bax) and neurotransmitter degradation (acetylcholinesterase) in the brains of our rats.
In the cortex and hippocampus of our rats, dietary VE did not affect mRNA concentrations of the measured genes (Table 4). Some of the analysed genes (e.g. acetylcholinesterase, β-site APP-cleaving enzyme 1)(Reference Tong, Zhou and Fung37, Reference Melo, Agostinho and Oliveira38) are thought to be regulated by oxidative stress, others experience age-related loss of activity and/or protein concentration (neprilysin, insulin-degrading enzyme)(Reference Caccamo, Oddo and Sugarman39, Reference Fukami, Watanabe and Iwata40). As an additional biomarker for oxidative stress, we determined haeme oxygenase type 1 (HO-1) mRNA concentrations in the brains of our rats. HO-1 is up-regulated in the situations of oxidative and nitrosative stress, presenting a cytoprotective effect against various forms of stress by releasing the vasoactive molecule carbon monoxide and the potent antioxidant bilirubin(Reference Calabrese, Scapagnini and Colombrita41). A significantly higher expression of HO-1 was observed in the neurons and astrocytes of AD hippocampus and cerebral cortex samples relative to age-matched controls(Reference Schipper, Cisse and Stopa42). Consistent with our F2-isoprostane data, mRNA concentrations of HO-1 were not affected by VE depletion or supplementation, indicating the absence of oxidative stress in the rat brains in the present study.
αTTP mRNA has been found in the human and rat brains, although at lower concentrations than in the liver(Reference Hosomi, Goto and Kondo43, Reference Copp, Wisniewski and Hentati44). The exact function of αTTP in the brain is not known, but it was suggested to function in maintaining αT concentrations in the brain(Reference Leonard, Terasawa and Farese45). In the present study, αTTP mRNA concentrations were not changed by VE depletion or by αT supplementation. Other groups, however, observed that VE deficiency led to transcriptional alterations in the cortex(Reference Hyland, Muller and Hayton46) and hippocampus(Reference Rota, Rimbach and Minihane47) of rats, as well as in αTTP− / − mice(Reference Gohil, Schock and Chakraborty48). Rota et al. (Reference Rota, Rimbach and Minihane47) have shown that certain genes related to the pathophysiology of neurodegenerative diseases were regulated in rat hippocampi by dietary VE. One main difference between these two studies is that we fed our rats for 6 months, while Rota et al. fed theirs for 9 months. In both studies, however, animals fed a VE-deficient diet had significantly reduced VE concentrations in the plasma and hippocampus, although the VE depletion of the brains remained incomplete, suggesting that the brain has a strong capability to retain VE. Additionally, the diets in our trial contained the synthetic antioxidant BHT to protect the diets from lipid peroxidation during storage and BHT might have exerted additional antioxidant effects in our rats by direct scavenging of reactive species and/or regenerating αT from its radical form(Reference Frank, Lundh and Parker49). However, with 10 mg/kg, the concentration of BHT in our diets was low compared with 2000–4000 mg/kg diet employed in the rat studies where antioxidant effects of BHT were observed(Reference Frank, Lundh and Parker50, Reference Kamal-Eldin, Frank and Razdan51). BHT is extensively metabolised in the liver, and both BHT and its metabolites are excreted with the bile, faeces and urine, which in turn results in a low rate of tissue accumulation of BHT(Reference Daniel and Gage52–Reference Conacher, Iverson and Lau54). Hence, potential antioxidant effects of BHT are likely to be more potent in the liver than in the extra-hepatic tissues. However, hepatic HO-1 mRNA concentrations were significantly higher in the dVE than in the sVE animals in conjunction with reduced micro-RNA expression(Reference Gaedicke, Zhang and Schmelzer55). Therefore, the absence of oxidative stress, as assessed by the concentrations of F2-isoprostanes and relative mRNA levels of HO-1, in the brains of our VE-depleted as well as αT-supplemented rats compared with control (sVE) is unlikely to be a result of the BHT in the diet.
Apoptosis seems to be an important process in AD, as suggested by increased levels of apoptosis markers in the brains of AD patients(Reference Honig and Rosenberg56). Bcl-2 and Bax, anti- and pro-apoptotic members, respectively, of the Bcl-2 family of signalling molecules, play a central role in apoptotic cell death. Both proteins function independently to regulate cell death, but can also form heterodimers. Therefore, the ratio of Bcl-2 to Bax is indicative of a pro- or anti-apoptotic cell status(Reference Lu, Moochhala and Kaur57). In the present study, neither Bcl-2 nor Bax mRNA (Table 4) and protein (Fig. 2) concentrations were different depending on the diet.
Conclusions
The present data suggest that stepwise reduction in dietary αT and γT for 6 months does not impair antioxidant status, induce apoptosis or alter the expression of selected genes in the brains of rats. Similarly, αT supplementation of already sVE animals does not improve antioxidant status, affect apoptosis or alter the expression of AD-relevant genes. In conclusion, VE does not appear to change the parameters involved in the aetiology and progression of AD in healthy animals. Furthermore, investigations into the impact of VE on these parameters in an AD disease model are warranted.
Acknowledgements
S. G. is an associated member of the PhD programme ‘Natural Antioxidants’ (GRK 820), supported by the German Research Foundation. G. R. was supported by a grant (no. 528/051) from the Union zur Foerderung von Oel-und Proteinpflanzen e.V.
G. R. designed the study. S. G. prepared the oils, conducted the feeding trial and determined antioxidant enzymes. S. G. and J. F. analysed tocopherols. X. Z. performed the western blot experiments. S. G., C. B.-S. and P. H. performed PCR analyses. I. W. and A. G. determined F2-isoprostanes. Y. L. and G. R. provided funding. S. G., J. F. and G. R. performed statistical calculations, and prepared and edited the manuscript. All authors reviewed the final manuscript. None of the authors had a known conflict of interest.








