Ultra-processed foods are defined by the NOVA classification system as industrialised foods made mostly from substances extracted from whole foods, with chemical modifications, added of unmodified and modified food substances with frequent addition of cosmetic additives and sophisticated packaging(Reference Monteiro, Cannon and Levy1). The FAO of the United Nations and national guidelines report the impact of the high consumption of ultra-processed foods on diet quality and health outcomes and recommend initiatives to promote the consumption of unprocessed or minimally processed foods and to reduce ultra-processed food intake(2–4).
Studies in different countries have shown that the high consumption of ultra-processed foods has negative effects on diet quality(Reference Louzada, Ricardo and Steele5–Reference Moubarac, Batal and Louzada7) and health, including metabolic syndrome(Reference Nasreddine, Tamim and Itani8), excess weight(Reference Silva, Giatti and Figueiredo9) and chronic non-communicable diseases(Reference Rauber, Louzada and Steele10), primarily because these foods are high in energy density, fats, carbohydrates, Na and additives. In addition, a study in Spain reported that higher ultra-processed food intake was associated with an increased risk of all-cause mortality(Reference Rico-campà, Martínez-gonzález and Alvarez-alvarez11), demonstrating the importance of studying the consumption of these foods and their impact at different stages of life.
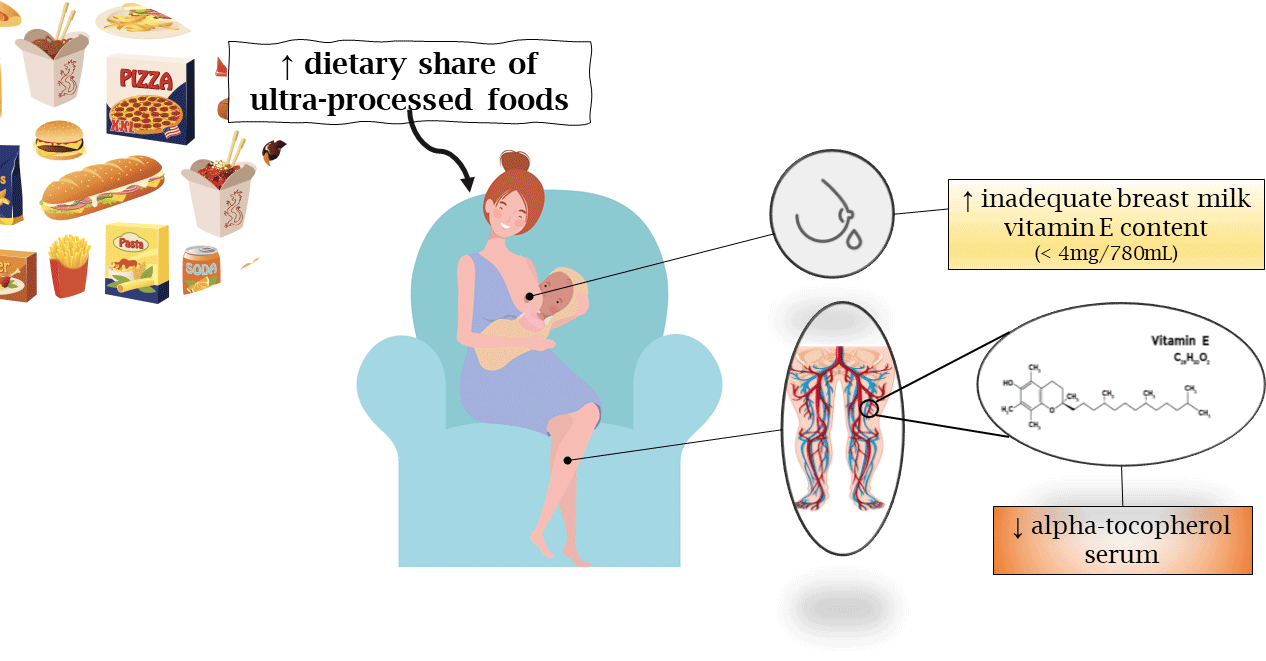
The consumption of ultra-processed foods has also been linked to dietary micronutrient intake. In Brazil, the higher dietary share of ultra-processed foods was associated with the lower dietary content of vitamin E(Reference Louzada, Martins and Canella12). These findings underscore the need to investigate the impact of ultra-processed foods on vitamin E status during lactation, since the maternal diet should ensure the adequate nutritional composition of breast milk to address the specific needs of the infant and avoid inadequate vitamin E status, which is common in newborns(Reference Debier and Larondelle13).
The vitamin E has important antioxidant roles in both intra-uterine and postnatal life, reacting with free radicals to prevent lipid peroxidation of cell membranes. This nomenclature represents eight forms synthesised by plants and the α-tocopherol is the bioactive constituent and the most abundant isomer found in human plasma and tissues(Reference Traber, Ross and Caballero14). Its absorption and circulation are strongly influenced by type of fat available in the meal. The RRR-α-tocopherol form present in the natural foods is preferentially secreted in VLDL than the racemic form found in many supplements or fortified foods (industrialised foods), causing a greater exchange to milk and greater circulation(Reference Clemente, Ramalho and Lima15).
The influence of ultra-processed food intake on the composition of breast milk has not been examined. Studies of breast milk(Reference Rebouças, Silva and Oliveira16,Reference Silva, Rebouças and Mendonça17) have found low concentrations of α-tocopherol considering the average volume of breast milk consumed daily by babies (780 ml) and the recommendation of vitamin E intake (4 mg/d)(18). Other data in monkeys indicate that a mixed diet of breast milk and industrialised foods containing synthetic α-tocopherol stereoisomers leads to lower brain 2R-α-tocopherol forms in infants(Reference Kuchan, Ranard and Dey19). If the effect is repeated, this insufficiency may expose the newborn to vitamin E deficiency (VED), since babies have low vitamin E status at birth(Reference Ribeiro, Lima and Medeiros20) due to limited placental transfer and a high demand for antioxidants to prevent oxidative stress and develop the nervous and immune system(Reference Debier and Larondelle13).
Thus, considering the increasing share of ultra-processed foods on the population’s diet and its impact on health and diet quality, the lack of research examining the association between ultra-processed food intake during lactation and breast milk composition and vitamin E biomarkers, the nutritional composition of ultra-processed foods (mix of natural and synthetic α-tocopherol stereoisomers), this study hypothesised that a higher share of ultra-processed foods in the diet might influence the vitamin E nutritional status in lactating women, especially on dietary intake of vitamin E and maternal serum and breast milk α-tocopherol concentrations.
Methods
Study design and ethical clearance
This study is a cross-sectional analysis using cohort study data(Reference Rodrigues21,Reference Silva22) collected between 2012 and 2018 in public health care institutions in the State of Rio Grande do Norte, Brazil, in accordance with ethical, legal and regulatory norms and standards for research involving human subjects, having been approved by the Federal University of Rio Grande do Norte’s Research Ethics Committee. The study’ purpose was to evaluate the vitamin A and E nutritional status of lactating women and their babies and the relationship between dietary intake and concentrations in serum and breast milk. Both summed a total of 351 lactating women with same sampling procedure, inclusion and exclusion criteria.
The participants were lactating women over 18 years old whose breast milk started being collected 30 d postpartum. The following inclusion criteria were used for the cohorts: women whose babies were born without birth defects and congenital disorders; women without infectious diseases and fat malabsorption; non-smokers; non-drinkers; and women who did not take vitamin E supplements during pregnancy(Reference Rodrigues21,Reference Silva22) . Women who reported implausible energy intake (defined as ≥20,920 kJ/d or ≤ 2,092 kJ/d) or taking daily supplements containing vitamin E were excluded. After applying the exclusion criteria, 294 women participated in this study (84 % of women in the main studies).
Sample size
The sample size was calculated to detect a minimum difference of 11 % between α-tocopherol concentrations in the mature breast milk evaluated according to the intake of vitamin E(Reference Silva, Rebouças and Mendonça17), based on a study with similar population in Brazil. Vitamin E intakes were adopted due to the lack of research investigating the connections between ultra-processed food intake and vitamin E levels in breast milk, and this composition increases after ingestion of high amounts of the vitamin, especially in supplementation cases(Reference Rebouças, Silva and Oliveira16). In addition, in Brazil, some ultra-processed foods are fortified with vitamins, especially margarines, fats, biscuits, breads and modified powder milk. Thus, their intake could influence the dietary vitamin E.
The G * Power 3.1.9 software(Reference Faul, Erdfelder and Lang23) was used with a significance level of 5 %, power of 80 % and expected effect size of 0·5, considering the comparison between three independent groups by ANOVA test. These groups of lactating women were divided per tertiles of energy contribution of ultra-processed foods to total energy intake foods. A sample size of 42 lactating women was estimated for each group with total of 126 women.
Procedures and measures
The participants answered a semi-structured questionnaire structured to collect information on socio-economic characteristics, health and dietary intake. Breast milk and blood (≈ 8 ml) were collected in the morning, after an overnight fasting(Reference Rodrigues21,Reference Silva22) . The milk was collected by manual expression of a single breast not previously suckled. The samples were transported under refrigeration temperature, centrifuged for extraction (blood) and stored at –20 °C for 1 week prior to analysis.
Data on food consumption were obtained using a 24-h dietary recall administered on three separate occasions at 30-d intervals between 7 and 100 d after birth (Fig. 1) and have been included 1 d on weekend(Reference Rodrigues21,Reference Silva22) . The recalls were administered by nutritionists or nutrition students trained and qualified with the help of photographic book for the identification of household food measurements. These data were collected in the hospital or at the participants’ houses.

Fig. 1. Data collection of lactating women, Rio Grande do Norte, Brazil (2012–2018).
The recall registered all foods, culinary preparations, supplements and drinks consumed by the respondent the day before the interview. Household food measurements were converted into g or ml based on household measurement standard equivalents(Reference Araújo and Guerra24,25) . The mean of the participants’ 24-h recalls was considered as their food consumption (the values of vitamin E intakes were not different between collections – P > 0·05).
The vitamin E and energy intake data were analysed using the software Virtual Nutri Plus (http://www.virtualnutriplus.com.br), through the creation of a database with information from the Brazilian Food Composition Table (TACO)(26) and the USDA National Nutrient Database for Standard Reference, version 2015(27). Dietary vitamin E was expressed in mg/4,184 kJ and mg/d. Vitamin E intake below the estimated average requirement of 16 mg/d was considered inadequate(18). No woman was supplemented with vitamin E.
The foods were grouped according to the NOVA classification system as follows: (a) unprocessed or minimally processed foods that include minimal processes applied to single fresh foods by the removal of parts, fractioning, freezing and packaging (fruits, vegetables, milk, eggs, poultry, meat, legumes and others). The processed culinary ingredients include substances extracted and purified from unprocessed or minimally processed foods used in the household or restaurants, such as oil, sugar and salt. The processed foods include relatively industrialised or artisanal simple foods manufactured by addition of culinary ingredients to unprocessed or minimally processed foods, while the ultra-processed foods include industrial formulations elaborated from substances extracted or obtained from foods or their synthetic constituents, by hydrogenation, hydrolysis, extruding, molding, reshaping, with frequent addition of cosmetic additives (flavouring agents, colours, emulsifiers, humectants and others) to imitate sensorial properties of foods and culinary preparations(Reference Monteiro, Cannon and Levy1). All culinary preparations were disaggregated into ingredients using standardised forms.
The percentage contribution of each NOVA food group relative to overall energy intake (dietary share) was then assessed. The lactating women were then grouped into tertiles of contribution of ultra-processed foods to total dietary energy intake (dietary share of ultra-processed foods). The ultra-processed foods were divided into nine groups of similar foods to identify the main ultra-processed food sources(Reference Louzada, Ricardo and Steele5).
The extraction of α-tocopherol from serum and breast milk was performed using methods adapted from Ortega and collaborators(Reference Ortega, López-Sobaler and Martínez28). Equal volumes of ethanol (95 %) were added to samples to precipitate proteins (Vetec) and extraction was conducted twice using 2 ml of hexane PA (Vetec) as an extraction reagent. After hexane’s evaporation in N, serum and milk residues were dissolved in 250 μl of absolute ethanol (Vetec) and 250 µl of dichloromethane (Vetec): methanol (Sigma-Aldrich, EUA) (2:1; v/v), respectively. The extracted α-tocopherol was analysed by HPLC using a Shimadzu LC-20AT chromatography system (Shimadzu Corporation), with a 20 µl injection loop, and a SPD-20A UV-VIS detector (Shimadzu Corporation), connected to a CBM-20A communicator (Shimadzu Corporation). Chromatographic separation was obtained using a LiChroCART 250-4 reverse phase column (Merck), with 100 % methanol, 292 nm wavelength and α-tocopherol standard (Sigma)(Reference Rodrigues21,Reference Silva22) .
The quality control on HPLC was evaluated. The linearity of the method was confirmed by the calibration curve coefficient in the concentration range of 3·4–53·7 µmol/l α-tocopherol (r 2 0·9998), a limit of quantification of 0·21 µmol/l, a limit of detection of 0·09 µmol/l and a CV of 0·01 % at 23·2 µmol/l for the standard and 0·05 % at 41·1 µmol/l for the milk samples. The method’s precision was evaluated through the recovery test (103 % for α-tocopherol acetate). Serum α-tocopherol values under 12 µmol/l(18) were taken to indicate VED.
The breast milk vitamin E (BMVE) adequacy was assessed according to the estimated daily supply of α-tocopherol from the milk analysed, based on a volume of 780 ml (the average volume of human milk consumed by infants aged 0–6 months) and the recommended adequate intake of vitamin E for infants (4 mg/d)(18), thus setting the cutoff value for ideal BMVE adequacy at 4 mg/780 ml or higher.
Statistical analysis
The dataset was analysed using SPSS 7.0 (IBM Corporation). The Kolmogorov–Smirnov normality test was applied to examine the distribution of the variables. The χ 2 test and Kruskal–Wallis test were used to identify differences in socio-demographic and anthropometric characteristics, and in serum, BMVE and vitamin E intake values across the tertiles of energy contribution of ultra-processed foods, respectively.
To verify the association between ultra-processed food intake and vitamin E biomarker, the population was divided according to the tertiles of the ultra-processed food contribution to the diet (percentage of energy intake). The ones who consumed the least belonged to the first tertile, while the ones who consumed the most belonged to the third tertile. After that, the following was estimated: (1) overall population’s average dietary vitamin E (mg/d), (2) adequate/inadequate BMVE adequacy (≥ 4 or < 4 mg/d), (3) milk α-tocopherol (µmol/l) and (4) serum α-tocopherol (µmol/l) (both vitamin E biomarkers) adjusted for family income per capita, since income is a confounding variable for the analysis of the consumption of ultra-processed foods(Reference Silva, Giatti and Figueiredo9,Reference Louzada, Martins and Canella12) and maternal age. Linear regression models were performed to evaluate the association between ultra-processed food intake (independent variable) and vitamin E biomarkers (outcome variables). The significance level was set at P < 0·05.
Results
Regarding socio-economic and health characteristics, 16·9 % (n 49) had not completed primary education and 4·5 % (n 13) were living below the poverty line (per capita income lower than 20 % of the minimum salary)(29). As for nutritional status, the high percentage of overweight and obese women stands out, being found in 51·4 % of the participants during pregnancy and in 70·7 % during lactation (Table 1).
Table 1. Socio-demographic and anthropometric characteristics of lactating women grouped in tertiles of energy contribution of ultra-processed foods to total energy intake, Rio Grande do Norte, Brazil (2012–2018)
(Numbers and percentages)

* Chi-square test.
† Cutoff point for poverty by the Bolsa Família Program(29).
‡ Atalah et al.(Reference Atalah, Castillo and Castro45) (BMI ratio by gestational age).
§ World Health Organization(46).
Mean energy intake was 8,991.4 kJ/d, being 16 % from ultra-processed foods, while 51 % from unprocessed or minimally processed foods, which are responsible for the highest density of vitamin E (1·20 mg/4,184 kJ) (Table 2). Breads and biscuits accounted for over half (57 %) of the energy intake from ultra-processed foods, followed by fats (mayonnaise and, mainly, margarines) (13 %) (Fig. 2).
Table 2. Mean energy from unprocessed or minimally processed foods, processed culinary ingredients, processed foods and ultra-processed foods in lactating women, Rio Grande do Norte, Brazil (2012–2018)
(Mean values and minimum–maximum)

* Monteiro et al. (Reference Monteiro, Cannon and Levy1,Reference Monteiro, Levy and Claro32) . The unprocessed or minimally processed foods include fresh foods or foods altered by removal of inedible parts, fractioning, freezing and packaging. For example, fruits, vegetables, grains, legumes, meat, poultry, fish, eggs and milk. The processed culinary ingredients include substances obtained directly from food or from nature and commonly used in culinary preparations and rarely consumed in the absence of unprocessed foods. For example, salt, sugar and oils. The processed foods are industrialised products or artisanal made by adding culinary ingredient to unprocessed or minimally processed foods. Ultra-processed foods are products that undergo industrial processes that include hydrogenation, hydrolysis, extruding, molding, reshaping and are often add cosmetic additives (flavouring agents, colours, emulsifiers, humectants and others) to imitate sensorial properties of foods and culinary preparations.

Fig. 2. Participation of ultra-processed foods grouped according to characteristics, with the highest values from left to right. Rio Grande do Norte, Brazil (2012–2018). ![]() , breads and biscuits;
, breads and biscuits; ![]() , milk products;
, milk products; ![]() , pasta;
, pasta; ![]() , fats;
, fats; ![]() , treats;
, treats; ![]() , sugary drinks;
, sugary drinks; ![]() , processed meats;
, processed meats; ![]() , cereal-based foods;
, cereal-based foods; ![]() , sauces and condiments.
, sauces and condiments.
The mean dietary share of ultra-processed foods in the first, second and third tertiles was 4·6, 13·8 and 30·17 %, respectively. No significant differences in characteristics were found between the tertiles (P > 0·05), except for age, where the percentage of young women (19–27 years) was greater in the highest tertile.
Mean vitamin E intake was 6·19 mg/d and below estimated average requirement (16 mg/d) in 100 % of the lactating women. Unprocessed or minimally processed and ultra-processed foods accounted for 44 and 27 % of total dietary vitamin E intake, respectively. However, there was no difference in vitamin E intake between women grouped in tertiles of energy contribution for the consumption of ultra-processed foods for the total energy intake (Table 3).
Table 3. Association between dietary share of ultra-processed foods and nutritional indicators of vitamin E. Linear regression model – crude and adjusted for family income per capita and maternal age. Rio Grande do Norte, Brazil (2012–2018)
(95 % confidence intervals)

* Evaluation of the adequacy of the estimated daily vitamin E supply from the analysed milk (≥4 or <4 mg of vitamin E/ 780 ml).
Mean serum α-tocopherol was 26·55 (7·98) µmol/l and 5 % (n 11) of the samples showed VED (< 12 µmol/l), despite consumption below recommendation in 100 % of women. The lowest tertile of dietary share of ultra-processed foods showed higher mean serum α-tocopherol values than other tertiles (Table 3).
Mean breast milk α-tocopherol was 8·40 (3·94) µmol/l. No differences were found between the tertiles (Table 3). The BMVE adequacy showed mean values of 2·82 (1·33) mg/780 ml of vitamin E, which is below the 4 mg cutoff point (78 % were below).
The regression analysis results showed a significant inverse association between dietary share of ultra-processed foods and BMVE adequacy (≥ 4 mg/d) (β = –0·144, P = 0·012) and concentration of serum α-tocopherol (µmol/l) (β = –0·168, P = 0·003) in both the crude model and model adjusted for family income per capita (Table 3).
Discussion
The evidence shows the negative impact of the consumption of ultra-processed foods on diet quality(Reference Silva, Giatti and Figueiredo9). It is known that maternal diet should ensure the adequate nutritional composition of breast milk(Reference Debier and Larondelle13), highlighting the importance of examining the relationship between the intake of these foods and the supply of nutrients in breast milk.
The findings show that the energy contribution of ultra-processed foods (16 %) was similar to that found for the Brazilian population (18·4 %)(30) and lower than values reported in the USA(Reference Steele, Popkin and Swinburn6) (57 %), Canada(Reference Moubarac, Batal and Louzada7) (47 %) and Chile(Reference Cediel, Reyes and Carvalán31) (28 %), suggesting that the consumption of unprocessed or minimally processed foods and culinary preparations made from these foods is high among the Brazilian population(30).
The most frequently consumed ultra-processed foods were breads and biscuits, followed by fats and processed meats (Fig. 2). Other studies have shown similar results, reporting that biscuits and candies (confectionery) comprised the highest proportional contribution to dietary energy intake(Reference Louzada, Ricardo and Steele5), processed meats accounted for a significant share of ultra-processed foods(Reference Rico-campà, Martínez-gonzález and Alvarez-alvarez11) and that breads were the ultra-processed foods that contributed most to energy intake(Reference Monteiro, Levy and Claro32). In contrast to other studies, margarine was one of the most frequently consumed ultra-processed foods because it is used as a substitute for other types of edible oils in developing countries(33).
During lactation, an increase in vitamin E intake is recommended and dietary reference intakes for vitamin E during this phase to ensure adequate breast milk composition(Reference Debier and Larondelle13,18) . Inadequate vitamin E intake increases susceptibility to vitamin E inadequacy, with consequences for the physiological functions of vitamin E, such as its roles as an antioxidant and in modulating cell signalling and cellular transcription, of which benefits include decreased cardiovascular risks. In the postpartum period, breast-feeding is the main way of preventing vitamin E inadequacy in infants, since newborns may have low body reserves of vitamin E. Breast milk, particularly colostrum, is a concentrated source of vitamin E with high absorptive capacity, reaffirming the importance of studying the relationship between diet and vitamin E biomarkers in lactating women(Reference Rodrigues21).
Vitamin E intake was under the recommended intake during lactation in all respondents, aligned with the findings of other studies(Reference Antonakou, Chiou and Andrikopoulos34,Reference Mata, Silva and Medeiros35) . The low intake of vitamin E reinforces the importance of characterising the risk of developing VED in both the mother and the infant and may be due to inadequate maternal consumption of classic sources of vitamin E, such as sunflower oil, nuts and olive oil(Reference Cozzolino and Cozzolino36). In contrast to the present study (Tables 2 and 3), Louzada (Reference Louzada, Martins and Canella12) reported that higher dietary share of ultra-processed foods had a negative impact on vitamin E intake. The absence of this association may have been due to the type of vitamin E in the foods, in addition to low consumption by the entire population studied. It is also important to highlight that the study by Louzada et al. included men and women, socio-economic categories and age groups, and that the participants consumed a wider variety of vitamin E food sources. In this regard, the fact that vitamin E food consumption among the participants was similar may be considered one of the limitations of the present study.
To the best of the authors’ knowledge, this is the first study to examine the association between the consumption of ultra-processed foods and breast milk adequacy and vitamin E biomarkers. This study’s findings show that a higher dietary share of ultra-processed foods containing synthetic α-tocopherol stereoisomers can lead to lower α-tocopherol serum and a tendency to BMVE inadequacy, even when vitamin E intake was low and similar among participants.
This association may be due the fact that ultra-processed foods can be fortified with racemic forms of vitamins, mainly in margarine, which have lower bioavailability than occurring natural or synthetic 2R forms, the unique form of α-tocopherol that should be counted towards vitamin E intake(Reference Clemente, Ramalho and Lima15,18) . Natural forms of vitamin E (like 2R-α-tocopherol) are the predominant isomers in breast milk(Reference Clemente, Ramalho and Lima15,Reference Kuchan, Moulton and Dyer37) , serum(Reference Gaur, Kuchan and Lai38) and cerebral tissue(Reference Kuchan, Ranard and Dey19), suggesting that natural vitamins have higher bioavailability and that there is discrimination against the synthetic 2R stereoisomers in mammary tissue and liver (and synthetic vitamin E appears not to be toxic); therefore, more 2R-α-tocopherol from unprocessed or minimally processed foods can be transferred to breast milk and blood, and consequently to the infant fed, than the vitamin present in the ultra-processed foods. In addition, Brazilian legislation(39) allows vitamin E fortification, generally in its racemic form (all-rac-α-tocopherol), but the dietary levels of synthetic α-tocopherol and stereoisomers in both serum and milk were not evaluated since only 2R forms of α-tocopherol are counted towards vitamin E intakes(18). This fact may be an important limitation of our study and may lead to interesting findings in future research.
Another finding that reinforces the impact of ultra-processed food consumption in the vitamin E during lactation is that in animal models (monkeys), infants fed with ultra-processed infant formulas containing synthetic forms of vitamin E had higher profile of synthetic stereoisomers and a lower circulating α-tocopherol and α-tocopherol in cerebral tissue. This result demonstrates that the synthetic stereoisomers competitively reduce the concentration of 2R-α-tocopherol in tissues and, thus, decreasing the level of the most potent stereoisomer(Reference Kuchan, Ranard and Dey19). But it is not yet known whether the same occurs in humans, and it is unclear how much 2S-α tocopherol is present in human tissues since this form is actively metabolised.
Other studies have pointed to low levels of vitamin E in breast milk(Reference Garcia, Ribeiro and de Araújo40,Reference Machado, Kamp and Nunes41) , but did not examine the relationship between vitamin E levels and consumption of ultra-processed foods. Although ultra-processed foods reduce vitamin E adequacy in breast milk and serum α-tocopherol concentrations, the concentrations are still considered adequate and do not suggest deficiency. Even so, the findings of this study highlight the effects of the consumption of ultra-processed foods during lactation on both mother and infant as maternal serum α-tocopherol levels are associated with low circulating levels in the child, even without maternal deficient serum levels(Reference Ribeiro, Lima and Medeiros20). Moreover, a reduced supply of vitamin E in the breast milk or maternal concentration can cause greater oxidative damage due to reduced antioxidant protection in the infant provided by vitamin E(Reference Brion, Bell and Raghuveer42) and gene expression genes involved in transcription regulation and synapse formation in the hippocampus are very sensitive to subtle changes in α-tocopherol concentration(Reference Rhodes, Rendeiro and Mun43). This in turn makes the infant, particularly premature babies, more susceptible to the development of clinical complications related to VED, such as hemolytic anaemia, retrolental fibroplasia, intracranial haemorrhage and bronchopulmonary dysplasia(Reference Brion, Bell and Raghuveer42). Additionally, in neonate, low α-tocopherol in cord blood might suppress adiponectin expression, suggesting a potential beneficial role of vitamin E in early life in preventing the development of childhood obesity(Reference Du, Luo and Nuyt44).
This study’s results reaffirm the importance of developing maternal and child nutrition care policies that promote the consumption of unprocessed or minimally processed foods, as well as sources of vitamin E. Further research should examine the relationship between ultra-processed food intake (and its composition regarding the different forms of vitamin E) among lactating women and clinical outcomes in exclusively breastfed babies to gain a deeper understanding of the impact of consumption on breast milk quality and, consequently, the health of infants whose only source of nutrients is breast milk.
Acknowledgements
The authors thank the scientific initiation students Danna Calina, Amanda Freitas, Juliana Amorim; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES), the Maternity School Januário Cicco (MEJC) and the University Hospital Ana Bezerra (HUAB).
This work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – Finance Code 001.
N. A.: analysed the data, interpreted the findings and wrote the article. K. D. S. R.: project administration, formulated the research question, designed the study, analysed the data, interpreted the findings and reviewed and edited the article. L. C. P. L.: analysed the data and reviewed the results. A. G. C. L. S., A. S. R., D. B., M. S. R. L., J. F. P. M.: carried out the study, analysed the data. R. D.: supervised the study and investigation, analysed the data. All authors reviewed the study.
There are no conflicts of interest.