The metabolic syndrome is characterised by a constellation of cardiovascular risk factors, including atherogenic dyslipidaemia, abnormal glucose tolerance, hypertension and visceral obesityReference Ruotolo and Howard1. Fat accumulation, a serious problem in the metabolic syndrome, is correlated with systemic oxidative stress in man. Oxidative stress may play critical roles in the pathogenesis of various features of the metabolic syndromeReference Brownlee2, via impairment of glucose uptake in muscle and fatReference Maddux, See, Lawrence, Goldfine, Godlfine and Evans3 and reduction in insulin secretion from pancreatic β cellsReference Matsuoka, Kajimoto, Watada, Kaneto, Kishimoto, Umayahara, Fujitani, Kamada, Kawamori and Yamasaki4. Increased oxidative stress may also underlie the pathophysiology of hypertensionReference Nakazono, Watanabe, Matsuno, Sasaki, Sato and Inoue5 and atherosclerosisReference Ohara, Peterson and Harrison6 by directly affecting vascular wall cells. The antioxidant activity of HDL is impaired in the metabolic syndromeReference Hansel, Giral, Nobecourt, Chantepie, Bruckert, Chapman and Kontush7, and increased oxidative stress in accumulated fat may contribute to this syndrome. Therefore, the reduction of oxidative stress may be a useful target for new therapies for patients with the metabolic syndromeReference Furukawa, Fujita, Shimabukuro, Iwaki, Yamada, Nakajima, Nakayama, Makishima, Matsuda and Shimomura8. Oxidative defence decreases with age because the defence systems may be compromised after the menopauseReference Berliner and Heinecke9. A reduced antioxidant defence is also seen specifically in postmenopausal women due to the lack of oestrogenReference Goudev, Kyurkchiev, Gergova, Karshelova, Georgiev, Atar, Kehayov and Nachev10. Therefore, postmenopausal women with the metabolic syndrome are at high risk of oxidative stress.
Diet plays an important role in the metabolic syndromeReference Azadbakht, Mirmiran, Esmaillzadeh and Azizi11–Reference Riccardi and Rivellese13. Although many studies have been conducted in patients with the metabolic syndromeReference Esposito, Marfella and Citotola14–Reference Klimes and Sebokova16, only one study addressed the issue of oxidative stress in these patientsReference Roberts, Won, Pruthi, Kurtovic, Sindhu, Vaziri and Barnard17. Several studies have focused on the effect of diet on oxidative stress, especially the effect of soya consumptionReference Jenkins, Kendall and Garsetti18–Reference Engelman, Alekel, Hanson, Kanthasamy and Reddy22. The antioxidant properties of soya isoflavones may protect against lipid oxidationReference Jenkins, Kendall and Garsetti18 and improve plasma antioxidant statusReference Vega-Lopez, Yeum, Lecker, Ausman, Johnson, Devaraj, Jialal and Lichtenstein19. Some studies have suggested an antioxidant action of soya isoflavonesReference Mahn, Borras and Knock20, Reference Wiseman, O'Reilly, Adlercreutz, Mallet, Bowey, Rowland and Sanders21 but others demonstrated little or no effect of soya-derived isoflavones on the biomarkers of oxidative stressReference Vega-Lopez, Yeum, Lecker, Ausman, Johnson, Devaraj, Jialal and Lichtenstein19, Reference Engelman, Alekel, Hanson, Kanthasamy and Reddy22. However, most of these studies have focused on healthy subjects and there has been little emphasis regarding the effect of soya consumption on the markers of oxidative stress specifically among postmenopausal women with the metabolic syndrome.
We hypothesised that soya consumption might be beneficial for reducing oxidative stress in the metabolic syndrome. We therefore evaluated the effects of soya consumption, in the form of isolated soya protein and roasted soya nuts with naturally occurring isoflavones, on plasma total antioxidant capacity (TAC) and malondialdehyde (MDA) in postmenopausal women with the metabolic syndrome.
Research design and methods
Participants
A total of 120 Tehranian postmenopausal women with the metabolic syndrome were screened for inclusion in the study. The present study was conducted in Tehran, Iran. The women were living in the same district of Tehran, so they were approximately in the same level of socio-cultural characteristics (moderate level). Women were considered postmenopausal if menstrual periods had been absent for more than 1 year and follicle-stimulating hormone, serum luteinising hormone, testosterone and oestradiol levels confirmed their statusReference Rozenberg, Bosson, Peretz, Caufriez and Robyn23. The metabolic syndrome was defined according to Adult Treatment Panel III guidelines24: (1) abdominal adiposity (waist circumference >88 cm); (2) low levels of serum HDL-cholesterol ( < 500 mg/l); (3) hypertriacylglycerolaemia ( ≥ 1500 mg/l); (4) elevated blood pressure ( ≥ 130/85 mmHg); (5) impaired glucose homeostasis ( ≥ 1100 mg/l). To be enrolled in the study, patients had to have three or more of the above-mentioned criteria to be classified as having the metabolic syndrome. Exclusion criteria were any secondary cause of hyperglycaemia, current or previous (in the preceding 6 months) use of oestrogen therapy, treatment with insulin or oral hypoglycaemic agents, untreated hypothyroidism, smoking, kidney or liver diseases or breast cancer. Finally, forty-two women were included in the present study; all of them had all five components of the metabolic syndrome. All participants provided informed written consent. The present study was approved by the research council and ethical committee of the National Nutrition and Food Technology Research Institute of Shaheed Beheshti University of Medical Sciences.
Study procedures
We used a randomised cross-over design. After 3 weeks of run-in on a usual diet (average of 55 % energy from carbohydrate, 15 % energy from protein and 30 % energy from fat), we randomly assigned women to a control diet (diet A, a red meat-Dietary Approaches to Stop Hypertension (DASH) diet), a DASH diet with soya nuts (diet B, soya nut period) or a DASH diet with soya protein (diet C, soya protein period), each one for 8 weeks (The rationale of intervention duration was based on previous interventions and we thought that in longer periods subjects may not follow the study completely.) Each woman received all three diets and had two wash-out periods (each wash-out for 4 weeks; the rationale of wash-out duration was based on previous studies) between the three diets. We set six different sequences of diet intakes (ABC, ACB, BCA, BAC, CBA, CAB) (Fig. 1). The randomisation was conducted at the end of the run-in.

Fig. 1 Design of the study. The control diet was a Dietary Approach to Stop Hypertension diet. This diet was rich in fruits, vegetables, whole grains, low-fat dairy products, and low in red meat, saturated fat, total fat, cholesterol, refined grains and sweets. The amount of Na intake was 2400 mg/d. The diet with soya nuts was the same as the control diet but we replaced red meat with soya nuts. Every 30 g soya nuts was considered as one serving of moderate-fat meat. The diet with soya protein was the same as the control diet but we replaced red meat with soya protein. Every 30 g soya protein was considered as one serving of low-fat meat.
Measurements were obtained before run-in, after run-in (baseline), after each diet and after each wash-out. Baseline measurements were considered after run-in and after each wash-out. Participants were asked not to change their habitual physical activity level for the duration of the study. Patients recorded their physical activities for 3 d each month.
Diets
We used three diets:
(1) Control diet: this diet was a DASH diet with 55 % energy from carbohydrates, 17 % energy from protein, and 28 % energy from total fat on average. This diet had one serving of red meat per d (red meat-DASH) and was rich in fruits, vegetables, whole grains, low-fat dairy products, and low in saturated fat, total fat, cholesterol, refined grains and sweets. The amount of Na intake was 2400 mg/dReference Karanja, Obarzanek and Lin25.
(2) Diet with soya nuts: this diet was the same as the control diet but we replaced red meat by soya nuts; a serving of 30 g soya nuts was considered as one serving of red meatReference Mahan, Escott-Stump, Mahan and Escott-Stump26.
(3) Diet with soya protein: this diet was the same as the control diet but we replaced red meat by soya protein. Every 30 g soya protein was considered as one serving of red meatReference Mahan, Escott-Stump, Mahan and Escott-Stump26.
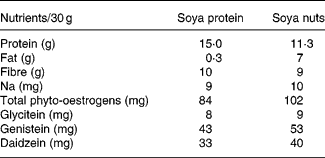
The nutrient composition of soya nuts and soya protein consumed by the study participants, based on our analysis, is shown in Table 1.
Table 1 Nutrient composition of soya protein and soya nuts used in the intervention
Energy requirements of each participant were calculated individually based on equations suggested by the Institute of Medicine, Food and Nutrition Board27. The participants were visited every 2 weeks, for 45–60 min/patient. They were in touch with the study nutritionist daily by phone. For measuring food intake, 3 d diet records were used at baseline and during intervention for each month. Every participant had to bring in her 3 d diet record and physical activity records every month, where they were reviewed by the study staff and used for checking diet compliance.
The study nutritionist explained the benefits of each diet for participants. They also received education in using an exchange list of foods, and in writing food diaries. The diets were individually prescribed using a ‘calorie count’ system and an exchange list was given to each patient for exchanging the food items and calculating calories. A 7 d menu cycle at six energy levels (7530, 7950, 8370, 8790, 9200 and 9620 kJ; 1800, 1900, 2000, 2100, 2200 and 2300 kcal) was developed for each diet.
To maximise treatment fidelity, group discussions were performed monthly, in which the food items that should be eaten were emphasised. Women also received education on the methods of preparing soya protein according to their menu and were encouraged to follow their diets. The investigators randomly took part in the counselling sessions and monitored the messages that the nutritionist was giving to each group. Patient compliance was assessed by analysing the 3 d food record diaries monthly and by the attendance at the meetings and monthly visits.
Measurements
Body weight was measured while the subjects were minimally clothed, without shoes, using digital scales and recorded to the nearest 0·1 kg. Height was measured in a standing position, without shoes, using a tape meter while the shoulders were in a normal state. Waist circumference was measured to the nearest 0·1 cm at the narrowest level over light clothing, using an unstretched tape meter, without any pressure to the body surface. The ferric-reducing ability of plasma method according to Benzie & Strain was used for plasma TAC measurementReference Benzie and Strain28. The EDTA plasma samples were stored at − 70°C until MDA measurements were determined by HPLC according to the method of Wong et al. Reference Wong, Knight, Hopfer, Zaharia, Leach and Sunderman29. Plasma phyto-oestrogen levels were measured by HPLC according to Franke et al. Reference Franke, Custer and Tanaka30, Reference Franke, Custer, Wang and Shi31 to check the soya trial compliance.
Statistical analysis
We used general linear models (repeated-measures ANOVA) to compare means of the markers of oxidative stress at the end of the soya nut, soya protein and control diets. Then, we used paired t tests to compare the end-of-treatment values of each group with each other group. The percentage change for each variable was also calculated by the formula (E–B)/B × 100, where E is the end-of-treatment values and B is the baseline values. We compared groups using the percentage change in both repeated-measures ANOVA and using paired t tests. We also determined the mean percentage change differences, which were derived by calculating the differences in percentage change for each variable in pair-wise group comparisons. This parameter gives the most direct estimate of the difference in response in comparing groups. We also calculated the percentage difference compared with control for each group (both soya protein and soya nut). The percentage difference compared with control for each variable was also calculated by the formula (X–C)/C × 100, where X is the end values of the soya protein or soya nut group and C is the end values of the control group. Interactions between soya intake and weight were not significant for any of the markers. Period effect and carryover effects were tested using the appropriate general linear models.
Pearson correlation coefficients were used to evaluate the relationship between soya-derived phyto-oestrogen intake (calculated from self-reported soya intake in 3 d diet records) and plasma phyto-oestrogen levels. All results were considered significant if the two-tailed P value was < 0·05. Statistical analysis was performed using SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL, USA) and SAS version 8.2 (1999; SAS Institute Inc., Cary, NC, USA).
Results
All participants (forty-two postmenopausal women with the metabolic syndrome) completed the entire cross-over study. Characteristics of the women at baseline are presented in Table 2. Calculated nutrient content and food-group servings of the 3 d diet records according to the patients' reports are shown in Table 3. Both the soya nut and soya protein diets were well tolerated. Only one individual complained of feeling bloated during the soya protein period.
Table 2 Baseline characteristics of participants (Mean values with their standard errors)

Table 3 Dietary intake of participants separately by intervention period

* Control diet: this diet had one serving of red meat per d and was rich in fruits, vegetables, whole grains, low-fat dairy products, and low in saturated fat, total fat, cholesterol, refined grains and sweets. The amount of Na intake was 2400 mg/d (Dietary Approach to Stop Hypertension (DASH) pattern).
† Soya protein diet: this diet was the same as the control diet (DASH diet) but we replaced red meat with soya protein.
‡ Soya nut diet: this diet was the same as the control diet (DASH diet) but we replaced red meat with soya nuts.
§ P values for differences among the three trial periods (repeated-measures ANOVA).
‖ Wash-out: in this period, patients used the same diet they were using before the study.
Participants' weights in the control, soya protein and soya nut periods were 71·6 (se 1·5), 71·5 (se 1·5) and 71·3 (se 1·5) kg at baseline and 70·1 (se 0·9), 70·7 (se 0·9) and 70·4 (se 0·8) kg at the end of the trials, which shows no significant changes during the trials.
Fig. 2 represents the baseline and end-of-trial values of MDA in the three different periods. There were no significant differences in the baseline values of MDA (0·76, 0·73 and 0·75 μmol/l; global P = 0·16). Significant differences between the end values of the control diet, soya protein regimen and soya nut consumption were seen (0·70, 0·64 and 0·63 μmol/l; global P < 0·01). Paired comparisons of the diets showed a significant difference between the end values of the control and soya protein diets (P < 0·01), and control and soya nut diets (P < 0·01). No significant differences were seen between the soya protein and soya nut diets (P = 0·44) with regard to the end values.

Fig. 2 Baseline and end-of-trial values of malondialdehyde (MDA) in three different periods. The control diet (□) was a Dietary Approach to Stop Hypertension diet. This diet was rich in fruits, vegetables, whole grains, low-fat dairy products, and low in red meat, saturated fat, total fat, cholesterol, refined grains and sweets. The diet with soya nuts (![]() ) was the same as the control diet but we replaced red meat with soya nuts. The diet with soya protein (
) was the same as the control diet but we replaced red meat with soya nuts. The diet with soya protein (![]() ) was the same as the control diet but we replaced red meat with soya protein. Values are means, with their standard errors represented by vertical bars. P values resulted from paired t tests. The global P value for the baseline values of MDA was 0·16 and the global P value for the end values of MDA was < 0·05. There were no significant differences in baseline values among the three groups. There was a significant difference between the soya protein and control diets (P < 0·01) as well as between the soya nut and control diets (P < 0·05), but there was no difference between the soya protein and soya nut diets (P = 0·44).
) was the same as the control diet but we replaced red meat with soya protein. Values are means, with their standard errors represented by vertical bars. P values resulted from paired t tests. The global P value for the baseline values of MDA was 0·16 and the global P value for the end values of MDA was < 0·05. There were no significant differences in baseline values among the three groups. There was a significant difference between the soya protein and control diets (P < 0·01) as well as between the soya nut and control diets (P < 0·05), but there was no difference between the soya protein and soya nut diets (P = 0·44).
Fig. 3 shows the baseline and end-of-diet values of TAC in the three different periods. The results showed a significant difference between the baseline values (1890, 1900 and 1950 μmol/l, respectively; global P < 0·01) and also end-of-diet values (1950, 2030 and 2110 μmol/l, respectively; global P < 0·01). Paired comparison of the diets showed a significant difference with regard to the end values between the control and soya protein diets (P < 0·01), control and soya nut diets (P < 0·01), and soya protein and soya nut diets (P < 0·01).

Fig. 3 Baseline and end-of-trial values of the plasma total antioxidant capacity (TAC) in three different periods. The control diet (□) was a Dietary Approach to Stop Hypertension diet. This diet was rich in fruits, vegetables, whole grains, low-fat dairy products, and low in red meat, saturated fat, total fat, cholesterol, refined grains and sweets. The diet with soya nuts (![]() ) was the same as the control diet but we replaced red meat with soya nuts. The diet with soya protein (
) was the same as the control diet but we replaced red meat with soya nuts. The diet with soya protein (![]() ) was the same as the control diet but we replaced red meat with soya protein. Values are means, with their standard errors represented by vertical bars. P values resulted from paired t tests. The general P value for the baseline values of TAC was < 0·05 and the general P value for the end values of TAC was < 0·05. There was no significant difference between the baseline values of the control and soya protein periods (P = 0·54) but there was a significant difference between the control and soya nut diets as well as between the soya protein and soya nut diets (both P < 0·01). The end values of TAC were significantly different between the control and soya nut, between the control and soya protein, and between the soya protein and soya nut diets (all P < 0·01).
) was the same as the control diet but we replaced red meat with soya protein. Values are means, with their standard errors represented by vertical bars. P values resulted from paired t tests. The general P value for the baseline values of TAC was < 0·05 and the general P value for the end values of TAC was < 0·05. There was no significant difference between the baseline values of the control and soya protein periods (P = 0·54) but there was a significant difference between the control and soya nut diets as well as between the soya protein and soya nut diets (both P < 0·01). The end values of TAC were significantly different between the control and soya nut, between the control and soya protein, and between the soya protein and soya nut diets (all P < 0·01).
Fig. 4 shows the percentage change of MDA and TAC in the three diets. Percentage changes in the control, soya protein and soya nut diets were significantly different for TAC (+3·2, +7·3 and +8·5 %; global P < 0·01) and MDA ( − 7·0, − 12·0 and − 14·0 %; global P < 0·01). The difference from control for TAC was +4·5 % (P < 0·01) in the soya nut period and 5·8 % (P < 0·01) on the soya protein regimen. Both soya nuts and soya protein reduced MDA significantly compared with the control diet (difference from control was − 7·9 % (P < 0·01) in the soya nut period and − 9·4 % (P < 0·01) on the soya protein diet). The results were not changed when we adjusted the means for the dietary Zn intake in further models (data not shown).

Fig. 4 Percentage change in malondialdehyde (MDA) and plasma total antioxidant capacity (TAC) in three diets: control (□), soya protein (■) and soya nut (Δ). The control diet was a Dietary Approach to Stop Hypertension diet. This diet was rich in fruits, vegetables, whole grains, low-fat dairy products, and low in red meat, saturated fat, total fat, cholesterol, refined grains and sweets. The diet with soya nuts was the same as the control diet but we replaced red meat with soya nuts. The diet with soya protein was the same as the control diet but we replaced red meat with soya protein. Values are means, with their standard errors represented by vertical bars. There was a significant effect of diet on TAC (P < 0·01) and MDA (P < 0·01) (repeated-measures ANOVA).
Compared with the control diet, plasma phyto-oestrogen increased significantly after the soya nut regimen (percentage change +64 %; P < 0·01) and the soya protein diet (percentage change +48 %; P < 0·01) (data not shown).
Discussion
The present study assessed the effects of soya protein and soya nut consumption on plasma TAC and MDA as a biomarker of oxidative stress in postmenopausal women with the metabolic syndrome. The present data suggested that both soya protein and soya nut intake raised the TAC and decreased MDA. This result is in line with a recent paper, which showed that plasma TAC was modestly higher (10 %) at the end of the soya protein phases than during the animal protein phases, regardless of the isoflavone content of the dietReference Vega-Lopez, Yeum, Lecker, Ausman, Johnson, Devaraj, Jialal and Lichtenstein19. An earlier multiple regression analysis also showed that after 12 weeks of soya protein consumption TAC was increasedReference Swain, Alekel, Dent, Peterson and Reddy32. A report on patients with hypercholesterolaemiaReference Bricarello, Kasinski, Bertolami, Faludi, Pinto, Relvas, Izar, Ihara, Tufik and Fonseca33 suggested a reduction in lipid peroxidation as estimated by thiobarbituric acid-reactive substances, after 6 weeks of soya milk consumption. Investigators mentioned that the antioxidant activity of soya might be related to its phyto-oestrogens or phytic acid contentReference Rufer and Kulling34, Reference Porres, Stahl, Cheng, Fu, Roneker, Pond and Lei35. The antioxidant effect of soya phyto-oestrogens may be due to donating hydrogen atoms to free radicals, so making them less reactiveReference Mitchell, Gardner, McPhail, Morrice, Collins and Duthie36. Another possible mechanism, shown in a mouse modelReference Cai and Wei37, may be to increase antioxidant enzyme concentrations. In another way, the phytate in soya may quench free radicals because of its metal-chelating abilityReference Rufer and Kulling34. Nevertheless, Engelman et al. Reference Engelman, Alekel, Hanson, Kanthasamy and Reddy22 showed that neither phytate nor isoflavones in soya protein isolate had a significant effect in reducing oxidative damage. It seems that the absorption of phytate in human is very lowReference Engelman, Alekel, Hanson, Kanthasamy and Reddy22.
The soya products used in the present study contained both protein and isoflavones. According to previous reports, full-fat flaxseed, despite high concentrations of antioxidants, had no significant effect on thiobarbituric acid-reactive substancesReference Cunnane, Hamadeh, Liede, Thompson, Wolever and Jenkins38, Reference Cunnane, Ganguli, Menard, Liede, Hamadeh, Chen, Wolever and Jenkins39, which might be related to the high content of α-linolenic acid in the full-fat flaxseed. In the present study, the effects of both soya protein and soya nuts on the markers of oxidative stress and TAC were similar, despite slightly higher amounts of phyto-oestrogens in soya nuts. Similarly, this may be due to the higher fat content of soya nuts.
In contrast to the present findings, Vega-Lopez et al. Reference Vega-Lopez, Yeum, Lecker, Ausman, Johnson, Devaraj, Jialal and Lichtenstein19 reported higher amounts of MDA after isoflavone-supplemented dietary periods and Wiseman et al. Reference Wiseman, O'Reilly, Adlercreutz, Mallet, Bowey, Rowland and Sanders21 reported no significant change in plasma MDA after soya consumption regardless of dietary isoflavones. In the present study, participants had the metabolic syndrome, which is associated with higher level of MDA. However, healthy subjects participated in the Wiseman et al. studyReference Wiseman, O'Reilly, Adlercreutz, Mallet, Bowey, Rowland and Sanders21. Conflicting results in different studies may be due to subject selection, doses of isoflavones and even interindividual variation in the ability to metabolise daidzein to equolReference Wiseman, O'Reilly, Adlercreutz, Mallet, Bowey, Rowland and Sanders21, Reference Engelman, Alekel, Hanson, Kanthasamy and Reddy22. Equol has antioxidant characteristics. So, higher amounts of equol production may increase the plasma TACReference Wiseman, O'Reilly, Adlercreutz, Mallet, Bowey, Rowland and Sanders21, Reference Engelman, Alekel, Hanson, Kanthasamy and Reddy22.
Moreover, some human subjects have higher rates of lipid peroxidation than others, even when consuming similar dietsReference Roberts and Morrow40. Kris-Etherton & WestReference Kris-Etherton and West41 mentioned that antioxidants may have beneficial effects on other pathways; for example, soya antioxidants may increase endothelial NO synthase activity which is associated with enhanced endothelial function.
There are multiple biomarkers of oxidative stress, which have strengths and limitationsReference Therond, Bonnefont-Rousselot, Davit-Spraul, Conti and Legrand42. There appears to be agreement that plasma concentrations of F2-isoprostanes may be the best biomarker of lipid peroxidation in healthy human subjectsReference Roberts, Montine, Markesbery, Tapper, Hardy, Chemtob, Dettbarn and Morrow43, Reference Basu44. Wiseman et al. Reference Wiseman, O'Reilly, Adlercreutz, Mallet, Bowey, Rowland and Sanders21 in a cross-over trial on healthy subjects concluded that the consumption of soya containing naturally occurring amounts of phyto-oestrogens reduced F2-isoprostane concentrations. Another cross-over study showed that dark soya sauce decreased F2-isoprostane more than placebo in healthy subjectsReference Lee, Isaac, Wang, Huang, Long, Jenner, Kelly and Halliwell45. We used plasma MDA concentration as a lipid peroxidation marker, because previous investigators suggested that this marker is probably best suited for use in clinical conditions such as the metabolic syndrome, where there is increased lipid peroxidation and thus elevated MDA productionReference Chirico, Smith, Marchant, Mitchinson and Halliwell46. However, plasma MDA reduction is an indirect marker of lipid peroxidation, and this result should be confirmed by direct assessment of lipid peroxidation in future studies.
Presently, no marker of oxidative stress can indicate both antioxidant status and oxidative stressReference Prior, Cao, Prior and Cao47; therefore, we evaluated TAC for assessing antioxidant status and MDA for measuring oxidative stress.
In the present study in all three diet periods, participants consumed the DASH diet, which includes many different antioxidants. Therefore, the possible interaction between different antioxidants in the body, which may increase the overall antioxidant status and obscure the effect of soya, is one of the limitations of the present study.
Soya products have gained considerable attention for their phyto-oestrogen contents and their possible role in reducing CVD risksReference Sacks, Lichtenstein, Van Horn, Harris, Kris-Etherton and Winston48, Reference Sacks49. Reduction of oxidative stress status may be one of the possible mechanism which needs further investigation. One of the major advantages of the present study and the consistent response of plasma TAC and MDA is that the subjects selected had increased oxidative stress resulting from the metabolic syndrome.
The acute effects on plasma TAC of the consumption of flavonoid-rich foods may be explained by changes in the concentration of the metabolic antioxidant uric acid. On the other hand, fructose has been known for more than 30 years to increase plasma urate levels consequent to its rapid metabolism by fructokinase. Fructose metabolism in this manner leads to a transient decrease in hepatic ATP and inorganic phosphate, which are important inhibitors of 5′-nucleotidase and AMP deaminase, respectively, and thus increased degradation of AMP to uric acid. High levels of methylxanthines in flavonoid-rich foods and their rapid absorption and metabolism to methyl uric acid derivatives could significantly increase plasma TACReference Lotito and Frei50. However, we did not measure uric acid levels in the present study.
In conclusion, the present findings suggest that substituting soya products instead of red meat in the DASH eating pattern may reduce MDA and increase the TAC in postmenopausal women with the metabolic syndrome.
Acknowledgements
The authors express appreciation to the participants of the present study for their enthusiastic support. None of the authors have any personal or financial conflicts of interest. The study was supported by a grant from the National Nutrition and Food Technology Research Institute, Shaheed Beheshti University of Medical Sciences.









