Obesity is a major health problem worldwide due to an increased risk for developing CVD, type 2 diabetes mellitus and hypertension(Reference Samartin and Chandra1). In the USA, analysis of data from the 2009–2010 National Health and Nutrition Examination Survey has shown obesity rates for adult men and women at 35·5 and 35·8 %, respectively, and 16·9 % for children and adolescents(Reference Flegal, Carroll and Kit2, Reference Ogden, Carroll and Kit3). In Europe, obesity rates varied in different countries. Adults in Italy, Spain, Portugal, Poland, Romania, Albania and the Czech Republic had the highest obesity rates at >25 %(Reference Berghöfer, Pischon and Reinhold4). Western and northern European countries tended to have lower obesity rates at 10–20 %. However, a trend analysis using data from the Diet, Obesity and Genes (DiOGenes) cohort that included subjects from Italy, the UK, The Netherlands, Germany and Denmark has predicted that the rate of obesity would rise significantly in these countries by 2015(Reference Saris and Harper5, Reference von Ruesten, Steffen and Floegel6).
Multiple studies have indicated that obesity is associated with an increased risk of developing infections, and greater susceptibility to infections has been linked to obesity-induced impairment of immune function(Reference Kanneganti and Dixit7, Reference Milner and Beck8). In animal studies using a diet-induced obesity model, obese mice have been shown to have an increased susceptibility to influenza virus infection compared with lean mice(Reference Smith, Sheridan and Harp9, Reference Karlsson, Sheridan and Beck10). Defects in natural killer cell, CD8+ lymphocyte and dendritic cell functions were observed in diet-induced obese mice that may have accounted for the higher infection rate(Reference Karlsson, Sheridan and Beck10, Reference Smith, Sheridan and Tseng11). In human subjects, higher rates of morbidity and mortality in the obese have been documented in multiple countries during the 2009 H1N1 influenza virus pandemic compared with normal-weight individuals(Reference Van Kerkhove, Vandemaele and Shinde12). Furthermore, the relationship between BMI and influenza infections was surveyed over twelve seasonal flu periods in Canada(Reference Kwong, Campitelli and Rosella13). In this survey, it has been reported that the obese had a greater risk of hospitalisation for respiratory complications during the flu season than the normal-weight population. A higher incidence of nosocomial and post-surgery infections has been reported in the obese compared with normal-weight populations(Reference Falagas and Kompoti14). Hospitalised obese patients, especially the critically ill, have a higher incidence of bloodstream, urinary tract and respiratory infections(Reference Bercault, Boulain and Kuteifan15–Reference Serrano, Khuder and Fath18). Obesity increases the risk of infection after cardiac and vascular, orthopaedic and gastric surgeries(Reference Falagas and Kompoti14, Reference Cantürk, Cantürk and Çetinarslan19–Reference Davenport, Xenos and Hosokawa21).
Innate immunity is affected by obesity, as is adaptive immunity. The innate immune system consists of first-line defenders against pathogens, including monocytes, neutrophils, dendritic cells and natural killer lymphocytes, and the adaptive immune system is composed of different subsets of T- and B-lymphocytes that promote prolonged antigen-specific responses to pathogenic organisms(Reference Dempsey, Vaidya and Cheng22). Previously, we evaluated the effects of dietary strawberries on systemic markers of inflammation in healthy obese subjects, such as the acute-phase proteins C-reactive protein and serum amyloid A, and the pro-inflammatory cytokines IL-6, IL-1β, IL-8 and TNF-α(Reference Zunino, Parelman and Freytag23). No differences in the levels of these circulating markers were found in the blood of the volunteers who consumed strawberry powder or strawberry-flavoured placebo preparations. There are limited data on the effects of dietary strawberries on the function of immune cells. Strawberries contain phytochemicals including the flavonoids catechin, several anthocyanins, quercetin and kaempferol that have shown anti-inflammatory activities(Reference Hannum24). The present analyses were aimed at determining whether or not dietary strawberries could influence the function of specific cell types of the immune system, and thereby have the potential to modulate the risk of infection in the obese population.
Methods
Subject recruitment and study design
The present study was conducted according to the guidelines described in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Institutional Review Board at the University of California Davis. Written informed consent was obtained from all subjects. The study design and subject recruitment was described previously(Reference Zunino, Parelman and Freytag23). Briefly, twenty human volunteers, both male (n 7) and female (n 13), with a BMI in the range of 30–40 kg/m2 and age between 20 and 50 years old completed the study. All volunteers had blood pressure, complete blood cell counts and blood chemistry profiles within the normal clinical range. Complete blood cell counts were performed on a Cell-Dyne 3200 instrument (Abbott Diagnostics). The study was a double-blind, randomised, cross-over trial for a total of 7 weeks. The volunteers were provided all meals for the duration of the study. The first week consisted of a baseline period when the subjects ate the prescribed meals without other interventions. Blood was drawn at the beginning and end of the first week and used as covariates in the analyses described below. For the next 3 weeks, the volunteers ate the prescribed meals with the addition of the foods containing either freeze-dried strawberry powder (California Strawberry Commission) or strawberry flavouring. The amount of the strawberry powder given to the volunteers was equivalent to four servings of frozen strawberries per d. Blood was drawn for analyses after 2 and 3 weeks on the intervention diets. For the last 3 weeks of the study, the subjects crossed over to the other intervention, and blood was again drawn after 2 and 3 weeks on the intervention diets. All blood draws were performed after a 12 h fasting period.
Ex vivo activation of peripheral blood mononuclear cells
Blood was collected from each subject into cell preparation tubes (Becton Dickinson) and peripheral blood mononuclear cells (PBMC) were prepared according to the manufacturer's protocol. PBMC were adjusted to a concentration of 1 × 106/ml in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10 % autologous serum, 1 mm-sodium pyruvate, 2 mm-l-glutamine, 60 mg penicillin/l, 100 mg streptomycin/l (Invitrogen) and 50 μmol β-mercaptoethanol/l (Sigma Chemical Company). For the activation of T-lymphocytes, ninety-six-well plates were pre-coated overnight at 4°C with 50 μl CD3 (clone OKT3) plus CD28 antibodies (eBioscience) per well with each antibody at a concentration of 3 mg/l. As a negative control for proliferation and cytokine production, each plate also included wells that were pre-coated with isotype control antibodies (eBioscience) at the identical volume and concentration. Wells in the pre-coated plates were washed three times with PBS (Sigma) before addition of 200 μl PBMC per well (2 × 105 cells). PBMC were incubated at 37°C in 5 % CO2 and cells and supernatants were removed after 24, 48 and 72 h for analysis of T-cell proliferation and cytokine production, respectively, as described below. For the activation of monocytes, 200 μl PBMC were stimulated in separate wells (ninety-six-well plates) with 10 μg/l of ultra-pure lipopolysaccharide (LPS; List Biological Laboratories, Inc.) or an equal volume of sterile endotoxin-free water as a negative control. PBMC were incubated at 37°C in 5 % CO2 and supernatants were removed after 24, 48 and 72 h for the analysis of monocyte cytokine production as described below.
Proliferation analysis
Measurements of T-lymphocyte proliferation were accomplished by pre-labelling PBMC using the PKH67 Green Fluorescent Cell Linker Kit (Sigma) and the manufacturer's recommended protocol. The PKH (Paul Karl Horan)-labelled PBMC were left unactivated (control) or activated with CD3/CD28 antibodies as described previously and aliquots of the cells were collected at 24, 48 and 72 h. At each time point, the PKH-labelled PBMC were further stained with phycoerythrin-conjugated anti-CD4 and allophycocyanin-conjugated anti-CD8 antibodies (Becton Dickinson) for 30 min on ice, washed and fixed in 1 % paraformaldehyde/PBS. As controls, aliquots of the cells were stained with phycoerythrin and allophycocyanin-conjugated isotype antibodies (Becton Dickinson). All staining was performed in duplicate. PBMC were collected on a FACSCanto flow cytometer using FACSDiva software (Becton Dickinson), and proliferation of CD4+ and CD8+ T-lymphocytes was analysed using the Proliferation Wizard feature of ModFit LT version 3.1 (Verity Software House). Non-proliferating cells from the negative control wells were used to find the parent peak used by the software for calculating the number of cells in each generation of the proliferating lymphocytes. For each sample, fifty thousand events were collected after excluding cellular debris and aggregated cells using appropriate gates.
Cytokine analyses
PBMC were plated into separate ninety-six-well plates and stimulated as described previously for measurements of cytokine production. Supernatants were collected every 24 h for 3 d to detect cytokines produced by activated T-lymphocytes or LPS-stimulated monocytes. For activated T-lymphocytes, the levels of interferon-γ, TNF-α, IL-4 and IL-10 were measured. For LPS-activated monocytes, the levels of TNF-α, IL-1β, IL-6 and IL-8 in the supernatants were measured. Cytokine concentrations were quantified using custom-made Milliplex cytokine detection kits (Millipore Corporation) and a Bioplex multiplex instrument (BioRad). All supernatant samples were analysed in duplicate.
Microarrays
For microarray analysis, two subjects were randomly selected.Briefly, 20 ml of heparinised blood were collected from each subject after 3 weeks on the control diet and, again, after 3 weeks on the strawberry-supplemented diet. Whole blood was diluted with 40 ml sterile RPMI-1640 medium containing 25 mm-HEPES and l-glutamine (Invitrogen) and then split into two 30 ml aliquots. Of these two aliquots of cells, one was stimulated with 10 mg/l of ultra-pure LPS (Escherichia coli O111:B4; List Biological Laboratories) and another was treated with sterile endotoxin-free water (Sigma). The blood samples were stimulated for 4 h at 37°C, 5 % CO2. Total cellular RNA from the leucocytes was isolated from the whole blood samples using the RNeasy Midi kit (Qiagen) according to the manufacturer's instructions. Briefly, erythrocytes were lysed in the erythrocyte lysis buffer EL. The resulting leucocyte pellets were lysed with buffer RLT and homogenised with QIAshredder spin columns (Qiagen). Purified total RNA was stored at − 80°C. RNA integrity was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies), and RNA abundance was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies).
Complementary DNA was generated from purified total RNA (5 μg) using the SuperScript III First-Strand Synthesis System (Invitrogen) followed by in vitro transcription to incorporate biotin labels and subsequent hybridisation to Human Genome U133 Plus 2·0 microarrays (38 500 genes; Affymetrix), according to the manufacturer's protocol. The arrays were washed and stained on an Affymetrix GeneChip Fluidics Station 450 and scanned on an Affymetrix GeneChip Scanner 3000. Affymetrix CEL files were imported into Partek Genomics Suite. The software uses the Robust Multichip Algorithm, a detection algorithm for defining the expression of each gene. Gene signals were converted into a log2 value and differentially expressed genes were obtained for the strawberry-supplemented group compared with the control group at a fold-change cut-off of 1·2. Statistical significance was designated at P< 0·05 after correcting with a step-up false discovery rate. Web-based tools from DAVID Bioinformatics (http://david.abcc.ncifcrf.gov/) were used to classify genes according to biological pathways in which changes in gene expression occurred.
Statistical analysis
The Statistical Analysis Systems statistical software package version 9.2 (SAS Institute) and the PROC MIXED procedure were used to fit the cross-over model with baseline values (blood draws 1 and 2) as covariates as described previously(Reference Zunino, Parelman and Freytag23). The means of the two baseline blood draws were used as covariates to adjust for variability among the subjects, and to reduce error and increase the precision in the tests. The Means procedure was used for determining means with their standard errors. If needed, data subsets were transformed using Box–Cox power transformations to conform to a normal distribution. All data were analysed for carry-over effects and no carry-over effects were observed for the parameters tested. Data are presented as means with their standard errors with significance set at P< 0·05.
Results
Proliferation of activated CD4+ and CD8+ T-lymphocytes
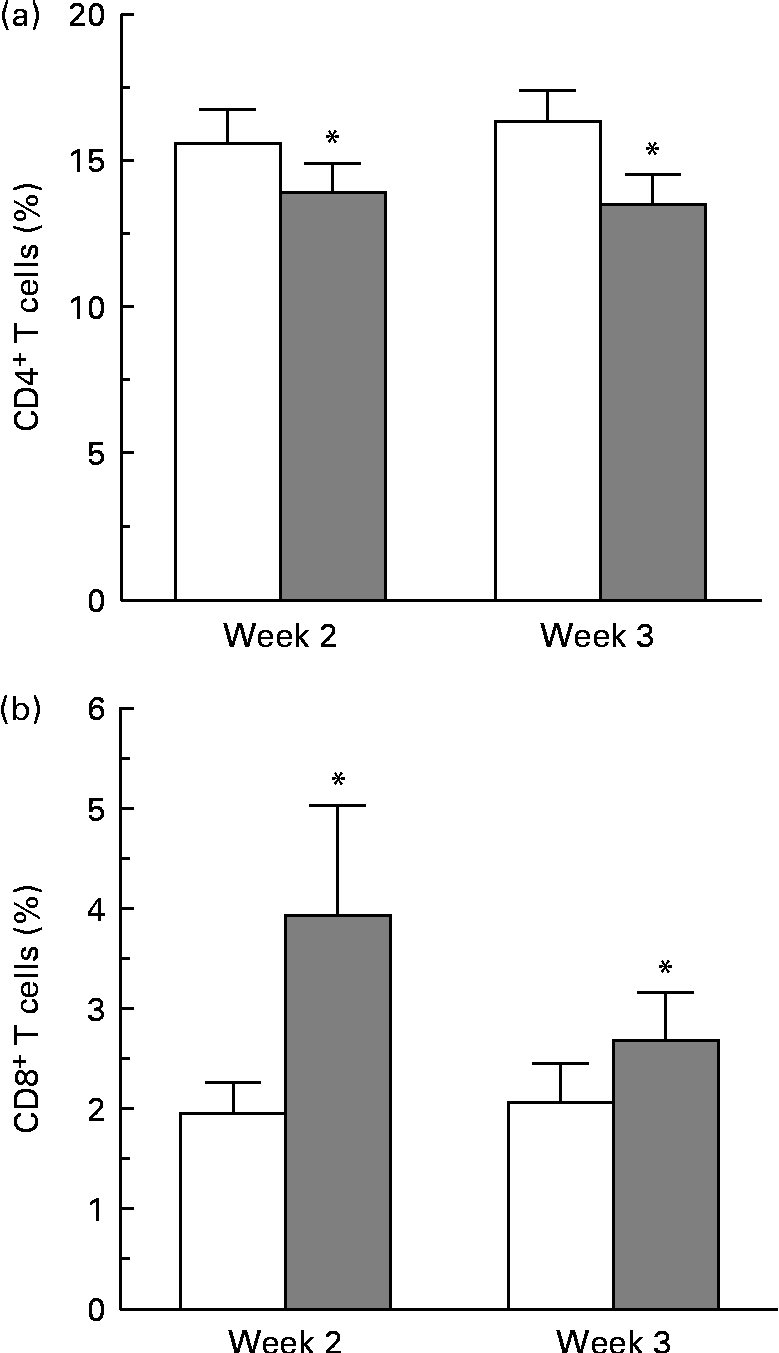
Complete blood cell counts were performed for all subjects at each blood draw, and included counts for basophils, eosinophils, neutrophils, lymphocytes, monocytes, erythrocytes and platelets. No differences in cell counts were observed between the two dietary groups. No differences were observed in the overall percentage of CD4+ and CD8+ cells at 24, 48 and 72 h, as determined by flow cytometry gating around the stained CD4+ and CD8+ cell populations. A more sensitive method was used to determine the changes in proliferative responses of these T-cell populations by pre-staining with a membrane-labelling dye (PKH) and analysing generations of dividing cells over time using ModFit specialty software. A change in the proliferative response of CD4+ cells was observed at 72 h. A modest decrease in the percentage of CD4+ T cells in the third generation of proliferating cells from the strawberry-fed group was observed at week 2 and week 3 compared with the control group (P= 0·016 for the overall diet effect; Fig. 1(a)). There was no concomitant increase or decrease in the percentage of CD4+ cells in later generations. There was an increase in the proliferative response of the CD8+ T-cell population 24 h after CD3/CD28 activation at weeks 2 and 3 (P= 0·029 for the overall diet effect; Fig. 1(b)). An increase in the percentage of CD8+ T cells in the second generation of proliferating cells was observed at the 24 h time point, suggesting an increased CD8+ T-cell responsiveness to the activation stimuli in the group fed the strawberry powder compared with the control group. No further differences in the proliferation response of CD8+ cells were observed at 48 and 72 h.

Fig. 1 Dietary strawberries induced changes in proliferative responses of activated CD4+ and CD8+ T-lymphocytes. Peripheral blood mononuclear cells were stained using the PKH67 Green Fluorescent Cell Linker Kit and the T-cell population was activated with CD3 and CD28 antibodies. Aliquots of the cells were collected at 24, 48 and 72 h, and further stained with phycoerythrin (PE)-conjugated anti-CD4 and allophycocyanin (APC)-conjugated anti-CD8 antibodies. As controls, aliquots of the cells were stained with PE- and APC-conjugated isotype antibodies. The cells were collected on a FACSCanto flow cytometer using FACSDiva software, and proliferation of CD4+ and CD8+ T-lymphocytes was analysed using the Proliferation Wizard feature of ModFit LT software. Values are means, with their standard errors represented by vertical bars. (a) A decrease in the percentage of CD4+ T cells in the third generation of proliferating cells was observed 72 h after activation at weeks 2 and 3 in the strawberry-fed group (![]() ) compared with the control group (□) (n 20 subjects, * P= 0·016 for the overall diet effect). (b) An increase in the percentage of CD8+ T cells in the second generation of proliferating cells was observed 24 h after activation at weeks 2 and 3 in the strawberry-fed group (
) compared with the control group (□) (n 20 subjects, * P= 0·016 for the overall diet effect). (b) An increase in the percentage of CD8+ T cells in the second generation of proliferating cells was observed 24 h after activation at weeks 2 and 3 in the strawberry-fed group (![]() ) compared with the control group (□) (n 20 subjects, * P= 0·029 for the overall diet effect).
) compared with the control group (□) (n 20 subjects, * P= 0·029 for the overall diet effect).
Cytokine production
PBMC were stimulated with CD3 plus CD28 to activate the T-cell population. Supernatants were collected after 24, 48 and 72 h and cytokines secreted by T helper 1 (interferon-γ and TNF-α) and T helper 2 (IL-4 and IL-10) lymphocytes were measured. The production of interferon-γ, TNF-α, IL-4 and IL-10 was maximal after 48 and 72 h activation. Therefore, these time points were chosen for measuring cytokine levels. No differences were observed for cytokine production by T-lymphocyte subsets between the subjects consuming strawberry powder or placebo preparations (Table 1). LPS was used to activate the monocyte population of PBMC, and supernatants were also collected at 24, 48 and 72 h. The production of monocyte-derived TNF-α, IL-1β, IL-6 and IL-8 was evaluated at the peak production time points of 24 and 48 h (Table 2). A significant increase in the production of TNF-α was observed in activated monocytes from the subjects who consumed strawberry powder compared with the placebo control group at 24 h (P< 0·02) and 48 h (P< 0·01) post-activation. The increase in TNF-α levels was observed after 2 weeks on the strawberry-enriched diet and maintained after 3 weeks on the diet. No differences in IL-1β, IL-6 or IL-8 levels were observed between the two intervention groups.
Table 1 Cytokine production from peripheral blood mononuclear cells activated with CD3/CD28 antibodies* (Unadjusted mean values with their standard errors, n 20)
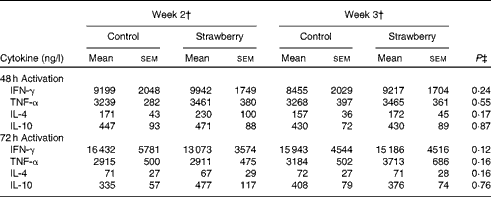
IFN-γ, interferon-γ.
* Baseline data (blood draws 1 and 2) were used as covariates.
† Week 2 data represent 2 weeks of intervention (blood draws 3 and 5), and week 3 data represent 3 weeks of intervention (blood draws 4 and 6) with the control diet (control) or the diet containing strawberry powder (strawberry).
‡ P value for the overall diet effect between the two intervention groups. P< 0·05 is significant. No carry-over effects were observed for these parameters.
Table 2 Cytokine production from peripheral blood mononuclear cells activated with lipopolysaccharide* (Unadjusted mean values with their standard errors, n 20)
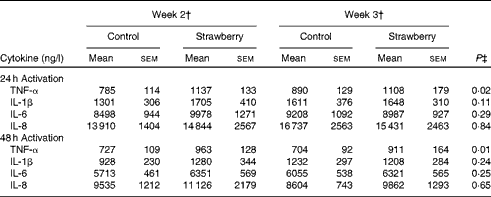
* Baseline data (blood draws 1 and 2) were used as covariates.
† Week 2 data represent 2 weeks of intervention (blood draws 3 and 5), and week 3 data represent 3 weeks of intervention (blood draws 4 and 6) with the control diet (control) or the diet containing strawberry powder (strawberry).
‡ P value for the overall diet effect between the two intervention groups. P< 0·05 is significant. No carry-over effects were observed for these parameters.
Gene expression in lipopolysaccharide-stimulated peripheral blood
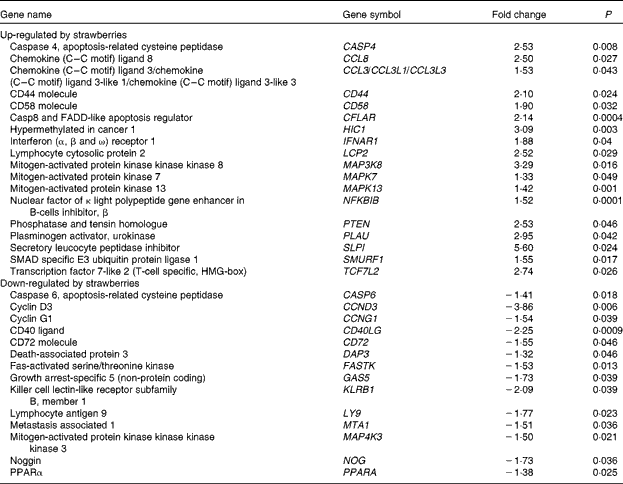
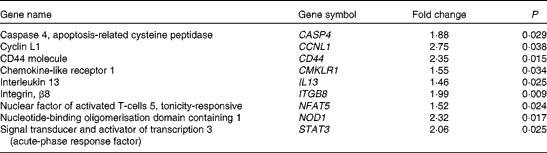
Microarrays that evaluated 38 500 genes were used to determine global changes in gene expression in unstimulated peripheral blood leucocytes and after stimulation with LPS for 4 h. Whole blood was collected from two human subjects and the vehicle or LPS was added with minimal handling of the blood. The vehicle-only treatment provided an opportunity to determine the changes in gene expression produced by dietary strawberries. Table 3 shows the expression for selected genes that were up- or down-regulated in peripheral blood leucocytes by dietary strawberries compared with the control diet (P< 0·05). The full lists of up- and down-regulated genes for the vehicle-treated blood samples are presented in Tables S1 and S2 (available online), respectively. Analysis of the LPS-treated blood cells showed no statistically significant down-regulation of gene expression. However, Table 4 shows the genes with significant up-regulation in the dietary strawberry group compared with the control diet group (P< 0·05). The full list of up-regulated genes in response to the LPS treatment is presented in Table S3 (available online).
Table 3 Selected genes regulated by dietary strawberries in peripheral blood leucocytes

FADD, Fas-associated via death domain; HMG, high mobility group.
Table 4 Selected genes up-regulated by dietary strawberries in lipopolysaccharide-stimulated peripheral blood leucocytes
Discussion
In the present study, we observed modulation of immune responsiveness by dietary strawberries in healthy obese subjects. The production of TNF-α increased in the monocyte population from the subjects fed strawberry powder after stimulation with LPS when compared with the control group fed the strawberry-flavoured foods. A modest but significant increase in the proliferative response of activated CD8+ T-lymphocytes was observed in subjects consuming strawberry powder compared with the control diet. A modest decrease in the percentage of CD4+ T cells in the third generation was observed after 72 h in the strawberry-fed group compared with the control group. However, since there was no concomitant change in percentages in later generations, the effect of strawberries on the proliferative response of CD4+ T cells remains unclear.
Obesity has been reported to impair both innate and adaptive immunity. A higher incidence of infection in the obese population has been well documented. Dietary strawberries may aid in sensitising or correcting the dysfunction of immune cells, thereby reducing the risk of infection in the obese. TNF-α is produced mainly by monocytes and T-lymphocytes, and is an important mediator of immune responsiveness to bacterial and viral infections(Reference Locksley, Killeen and Lenardo25–Reference Guidotti and Chisari28). The importance of this cytokine in fighting off invading pathogens is highlighted by studies with patients receiving anti-TNF-α therapies, who have an increased risk of developing infections(Reference Dixon, Watson and Lunt29–Reference Atzeni, Sarzi-Puttini and Botsios33). Elevated levels of circulating TNF-α have been associated with an increased risk for developing insulin resistance, diabetes and CVD(Reference Vykouhal and Davies34). However, immunological responses, such as production of TNF-α from activated monocytes, are generally self-limiting, and a long-term contribution to obesity-associated risk factors would not be expected. The mean blood level of TNF-α for the healthy obese subjects in the present study was approximately 3·2 ng/l(Reference Zunino, Parelman and Freytag23). There was no difference in circulating levels of TNF-α between the control group and the group supplemented with strawberry powder (P= 0·45)(Reference Zunino, Parelman and Freytag23). Other studies have reported serum TNF-α levels of 3·6 and 4·9 ng/l for normal-weight individuals(Reference Ziccardi, Nappo and Giugliano35, Reference Straczkowski, Kowalska and Nikolajuk36), indicating that the obese volunteers in the present study did not have elevated circulating TNF-α.
The present data suggest that dietary strawberries modulate the sensitivity of monocytes to a bacterial challenge (LPS) and may improve the effectiveness of an immune response to invading pathogens in the obese population at risk for infection. LPS is a cell-wall component of Gram-negative bacteria that directly activates monocytes to produce pro-inflammatory cytokines, such as TNF-α, by signalling through Toll-like receptors(Reference Beutler37, Reference Wong, Chugn and Sultzer38). The type of LPS used in the present experiments was a highly purified preparation that was devoid of contaminating endotoxin proteins. Endotoxin proteins in crude preparations of commercially available LPS can act as stimulators of lymphocytes(Reference Sultzer and Goodman39–Reference Hirschfeld, Ma and Weis41). Therefore, we are confident that the increased TNF-α originated from monocytes and not the T-cell population.
CD8+ lymphocytes are cytolytic cells that are important for clearing cancer and virally infected cells(Reference Harty, Tvinnereim and White42). Autologous serum was used in the cell-culture medium during the activation of PBMC to maximise the exposure of the cells to any polyphenol metabolites or other components that remained in the circulation after the 12 h fasting period. The increase in the proliferation of CD8+ cells was observed only at the 24 h time point, which may be reflective of continued exposure to bioactive serum components during the first 24 h and these components may have been depleted over time. The increased sensitivity of CD8+ T cells to activation stimuli in the dietary strawberry group, although modest, may be advantageous in obese people who are at risk for developing viral infections, and who are at higher risk than normal-weight individuals for developing cancer.
Dietary supplementation of obese volunteers with strawberry powder produced changes in basal mRNA expression in both the untreated and LPS-treated leucocyte populations. The altered expression profiles from the untreated cells showed that the dietary intervention with strawberries had an impact on gene transcription in immune cells without the need for external stimulation with LPS. Most notable is that mitogen-activated protein 3 kinase kinase kinase 8 (MAP3K8) was significantly induced by the strawberry intervention compared with the placebo. This result is consistent with increased TNF-α production induced by LPS in PBMC derived from the subjects who consumed strawberries, as MAP3K8, also known as tumour progression locus 2, is required for the up-regulation of TNF-α by LPS in macrophages(Reference Dumitru, Ceci and Tsatsanis43, Reference Symons, Beinke and Ley44). Dietary strawberries increased the mRNA expression of CD44 and CD58 compared with the cells from the control group. CD44 and CD58 are adhesion molecules necessary for cell–cell adhesion, homing and signalling in leucocytes(Reference Ponta, Sherman and Herrlich45, Reference Springer, Dustin and Takashi46). Chemokine (C–C) ligand 8 (CCL8), chemokine (C–C) ligand 3 (CCL3) and secretory leucocyte peptidase inhibitor were up-regulated in the leucocyte population by dietary strawberries. CCL8, also known as monocyte chemotactic protein-2, is notable for its ability to block CD4/CCR5-mediated entry and replication of HIV-1(Reference Proost, Wuyts and Van Damme47, Reference Gong, Howard and Turpin48). CCL3 preferentially recruits CD8+ lymphocytes(Reference Taub, Conlon and Lloyd49) and is an important mediator of anti-bacterial responses by memory CD8+ T cells(Reference Narni-Mancinelli, Campisi and Bassand50). Secretory leucocyte peptidase inhibitor has anti-bacterial, anti-fungal and anti-viral activities and protects epithelial cells in the lung from proteolytic damage due to pathogenic organisms and aberrant neutrophil activation, as in asthma(Reference Scott, Weldon and Taggart51, Reference Zani, Tanga and Saidi52).
LPS stimulation did not result in the significant down-regulation of gene expression between the dietary groups, but increased expression of genes was observed in LPS-treated cells from the strawberry group compared with the control. As with the untreated blood leucocytes, the LPS treatment increased the expression of mRNA for CD44 and Caspase 4 in the strawberry group. Caspase 4 is an ‘inflammatory’ caspase and is required for the activation of the inflammasome of the innate immune system in response to pathogens(Reference Martinon and Tschopp53, Reference Sollberger, Strittmatter and Kistowska54). Nucleotide-binding oligomerisation domain containing 1 (NOD1), a part of an intracellular sensing system involved in resistance to infectious pathogens(Reference Philpott and Girardin55), was also up-regulated in LPS-treated leucocytes from the group receiving strawberries. Although microarrays for gene expression profiles were only performed with two subjects, the data show an interesting array of genes that appear to be important in modulating immune function after ingestion of strawberries. An advantage of the study design for the microarray analysis was that whole blood was used for the stimulation, so there was minimal handling of the samples, suggesting that these data are reflective of in vivo immune responsiveness. A disadvantage of using a mixed population of cells is that the cellular origin for the changes in gene expression is often unclear. However, these profiles provide the first report of changes in the gene expression of immune cells after the dietary intervention with strawberries in obese human subjects, and point to a greater role of this fruit in modulating immune responsiveness than was previously known.
There have been numerous reports on the effects of different fruit-derived polyphenols on immune function(Reference González-Gallego, García-Mediavilla and Sánchez-Campos56). However, the majority of these studies have been performed in vitro with supra-physiological levels of the aglycone forms of these polyphenols, which are generally found at low levels in vivo because they are rapidly metabolised. Studies of the effects of polyphenol metabolites on immune function are scarce. There are animal and human studies that indicate a role for fruit-derived components in modulating responses of immune cells. For example, increased fruit and vegetable consumption in the elderly has been reported to increase the antibody response to a pneumococcal vaccine and also increase the cytotoxicity of natural killer lymphocytes(Reference Sanderson, Elsom and Kirkpatrick57). Bub et al. (Reference Bub, Watzl and Blockhaus58) reported that consumption of fruit juice increased lymphocyte proliferation, although these authors did not define the subset of lymphocytes that were affected by the intervention. An increase in natural killer cytotoxicity has also been noted in this latter study. In animal studies, mice fed a bioactive ethyl acetate fraction derived from Prunus cerasus (sour cherry) had increased proliferative responses of both B- and T-lymphocytes, and increased IgM and IgG production from B-lymphocytes(Reference Abid, Khajuria and Parvaiz59).
The results from the present study suggest that dietary strawberries have a sensitising effect on different populations of immune cells from obese individuals who may have an impaired immune response to pathogenic organisms. Given the delicate balance necessary to maintain immunological homeostasis, small changes in immune responsiveness that are induced by dietary interventions in relatively healthy obese subjects should be expected. Previous analyses showed a carry-over effect for a limited number of serum lipid parameters and serum inflammatory markers, indicating that our study design may have benefited from an appropriate washout period(Reference Zunino, Parelman and Freytag23). The present analyses of immunological responses did not show carry-over effects, suggesting that the modulation of immune cell function by dietary strawberries may have been more rapid than the turnover of serum lipids. The subtle changes that were observed for lymphocyte proliferation suggest that the study may have been enhanced by increasing the number of subjects and the length of the intervention period. However, the 3-week intervention period appeared to be appropriate for detecting the changes in monocyte sensitivity to LPS stimulation, since changes in TNF-α production were observed by week 2. The intervention was equivalent to four servings of strawberries per d divided between breakfast and dinner. Approximately four servings of fruit per d are recommended by the 2010 dietary guidelines of the US Department of Agriculture and Department of Health and Human Services(60). Although most individuals consume a mixture rather than only one type of fruit per d, these data provide evidence of potential health benefits of strawberries that may be applicable to other darkly coloured fruits in the maintenance and enhancement of immune function.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513000937
Acknowledgements
The present study was supported by the United States Department of Agriculture (USDA) Current Research Information System project no. 5306-51530-013-00D and 5306-51530-018-00D, and a grant from the California Strawberry Commission, Watsonville, CA, USA. S. J. Z. was a member of the scientific advisory committee for the California Strawberry Commission during the study period. The authors' contributions were as follows: S. J. Z. and D. H. H. designed the research; D. H. S., T. L. F and L. Z. conducted the research; S. J. Z., B. E. M., J. S. G. and L. Z. analysed the data; S. J. Z. wrote the paper with input from all authors and had primary responsibility for final content. All authors read and approved the final manuscript. USDA is an equal opportunity provider and employer. The authors report no conflict of interest.