As a free amino acid which can conjugate with bile acids, taurine (TAU) has been demonstrated to have a strong hypocholesterolaemic effect in mammals by stimulating the conversion of cholesterol to bile acids in the liver(Reference Chen, Guo and Chang1). The hypolipidaemic effect of TAU in terrestrial animals has also been widely reported(Reference Militante and Lombardini2), although lipid-increasing effects of TAU have been observed in some studies(Reference Kishida, Miyazato and Ogawa3,Reference Ebihara, Miyazato, Ogawa and Kishida4) .
In fish, TAU plays an unique role in bile acid conjugation and has been identified as an conditionally essential nutrient(Reference Salze and Davis5,6) . In marine teleosts, especially, the concentration of TAU is high. However, precise functions of TAU in the metabolism of cholesterol and lipids in fish remain unclear. The limited studies reported with fish suggested that although the promoting effects on bile acid synthesis were consistently observed(Reference Kim, Matsunari and Takeuchi7–Reference Yun, Ai and Mai9), the effects of TAU on cholesterol and lipid metabolism in fish were probably different from those observed in mammals and seemed more varied with fish species and fish tissue(Reference Han, Koshio and Jiang10–Reference Magalhães, Martins and Martins14). A recent study with juvenile totoaba showed that dietary TAU supplementation increased the concentrations of cholesterol and TAG in the plasma(Reference Satriyo, Galaviz and Salze13). However, a study with yellow catfish showed that the contents of cholesterol and TAG in the serum decreased with increasing dietary TAU levels(Reference Li, Lai and Li12), while another study with white seabream showed that dietary TAU supplementation reduced the TAG levels in the plasma but did not affect the cholesterol level(Reference Magalhães, Martins and Martins14). Different results have also been observed in studies with other fish species such as Japanese flounder and white grouper(Reference Han, Koshio and Jiang10,Reference Koven, Peduel and Gada11) . In Japanese flounder, neither the total cholesterol (TC) nor the TAG contents were affected by dietary TAU(Reference Han, Koshio and Jiang10). In white grouper, however, the response of lipid accumulation to dietary TAU differed among different tissues(Reference Koven, Peduel and Gada11). With increasing dietary TAU levels, the total lipids and their constituent fatty acids in the liver decreased, but the total lipids and the fatty acids of all lipid classes in the eyes increased, while the lipid stores in the muscle were not significantly affected. Considering the large discrepancy among these results, it is worthwhile to investigate the interaction among TAU, cholesterol and lipids in fish.
Regarding the mechanisms involved in TAU actions on bile acids, cholesterol and lipids, the mammal studies have well revealed that TAU stimulated the expression of cholesterol 7α-hydroxylase (CYP7A1)(Reference Ebihara, Miyazato, Ogawa and Kishida4,Reference Nishimura, Umeda and Oda15–Reference Murakami, Fujita and Nakamura18) , the rate-limiting enzyme for bile acid synthesis, and farnesoid X receptor (FXR (nuclear receptor subfamily 1, group H, member 4, NR1H4)) activation might be involved in this process(Reference Parks, Blanchard and Bledsoe19). Besides, regulation of lipoproteins and the LDL receptor contributes to the effects of TAU on cholesterol metabolism(Reference Kishida, Miyazato and Ogawa3,Reference Ebihara, Miyazato, Ogawa and Kishida4,Reference Murakami, Kondo and Toda20) . At the level of cellular signalling transduction, recent studies indicated that mitogen-activated protein kinase (MAPK) signalling pathways may play important roles in actions of TAU on the metabolism of bile acids and cholesterol(Reference Wang, Mei and Yuan21,Reference Guo, Gao and Cao22) . However, in fish, no information has been available regarding the mechanisms involved in TAU actions on bile acids, cholesterol and lipids. Studies with fish are evidently needed to investigate whether teleosts share the same mechanisms with mammals, especially considering that inconsistent apparent TAU effects have been observed between fish and mammals.
The present study was conducted with a marine teleost, tiger puffer. Tiger puffer is not only an important aquaculture species in Asia but also a good model marine fish due to the detailed genome information(Reference Fernandes, Mackenzie and Elgar23–Reference Wongwarangkana, Fujimori and Akiba26). What is more interesting about tiger puffer is that this fish has a unique lipid storage pattern. They have no intraperitoneal adipose tissue and a very low lipid content in the muscle and thus store lipids predominantly in the liver(Reference Kaneko, Yamada and Han27). Additionally, we are curious about whether this special lipid storage pattern would influence the TAU–lipid interaction. With a feeding trial on tiger puffer, the present study aimed to comprehensively assess the effects of dietary TAU supplementation on the metabolism of bile acids, cholesterol and lipids. The possible mechanisms indicated by mammal studies were also investigated in the present study at the gene transcription level. Results of the present study will not only be beneficial to better understanding the TAU–lipid interaction but also provide useful information about the regulation of lipid metabolism in fish that store lipids in the liver.
Methods
Experimental diets
Three experimental diets differing only in TAU content were used in the present study (Table 1). The control diet used a low-fishmeal formulation, containing a basic TAU level of 1·7 g/kg DM(Reference Salze and Davis5). Crystalline TAU (purity > 99 %; Shanghai Macklin Biochemical Co. Ltd) was supplemented into the control diet, replacing wheat meal, to obtain two treatment diets with a medium (8·2 g/kg, M-TAU) or high-TAU level (14·0 g/kg, H-TAU) (Table 2). The experimental diets were prepared following the routine procedures in our laboratory(Reference Xu, Mu and Zhang28).
Table 1. Formulation and proximate composition of the experimental diets (g/kg DM basis)
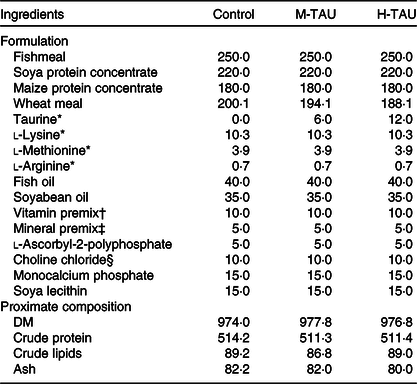
M-TAU, medium-taurine group; H-TAU, high-taurine group.
* Crystalline l-amino acids were purchased from Shanghai Macklin Biochemical Co. Ltd. Purity > 99 %.
† Vitamin premix (mg/kg diet): thiamin, 25 mg; riboflavin, 45 mg; pyridoxine HCl, 20 mg; vitamin B12, 0·1 mg; vitamin K3, 10 mg; inositol, 800 mg; pantothenic acid, 60 mg; niacin, 200 mg; folic acid, 20 mg; biotin, 1·2 mg; retinyl acetate, 32 mg; cholecalciferol, 5 mg; α-tocopherol, 120 mg; wheat middlings, 8·66 g.
‡ Mineral premix (mg or g/kg diet): MgSO4·7H2O, 1200 mg; CuSO4·5H2O, 10 mg; ZnSO4·H2O, 50 mg; FeSO4·H2O, 80 mg; MnSO4·H2O, 45 mg; CoCl2·6H2O (1 %), 50 mg; NaSeSO3·5H2O (1 %), 20 mg; Ca(IO3)2·6H2O (1 %), 60 mg; zoelite, 3·49 g.
§ Choline chloride, 35 % purity (with corncob as a carrier).
Table 2. Amino acid composition of experimental diets (g/kg DM)

M-TAU, medium-taurine group; H-TAU, high-taurine group.
Experimental fish and feeding procedure
Juvenile tiger puffer with an average initial body weight of 20·05 (sd 0·06) g was purchased from Tangshan Haidu Seafood Co. Ltd. The feeding trial was conducted in a flow-through seawater system in a local farm, Huanghai Aquaculture Co. Ltd. Before the start of the feeding trial, experimental fish were reared in polyethylene tanks and fed the control diet for 7 d to acclimate to the experimental conditions. At the beginning of the feeding trial, experimental fish were distributed into nine polyethylene tanks (200 litres) and each diet was randomly assigned to triplicate tanks (thirty fish in each tank). Fish were hand-fed to apparent satiation three times daily (08.00, 14.00 and 20.00 hours). The feeding trial lasted for 8 weeks. During the experiment, the water temperature ranged from 22 to 27 °C; salinity, 24–26 and dissolved O2, >6 mg/l. The tanks were cleaned by syphoning out residual feed and faeces 2 h after each feeding.
At the end of the feeding trial, after being fasted for 12 h, fish were anaesthetised with eugenol (1:10 000) and then the number and weight of fish in all tanks were recorded. After that, three randomly selected whole fish were collected from each tank for the analysis of proximate composition. Ten more randomly selected fish per tank were dissected to collect the samples of serum, liver and muscle for other assays. Gallbladder samples were not collected. Blood was collected from the caudal vein and allowed to clot first at room temperature for 2 h and then at 4 °C for 6 h. After that, centrifugation (836 g, 10 min, 4 °C) was conducted and the straw-coloured supernatants were collected as serum samples. After dissection, four small pieces of liver tissue (the small tip part) and two pieces of muscle tissue (dorsal muscle, about 3 cm × 1·5 cm) were collected for subsequent potential use. Faeces samples were not successfully collected because the faeces were not shaped, but easily diffused into water. All tissue samples were frozen with liquid N2 immediately and then stored at −86 °C before use. All sampling protocols, as well as fish rearing practices, were reviewed and approved by the Animal Care and Use Committee of Yellow Sea Fisheries Research Institute.
Analysis of proximate composition, lipid content, amino acids and fatty acids
The proximate composition analysis of experimental diets (triplicate assays for each diet) and whole fish (three individual fish per tank) was performed according to the standard methods of Association of Official Analytical Chemists. Briefly, samples of diets and fish were oven-dried at 105 °C to constant weight for moisture assay. Protein was determined by measuring N (N × 6·25) using the Kjeldahl method, lipids by diethyl ether extraction using the Soxhlet method and ash by combustion at 550°C. The lipid content of fish liver was assayed with the Soxhlet method, but the lipids in muscle were extracted and analysed with the chloroform–methanol method according to Folch et al. (Reference Folch, Lees and Sloane Stanley29).
Compositions of amino acids and TAU in the diets, as well as the TAU content in the liver and serum, were determined using an automatic amino acid analyser (L-8900; Hitachi High-Technologies Corp.). The fatty acid compositions of fish liver (a pooled sample for each tank with three samples from different fish) were analysed with GC (HP6890; Agilent Technologies Inc.) according to the methods described in our previous studies(Reference Xu, Cao and Wei30).
Analysis of TAG, cholesterol, NEFA, and total bile acids, and lecithin cholesterol acyl transferase concentration
All these parameters were assayed using commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions. An ELISA kit was used in the assay of hepatic lecithin cholesterol acyl transferase (LCAT) concentration. Tissue samples were homogenised in normal saline (v/w = 9:1) and centrifuged (694 g, for 20 min), and then the supernatant was collected for following assays. Competition method was used in the ELISA assay. Briefly, antigen in samples interacts with antibodies pre-coated on the well in competition with recognition antigen labelled with horse radish peroxidase. Tetramethylbenzidine was used to react with horse radish peroxidase to generate coloured solution for spectrophotometry. Zebrafish (Danio rerio) anti-rabbit polyclonal antibodies were used in the assays. Standard curves were plotted based on optical density of standard assays (ELISAcalc software; logistic model), and experimental samples were assayed at the same time. The inter- and intra-assay CV for the assays were <10 and <13 %, respectively. Pooled serum or liver samples with three samples from different fish of each tank were used for these assays.
Quantitative analysis of hepatic lipidomics and bile acid omics
The quantitative analysis of hepatic lipidomics and bile acid omics was analysed in collaboration with Shanghai Biotree Biotech Co. Ltd. Ultra-high-performance liquid tandem chromatography quadrupole time-of-flight MS was used in the lipidomics analysis according to Tu et al. (Reference Tu, Yin and Xu31). High-throughput target-based ultra-high-performance liquid tandem chromatography-MS/MS was used in the bile acid omics analysis according to Han et al. (Reference Han, Liu and Wang32). A pooled liver sample for each tank with three samples from different fish was used for these analyses (three replicates for each dietary treatment). The detailed procedures of these analyses can be found in the online Supplementary material.
Quantitative real-time PCR analysis
Total RNA in the liver samples (pools of five samples from different fish of each tank) was extracted using RNAiso Plus (TaKaRa) and reverse-transcribed with a PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa) according to the user’s manual.
Specific primers for target genes and the reference genes (18SRNA and β-actin) were designed based on the sequences available in the GenBank database (Table 3). The amplification efficiency for all primers, which was estimated by standard curves based on a six-step 4-fold dilution series of target template, was within 95–105 %, and the coefficients of linear regression (R 2) were more than 0·99. SYBR Green Real-time PCR Master Mix (TaKaRa) and a quantitative thermal cycler (Roche LightCycler 96) were used for the real-time quantitative PCR. The detailed programme was similar to those described by Xu et al.(Reference Xu, Dong, Ai and Mai33). The mRNA expression levels were calculated with the quantitative real-time-PCR method: 2−ΔΔCT(Reference Livak and Schmittgen34).
Table 3. Sequences of the primers used in this work

Tm, melting temperature; PL, product length; cyp7a1, cholesterol 7α-hydroxylase; F, forward; R, reverse; hmgcr, 3-hydroxy-3-methylglutaryl-CoA reductase; abcg, ATP-binding cassette subfamily G; fxr, farnesoid X receptor (nuclear receptor subfamily 1, group H, member 4, nr1h4); lxra, liver X receptor alpha (nuclear receptor subfamily 1, group H, member 3, nr1h3); hnf4a, hepatocyte nuclear factor 4, alpha; lrh-1, liver receptor homolog-1 (nuclear receptor subfamily 5, group A, member 2, nr5a2); mttp, microsomal TAG transfer protein; ldlr, LDL receptor; scarb1, scavenger receptor class B, member 1; hdlbp, HDL-binding protein; fas, fatty acid synthase; cpt-1, carnitine palmitoyltransferase-1; srebf1, sterol regulatory element-binding factor 1; bsal, bile salt-activated lipase-like; mek1, mitogen-activated protein kinase kinase 1 (map2k1); mek2, mitogen-activated protein kinase kinase 2 (map2k2); erk2, mitogen-activated protein kinase 1 (mapk1); jnk1, mitogen-activated protein kinase 8 (mapk8); jnk2, mitogen-activated protein kinase 9 (mapk9); c-jun, c-Jun protein.
Statistical methods
All percentage data were arcsine-transformed before analysis. All data were subjected to one-way ANOVA in SPSS 16.0 for Windows. Tukey’s multiple range test was used to detect the significant differences between the means. The significance level was P < 0·05. The results are presented as mean values of triplicate tanks with their pooled standard errors.
Results
Lipids, taurine and fatty acid compositions in fish
No significant (P > 0·05) difference was observed either in the lipid content of liver and muscle or in the proximate composition of whole fish (Table 4). TAU accumulation in the liver and serum significantly (P < 0·05) increased with increasing dietary TAU levels. No significant (P > 0·05) difference was observed in the liver fatty acid composition among dietary groups (online Supplementary Table S1).
Table 4. Proximate composition of whole fish, lipid contents in fish tissues and taurine accumulation in fish tissues
(Mean values with their standard errors, n 3)

M-TAU, medium-taurine group; H-TAU, high-taurine group.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
In addition, fish eat and grew normally during the feeding trial. The average final body weight and average feed efficiency ratio were 55·9 g and 0·7, respectively. Group H-TAU showed the best growth performance, and the control group showed the worst.
TAG, cholesterol and total bile acid contents in the liver and serum
For the liver, the TC content in group H-TAU was significantly (P < 0·05) higher compared with groups Control and M-TAU (Table 5). The HDL-cholesterol content in group H-TAU was significantly (P < 0·05) higher than that in the control group, and group M-TAU showed an intermediate value. The hepatic total bile acid (TBA) content showed a similar trend to HDL-cholesterol in response to dietary TAU.
Table 5. TAG, cholesterol and total bile acid (TBA) contents in the liver and serum of tiger puffer fed experimental diets
(Mean values with their standard errors, n 3)

M-TAU, medium-taurine group; H-TAU, high-taurine group; TC, total cholesterol.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
The TC and HDL-cholesterol contents in the serum showed similar trends to their counterparts in the liver in response to dietary TAU, that is, significantly (P < 0·05) higher in group H-TAU compared with the control group. However, the TBA content in serum showed an opposite trend to liver TBA content in response to dietary TAU. The TBA content in the serum significantly (P < 0·05) decreased with increasing dietary TAU levels.
Hepatic lipidomics
Raw data were first normalised with respect to total ion current(Reference Dunn, Broadhurst and Begley35). The results from positive and negative ion modes were combined. After management of raw data, a total of 690 peaks were extracted, successfully identified and quantified. However, only ten, six and twelve individual lipids showed significant difference in concentration between M-TAU and control, H-TAU and control, and H-TAU and M-TAU, respectively (online Supplementary Table S2, Fig. 1). Generally, dietary supplementation of TAU decreased the contents of a series of phospholipids but increased those of several TAG. Group H-TAU had higher contents of ceramides than groups control and M-TAU.

Fig. 1. Heatmap of lipids with significantly different concentrations between the control and medium-taurine (M-TAU) groups (A), the control and high-taurine (H-TAU) groups (B), and the M-TAU and H-TAU groups (C). PC, phosphatidylcholine; PE, phosphatidylethanolamine; Cer, ceramide.
Hepatic bile acid profiles
While forty-one bile acids (see the list in online Supplementary Table S3) can be quantitatively analysed with the method used in the present study, only three conjugated bile acids, taurocholic acid, taurochenodeoxycholic acid and taurodeoxycholic acid, had concentrations above the lowest limit of quantitation in the present analysis. Trace amount of cholic acid can be detected in some samples, but not in all samples. The bile acid profile of the liver was averagely included 94·48 % taurocholic acid, 4·17 % taurochenodeoxycholic acid and 1·35 % taurodeoxycholic acid. The concentration of these three conjugated bile acids was significantly (P < 0·05) increased by dietary TAU supplementation (Table 6).
Table 6. Hepatic bile acid profile of tiger puffer fed experimental diets (nmol/g wet liver tissue)
(Mean values with their standard errors, n 3)

M-TAU, medium-taurine group; H-TAU, high-taurine group.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Hepatic relative mRNA expression
Regarding the expression of genes related to bile acid and cholesterol metabolism, the hepatic relative mRNA expression of cyp7a1, hmgcr, abcg5, abcg8 and fxr significantly (P < 0·05) increased with increasing dietary TAU levels (Fig. 2). All these genes have the lowest expression in the control group and the highest expression in the group H-TAU.

Fig. 2. Relative mRNA expression of genes related to bile acid and cholesterol metabolism in the liver of experimental fish. a,b Mean values for each gene with unlike letters were significantly different (P < 0·05).  , Control group;
, Control group;  , medium-taurine group;
, medium-taurine group;  , high-taurine group. cyp7a1, Cholesterol 7α-hydroxylase; hmgcr, 3-hydroxy-3-methylglutaryl-CoA reductase; abcg, ATP-binding cassette subfamily G; fxr, farnesoid X receptor; lxra, liver X receptor alpha; hnf4a, hepatocyte nuclear factor 4, alpha; lrh-1, liver receptor homolog-1.
, high-taurine group. cyp7a1, Cholesterol 7α-hydroxylase; hmgcr, 3-hydroxy-3-methylglutaryl-CoA reductase; abcg, ATP-binding cassette subfamily G; fxr, farnesoid X receptor; lxra, liver X receptor alpha; hnf4a, hepatocyte nuclear factor 4, alpha; lrh-1, liver receptor homolog-1.
The hepatic mRNA expression of lipoproteins, ApoA1 and ApoA4, also significantly (P < 0·05) increased with increasing dietary TAU levels (Fig. 3), while other lipoprotein-related genes, apob100, mttp, apoe1, ldlr, scarb1 and hdlbp, showed no significant difference in hepatic gene expression among dietary groups.

Fig. 3. Relative mRNA expression of lipoprotein-related genes in the liver of experimental fish. a,b Mean values for each gene with unlike letters were significantly different (P < 0·05).  , Control group;
, Control group;  , medium-taurine group;
, medium-taurine group;  , high-taurine group. mttp, Microsomal TAG transfer protein; ldlr, LDL receptor; scarb1, scavenger receptor class B, member 1; hdlbp, HDL-binding protein.
, high-taurine group. mttp, Microsomal TAG transfer protein; ldlr, LDL receptor; scarb1, scavenger receptor class B, member 1; hdlbp, HDL-binding protein.
Regarding the lipid metabolism-related genes, group H-TAU showed significantly (P < 0·05) higher hepatic gene expression of fatty acid synthase (fas) than the control group, as well as significantly (P < 0·05) higher gene expression of pparγ and bile salt-activated lipase (bsal) than groups control and M-TAU (Fig. 4).

Fig. 4. Relative mRNA expression of lipid metabolism-related in the liver of experimental fish. a,b Mean values for each gene with unlike letters were significantly different (P < 0·05).  , Control group;
, Control group;  , medium-taurine group;
, medium-taurine group;  , high-taurine group. fas, Fatty acid synthase; cpt-1, carnitine palmitoyltransferase-1; srebf1, sterol regulatory element-binding factor 1; bsal, bile salt-activated lipase-like.
, high-taurine group. fas, Fatty acid synthase; cpt-1, carnitine palmitoyltransferase-1; srebf1, sterol regulatory element-binding factor 1; bsal, bile salt-activated lipase-like.
Regarding the key proteins in MAPK signalling pathways, the gene expression of erk2 and jnk2 significantly (P < 0·05) increased with increasing dietary TAU levels, while mek1 had significantly (P < 0·05) higher gene expression in group H-TAU than in groups control and M-TAU (Fig. 5).

Fig. 5. Relative mRNA expression of key proteins in mitogen-activated protein kinase (MAPK) signalling pathways in the liver of experimental fish. a,b Mean values for each gene with unlike letters were significantly different (P < 0·05).  , Control group;
, Control group;  , medium-taurine group;
, medium-taurine group;  , high-taurine group. mek1, Mitogen-activated protein kinase kinase 1 (map2k1); mek2, mitogen-activated protein kinase kinase 2 (map2k2); erk2, mitogen-activated protein kinase 1 (mapk1); jnk1, mitogen-activated protein kinase 8 (mapk8); jnk2, mitogen-activated protein kinase 9 (mapk9); c-jun, c-Jun protein.
, high-taurine group. mek1, Mitogen-activated protein kinase kinase 1 (map2k1); mek2, mitogen-activated protein kinase kinase 2 (map2k2); erk2, mitogen-activated protein kinase 1 (mapk1); jnk1, mitogen-activated protein kinase 8 (mapk8); jnk2, mitogen-activated protein kinase 9 (mapk9); c-jun, c-Jun protein.
Hepatic concentration of lecithin cholesterol acyl transferase
The hepatic concentration of LCAT assayed by the ELISA method was significantly (P < 0·05) higher in group H-TAU compared with groups control and M-TAU, and no significant difference was observed between the latter two groups (Fig. 6).

Fig. 6. Concentration of lecithin cholesterol acyl transferase in the liver of experimental fish, assayed with the ELISA method. a,b Mean values with unlike letters were significantly different (P < 0·05). M-TAU, medium-taurine group; H-TAU, high-taurine group.
Discussion
As expected, in the present study, dietary TAU supplementation stimulated the hepatic bile acid biosynthesis in tiger puffer, which was evidenced by both the increased hepatic bile acid contents and the up-regulated hepatic gene expression of CYP7A1, the rate-limiting enzyme for bile acid synthesis(Reference Chiang36). The up-regulated gene expression of ATP-binding cassette subfamily G5 and ATP-binding cassette subfamily G 8, which play essential roles in the selective sterol excretion by the liver into bile(Reference Yu, Hammer and Li-Hawkins37,Reference Wang, Mitsche and Lütjohann38) , by TAU supplementation provided additional evidences. These results were in good accordance with other fish studies with species such as Japanese flounder(Reference Kim, Matsunari and Takeuchi7), red sea bream(Reference Takagi, Murata and Goto8) and turbot(Reference Yun, Ai and Mai9). These studies have demonstrated that TAU can stimulate the biosynthesis and excretion of bile acids, and this stimulation could be due to the roles of TAU in conjugating with bile acids. In teleosts, virtually all described bile acid conjugations occur with TAU(Reference Hofmann, Hagey and Krasowski39). For example, in the marine teleost Japanese flounder, TAU has been found to be the major compound to conjugate with cholesterol derivatives in the liver to produce bile salts(Reference Kim, Matsunari and Takeuchi7). Similarly, for tiger puffer in the present study, TAU, which was well accumulated in the liver, rather than glycine was the only compound to conjugate with bile acids at the current detection level. Only three conjugated bile acids, taurocholic acid, taurochenodeoxycholic acid and taurodeoxycholic acid, was detected in the present study. This result was similar to most species of aquaculture interest, which only secrete C24 bile acids, cholic acid and chenodeoxycholic acid, although noteworthy exceptions exist in sturgeons, paddlefish, cyprinids, gilthead sea bream and red sea bream(Reference Hofmann, Hagey and Krasowski39).
In contrast with the hepatic bile acid content, the TBA concentration in the serum decreased with increasing dietary TAU levels. The enterohepatic circulation of bile acids has been well known(Reference Hofmann, Hagey and Krasowski39,Reference Dawson and Said40) . It has been demonstrated that most bile acids are re-absorbed in distal ileum, and the re-absorbed bile acids are then routed to the liver where they will be recycled to the gallbladder. Studies with rats have showed that TAU inhibited the re-absorption of bile acids from the distal ileum(Reference Chen, Nishimura and Oda41–Reference Nishimura, Yamamoto and Ota43). Same mechanisms could be used to explain the reduction of serum bile acids by TAU in the present study.
Different from the bile acid results which showed good consistency between fish and mammals, the effects of TAU supplementation on cholesterol content in tiger puffer seemed different from those observed in mammals. The hypocholesterolaemic effect of TAU has been consistently observed in many independent experiments performed on rat and mouse with exogenous hypercholesterolaemia caused by high-cholesterol–sodium cholate loading diet(Reference Chen, Guo and Chang1). However, in the present study, the TC content in both liver and serum of tiger puffer was increased by TAU. Meanwhile, this was the first time in fish observing the stimulating effect of TAU on hepatic gene expression of 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis. Since normal or even low-cholesterol diets were used in the present study, the difference in abundance of dietary cholesterol could be a factor resulting in different results regarding effects of dietary TAU on cholesterol contents. In a study with turbot using exogenous cholesterol supplementation, when 1·0 % exogenous cholesterol was added, TAU supplementation decreased the serum TC concentration(Reference Yun, Ai and Mai9). However, another fish study with juvenile totoaba-fed normal diets showed that plasma TC content increased linearly with dietary TAU (R 2 0·75, P < 0·001)(Reference Satriyo, Galaviz and Salze13), which was similar to the present study. Nevertheless, the effects of TAU on cholesterol content in fish studies seemed much varied. A study with yellow catfish-fed low-cholesterol diets showed that serum TC content decreased with increasing dietary TAU levels(Reference Li, Lai and Li12), whereas the study with white seabream-fed low-cholesterol diets showed that the plasma TC concentration was not affected by dietary TAU supplementation(Reference Magalhães, Martins and Martins14). Protein sources, especially soya products, have been reported to interact with cholesterol in the intestine(Reference Anderson and Bush44–Reference Murashita, Akimoto and Iwashita48), whereas most TAU studies with fish used high levels of plant protein sources in the diets. The difference in protein source profile may contribute to the varied results among different fish studies. Another probable explanation of the inconsistent results among fish studies was that the effects of TAU on cholesterol metabolism in fish could be species-specific. Similar to tiger puffer, totoaba also have low lipid contents in the muscle and viscera, relatively high-lipid level in the liver and relatively low lipid requirement(Reference Rueda-López, Lazo and Reyes49–Reference Barreto-Curiel, Focken and D’Abramo52). Whether these characteristics are associated with the stimulation of cholesterol synthesis by TAU warrants further studies.
Besides the TC contents, the HDL-cholesterol contents in both liver and serum were also elevated by the TAU supplementation. Elevation of HDL-cholesterol by TAU has been widely observed in mammal studies(Reference Mochizuki, Oda and Yokogoshi53–Reference Chen, Suruga and Nishimura57), and in most of these studies, the elevation of HDL-cholesterol was concomitant with the decrease of serum TC. HDL particles have been demonstrated to be able to transport cholesterol to liver for bile acid synthesis and thus reduce its accumulation in blood. In the present study, in accordance with the increase of HDL-cholesterol, the hepatic transcription of ApoA1 and the hepatic LCAT concentration were increased by dietary TAU. ApoA1 is the major apo of plasma HDL(Reference Chapman58). The increase of HDL-cholesterol by TAU may be initiated from the stimulation of ApoA1 expression. Required for remodelling HDL particles into their spherical forms(Reference Clay, Pyle and Rye59), LCAT is a central enzyme synthesised mainly in the liver and functioning on the surface of HDL(Reference Kosek, Durbin and Jonas60,Reference Hirsch-Reinshagen, Donkin and Stukas61) , where it converts cholesterol and phosphatidylcholines to cholesteryl esters and lysophosphatidylcholines, for the backward transport of cholesterol ester to the liver. The up-regulation of hepatic LCAT by dietary TAU evidenced the up-regulation of serum cholesterol removal. The still increase of serum TC by dietary TAU suggested that the removing effects of TAU on serum cholesterol may be masked by the stimulation of cholesterol biosynthesis per se by TAU.
Regarding LDL-cholesterol, in the present study, neither the LDL-cholesterol content in serum and liver nor the hepatic transcription of LDL receptor, which removes VLDL and LDL from circulation via binding to ApoB100 and ApoE moieties on them, was affected by dietary TAU supplementation. The hepatic mRNA expression of ApoB100 and ApoE1, as well as microsomal triglyceride TAG transfer protein, which is required for the secretion of plasma lipoproteins that contain ApoB(Reference Walsh, Iqbal and Josekutty62), was also not affected by TAU. These results were obviously different from what observed in mammal studies. In mammal studies, it has been reported that TAU lowers cholesterol concentration by reducing ApoB and VLDL secretion from the liver and improves cholesterol clearance from the circulation by up-regulating LDL receptor-binding capacity led to increase of LDL uptake(Reference Kishida, Miyazato and Ogawa3,Reference Ebihara, Miyazato, Ogawa and Kishida4,Reference Murakami, Kondo and Toda20,Reference Murakami, Kondo and Nagate56,Reference Chang, Chou and Chiu63) . The decrease of plasma LDL-cholesterol has also been observed in a study with turbot, but in that study a high-cholesterol level was used in the diet(Reference Yun, Ai and Mai9). The non-response of LDL to dietary TAU in the present study could be related to the significant stimulation of cholesterol biosynthesis. The necessity of transport of excess cholesterol out of liver via LDL may counteract the opposite transportation effects of TAU. Another possibility was that TAU does not affect LDL metabolism at all in fish species that store lipids in the liver.
Regarding the effects of dietary TAU supplementation on lipid accumulation, neither the lipid content in the whole body, liver and muscle, nor the TAG content in the serum and liver was affected by dietary TAU supplementation. Different from the mammal studies, most of which extensively demonstrated the hypolipidaemic effects of TAU(Reference Militante and Lombardini2,Reference Murakami64–Reference Chen, Guo and Zhang66) , the relevant fish studies showed various results regarding the effects of TAU on lipid accumulation.
In the study with totoaba, plasma TAG increased quadratically (R 2 0·53, P < 0·001) with dietary TAU(Reference Satriyo, Galaviz and Salze13), but in the studies with white seabream and yellow catfish, dietary TAU supplementation decreased the plasma TAG level(Reference Magalhães, Martins and Martins14), while the blood TAG of Japanese flounder was not affected by dietary TAU(Reference Han, Koshio and Jiang10). In white grouper, TAU supplementation decreased the livers’ total lipids but appeared to have little effect on lipid stores in the muscle(Reference Koven, Peduel and Gada11). With respect to body lipid content, while there is a general trend of decreasing body lipid content in response to limiting levels of dietary TAU(Reference Yun, Ai and Mai9,Reference Espe, Ruohonen and El-Mowafi67–Reference Salze, Spangler and Cobine70) , the opposite trend or lack of effect has also been observed in different species such as Atlantic salmon and sable fish(Reference Espe, Ruohonen and El-Mowafi67,Reference Espe, Ruohonen and El-Mowafi71,Reference Johnson, Kim and Watson72) . Evidence also suggests differences in the lipid-regulating effects of TAU depending on fish size(Reference Qi, Ai and Mai69). These results strongly suggested that the effects of TAU on lipid accumulation in fish could vary with many factors such as fish species, fish size, tissue type and dietary formulation.
In the present study, in spite of being not effective in regulating lipid contents, TAU supplementation increased the hepatic transcription of lipogenic genes fas and pparγ, indicating the potential of TAU in accelerating lipogenesis. The lipidomics results also showed that TAU up-regulated the concentration of many TAG. In addition, the transcription of bile salt-activated lipase-like, which is a major lipase in fish, and that of ApoA4, which acts primarily in intestinal lipid absorption, were up-regulated by the TAU supplementation, indicating the potential of TAU in facilitating lipid digestion. The biological activities of TAU in facilitating lipid emulsion and absorption via bile salts have been well documented(Reference Huxtable73–Reference Richard, Colen and Aragão75). The increase of lipid content in tiger puffer by TAU supplementation may appear after a longer feeding period. Moreover, the present results indicated again the discrepancy in lipid-regulating effect of TAU between high-fat mammal models and fish-fed normal diets. Besides lipogenesis, this discrepancy is also observed in fatty acid β-oxidation and energy expenditure. In mammals, increasing fatty acid β-oxidation and energy expenditure in adipose tissues is one of the mechanisms underlying the TAG ameliorating and anti-obesity effects of TAU(Reference Murakami64,Reference Puigserver, Wu and Park76,Reference Fukuda, Yoshitama and Sugita77) . However, in the present study, the transcription of cpt-1 and pparα2, which play important roles in fatty acid β-oxidation, was not affected by dietary TAU.
Additionally, the lipidomics results showed that on the contrary to TAG, however, the hepatic abundance of several phosphatidylcholines and phosphatidylethanolamines, including some lysophospholipids, was down-regulated by dietary TAU supplementation. The decrease in phospholipids by TAU could be related to the increase of HDL-cholesterol. It was possible that more hepatic phospholipids were transported to blood to facilitate the function of HDL. However, it was difficult to explain the regulation of each individual phospholipid by TAU based on our current knowledge. These phospholipids regulated by TAU may be preferences of cholesterol metabolism on the surface of HDL.
Moreover, from the lipidomics results, we can see that the concentration of ceramide, Cer(t18:1/24:4) and Cer(t15:0/22:0), was higher in the high-TAU group compared with other groups. It has been well known that TAU has considerable accumulation in animal brain(Reference Kim, Matsunari and Takeuchi7,Reference Pasantes-Morales, Chatagner and Mandel78) and can serve as neurotransmitter and neuromodulator(Reference Huxtable73,Reference Kuriyama79) . The present result indicated that Cer(t18:1/24:4) and Cer(t15:0/22:0) might have important roles in neuromodulation of tiger puffer.
The mechanisms involved in the regulation of bile acids by TAU have been investigated in a number of previous studies(Reference Chen, Guo and Chang1,Reference Hoang, Jia and Jun80) . In the promoters of mammal CYP7A1 genes, binding sites for transcription factors such as liver X receptor α and hepatocyte nuclear factor 4, α, and liver receptor homolog-1 have been observed and these transcription factors have been reported to be important regulators of CYP7A1 transcription(Reference Lehmann, Kliewer and Moore81–Reference Chiang, Kimmel and Stroup84). However, in the present study, transcription of all these transcription factors was not affected by dietary TAU in spite of the increase of CYP7A1 transcription. The promoter sequence of tiger puffer CYP7A1 has not been available. Differences in binding sites for the transcription factors mentioned above probably exist between tiger puffer and mammals.
However, the present results showed that the transcription of FXR, which is a bile acid receptor and plays a critical role in the regulation of bile acid synthesis and homoeostasis, was significantly increased by dietary TAU. FXR acts as a negative regulator of CYP7A1. Stimulation of FXR transcription by dietary TAU reflected feedback regulation of CYP7A1 by increased hepatic bile acid pool. In C57BL/6 mice, however, Lam et al. (Reference Lam, Chen and Suruga42) reported that TAU may interrupt the activation of FXR via some unknown pathways. Mammal studies showed that bile acids such as lithocholic acid, chenodeoxycholic acid and deoxycholic acid are potential ligands that activate FXR(Reference Lehmann, Kliewer and Moore81–Reference Miyazaki, Honda and Matsuzaki85). However, it remains unclear which bile acid is effective ligand of FXR in tiger puffer.
Regarding the hepatic receptors of lipoproteins, besides LDL receptor, scavenger receptor class B, member 1 and HDL-binding protein, which are potential receptors of HDL, were also analysed. The function of scavenger receptor class B, member 1 and HDL-binding protein has not been well understood. The significant effects of TAU on HDL-cholesterol content but not on transcription of scarb1 and hdlbp in the present study indicated that there might be no high-affinity interaction between HDL and these receptors.
Regarding the cellular signalling transduction related to TAU function, very little information has been available. It has been reported that TAU stimulated alkaline phosphatase activity and collagen synthesis in cultured osteoblasts via activation of the extracellular signal-regulated kinase (ERK) pathway(Reference Park, Kim and Kim86,Reference Yuan, Lu and Luo87) . A study with HepG2 cell showed that TAU could enhance cyp7a1 expression by inducing hnf4α and inhibiting mek1/2 and p-c-jun expression(Reference Guo, Gao and Cao22). In the present study, transcription of mek1, erk2 and jnk2 was up-regulated by dietary TAU. As the first time in fish, this result preliminarily indicated that the MAPK signalling pathway might be involved in the exertion of TAU function. Since the MAPK signalling pathway is involved in various physiological processes, future studies are needed to elucidate its precise role in regulating the effects of TAU on the metabolisms of bile acids, cholesterol and lipids.
In conclusion, dietary TAU supplementation stimulated the hepatic biosynthesis of both bile acids and cholesterol in tiger puffer and increased the HDL-cholesterol content in both liver and serum. TAU tended to increase the contents of some individual TAG and ceramides but decrease the contents of individual phosphatidylcholines and phosphatidylethanolamines. Taurocholic acid and taurochenodeoxycholic acid were the major bile acids in the liver of tiger puffer. It was possible that FXR, but not liver X receptor α, hepatocyte nuclear factor 4, α, and liver receptor homolog-1, was involved in the regulation of CYP7A1 by TAU and that the MAPK signalling pathway was involved in the exertion of TAU functions. As the state of knowledge about TAU physiology in fish remains fragmented and limited, additional research is evidently necessary to elucidate the mechanisms by which TAU exerts its functions.
Acknowledgements
The authors thank Yingming Yang for his help in fish rearing.
This work was supported by National Key R&D Program of China (2018YFD0900400), Central Public-Interest Scientific Institution Basal Research Fund (2018HY-ZD0505), China Agriculture Research System (CARS-47-G15) and National Natural Science Foundation of China (31772862).
H. X. and M. L. designed this research. Q. Z. and Z. L. conducted the feeding trial. Q. Z. and Y. W. analysed the fish proximate composition and amino acid contents. H. X., Q. Z., B. S. and L. J. analysed other parameters and performed statistical analysis. H. X., S.-K. K. and S. C. wrote the manuscript. All authors read and approved the final manuscript. H. X. and M. L. had primary responsibility for final content.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520000161)















